FSH Stimulation with Short Withdrawal Improves Oocyte Competence in Italian Mediterranean Buffalo (Bubalus bubalis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
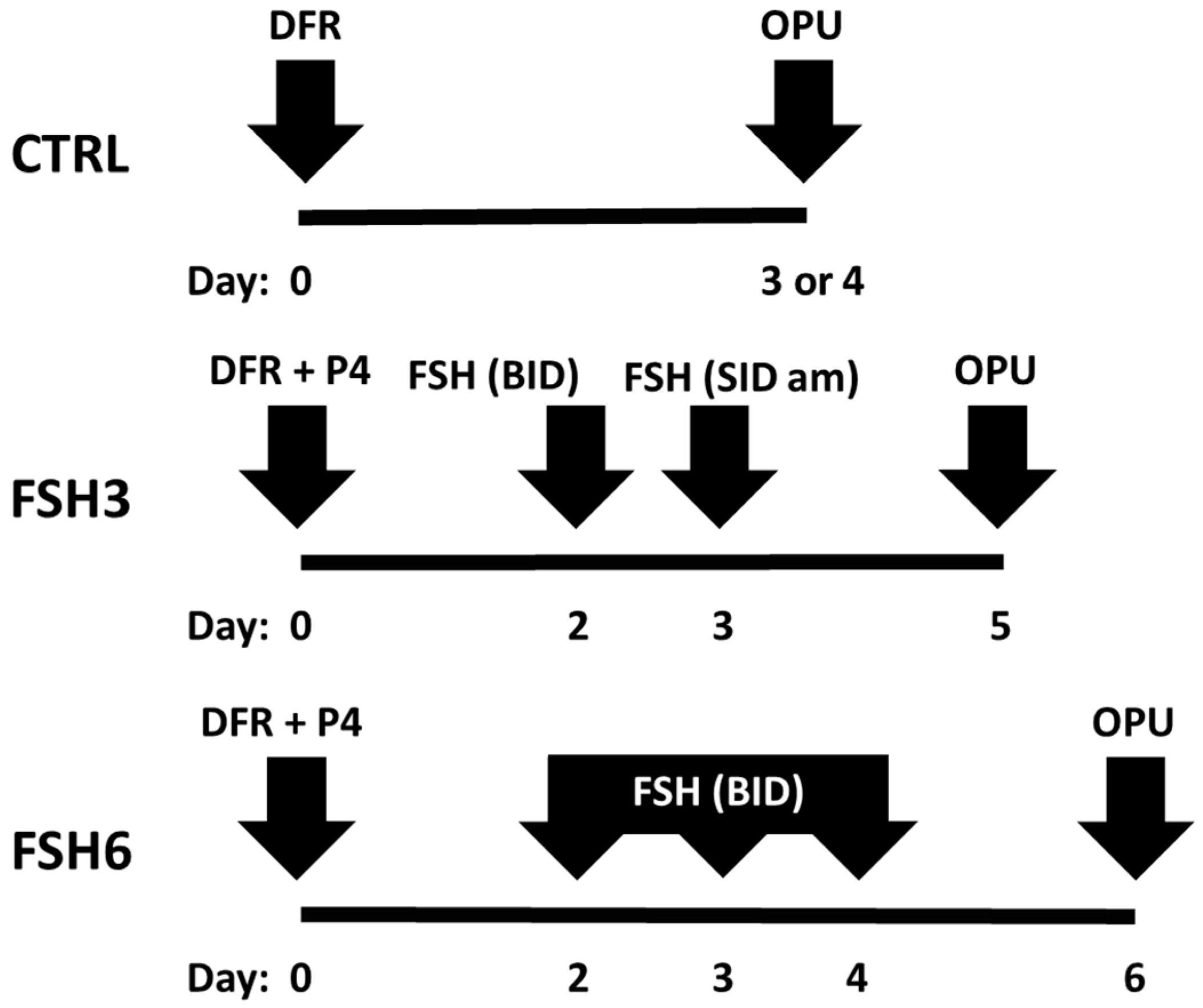
2.2. Experimental Design
2.3. Ovum Pick-Up Procedure and COC Processing
2.4. In Vitro Embryo Production
2.5. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zicarelli, L. Influence of seasonality on buffalo production. In The Buffaloes (Bubalus bubalis)—Production and Research; Presicce, G.A., Ed.; Bentham Science Publishers: Sharjah, UAE, 2016; pp. 196–224. [Google Scholar]
- Cruz, L.C. Trends in buffalo production in Asia. Ital. J. Anim. Sci. 2007, 6 (Suppl. S2), 9–24. [Google Scholar] [CrossRef]
- Misra, A.K.; Joshi, B.V.; Agrawala, P.L.; Kasiraj, R.; Sivaiah, S.; Randareddi, N.S.; Siddiqui, M.U. Multiple ovulation and embryo transfer in Indian buffaloes (Bubalus bubalis). Theriogenology 1990, 33, 289. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Soares, J.G.; Bayeux, B.M.; Silva, J.C.B.; Mingoti, R.D.; Carvalho, N.A.T. Assisted reproductive technologies (ART) in water buffaloes. Anim. Reprod. 2018, 15 (Suppl. S1), 971–983. [Google Scholar] [CrossRef]
- Salzano, A.; De Canditiis, C.; Della Ragione, F.; Prandi, A.; Zullo, G.; Neglia, G.; Campanile, G. Evaluation of factors involved in the failure of ovum capture in superovulated buffaloes. Theriogenology 2018, 122, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, B. In vitro embryo production in buffalo species: State of the art. Theriogenology 2002, 57, 237–256. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Yang, B.; Chen, M.; Huang, F.; Pang, C.; Liao, C. Repeated Ovum Pick-Up and In-vitro Embryo Production in Buffalo. Ital. J. Anim. Sci. 2007, 6 (Suppl. S2), 759–761. [Google Scholar] [CrossRef]
- Neglia, G.; Gasparrini, B.; Vecchio, D.; Boccia, L.; Varricchio, E.; Di Palo, R.; Zicarelli, L. Long term effect of Ovum Pick-up in buffalo species. Anim. Reprod. Sci. 2011, 123, 180–186. [Google Scholar] [CrossRef]
- Danell, B. Oestrous Behaviour, Ovarian Morphology and Cyclical Variation in Folicular System and Endocrine Pattern in Water Buffalo Heifers. Ph.D. Thesis, Faculty of Veterinary Medicine, Swedish University of Agricultural Sciences, Uppsala, Sweden, 1987; p. 124. [Google Scholar]
- Gimenes, L.U.; Fantinato Neto, P.; Arango, J.S.P.; Ayres, H.; Baruselli, P.S. Follicular dynamics of Bos indicus, Bos taurus and Bubalus bubalis heifers treated with norgestomet ear implant associated or not to injectable progesterone. Anim. Reprod. 2009, 6, 256. [Google Scholar]
- Di Francesco, S.; Boccia, L.; Campanile, G.; Di Palo, R.; Vecchio, D.; Neglia, G.; Zicarelli, L. The effect of season on oocyte quality and developmental competence in Italian Mediterranean buffaloes (Bubalus bubalis). Anim. Reprod. Sci. 2011, 123, 48–53. [Google Scholar] [CrossRef]
- Di Francesco, S.; Novoa, M.V.S.; Vecchio, D.; Neglia, G.; Boccia, L.; Campanile, G.; Zicarelli, L. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 2012, 77, 148–154. [Google Scholar] [CrossRef]
- Gasparrini, B.; Neglia, G.; Di Palo, R.; Vecchio, D.; Albero, G.; Esposito, L.; Campanile, G.; Zicarelli, L. Influence of oocyte donor on in vitro embryo production in buffalo. Anim. Reprod. Sci. 2014, 144, 95–101. [Google Scholar] [CrossRef]
- Baldrighi, J.; Sá Filho, M.; Batista, E.; Lopes, R.; Visintin, J.; Baruselli, P.; Assumpção, M. Anti-Mullerian Hormone Concentration and Antral Ovarian Follicle Population in Murrah Heifers Compared to Holstein and Gyr Kept Under the Same Management. Reprod. Domest. Anim. 2014, 49, 1015–1020. [Google Scholar] [CrossRef]
- Liang, A.; Salzano, A.; D’Esposito, M.; Comin, A.; Montillo, M.; Yang, L.; Campanile, G.; Gasparrini, B. Anti-Mullerian hormone (AMH) concentration in follicular fluid and mRNA expression of AMH receptor type II and LH receptor in granulosa cells as predictive markers of good buffalo (Bubalus bubalis) donors. Theriogenology 2016, 86, 963–970. [Google Scholar] [CrossRef]
- Bols, P.E.J.; Ysebaert, M.T.; Van Soom, A.; De Kruif, A. Effects of long term treatment with bovine somatotropin on follicular dynamics and subsequent oocyte and blastocyst yield during an OPU-IVF program. Theriogenology 1998, 49, 983–995. [Google Scholar] [CrossRef]
- Sirard, M.A.; Picard, L.; Dery, M.; Coenen, K.; Blondin, P. The time interval between FSH administration and ovarian aspiration influences the development of cattle oocytes. Theriogenology 1999, 51, 699–708. [Google Scholar] [CrossRef]
- Tripp, M.W.; Ju, J.C.; Hoagland, T.A.; Riesen, J.W.; Yang, X.; Zinn, S.A. Influence of somatrotopin and nutrition on bovine oocyte retrieval and in vitro development. Theriogenology 2000, 53, 1581–1590. [Google Scholar] [CrossRef]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.A. Manipulation of Follicular Development to Produce Developmentally Competent Bovine Oocytes. Biol. Reprod. 2002, 1, 38–43. [Google Scholar] [CrossRef]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.P.; Gordon, I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef]
- Blondin, P.; Sirard, M.A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol. Reprod. Dev. 1999, 41, 54–62. [Google Scholar] [CrossRef]
- Hagemann, L.J.; Beaumont, S.E.; Berg, M.; Donnison, M.J.; Ledgard, A.; Peterson, A.J.; Schurmann, A. Development during single IVP of bovine oocytes from dissected follicles: Interactive effects of estrous cycle stage, follicle size and atresia. Mol. Reprod. Dev. 1999, 53, 451–458. [Google Scholar] [CrossRef]
- Hendriksen, P.J.M.; Vos, P.L.A.M.; Steenweg, W.N.M.; Bevers, M.M.; Dieleman, S.J. Bovine follicular development and its effect on the in vitro competence of oocytes. Theriogenology 2000, 53, 11–20. [Google Scholar] [CrossRef]
- Blondin, P.; Vigneault, C.; Nivet, A.; Sirard, M. Improving oocyte quality in cows and heifers—What have we learned so far? Anim. Reprod. 2012, 9, 281–289. [Google Scholar]
- Oussaid, B.; Mariana, J.C.; Poulin, N.; Fontaine, J.; Lonergan, P.; Beckers, J.F.; Cognie, Y. Reduction of the developmental competence of sheep oocytes by inhibition of LH pulses during the follicular phase with a GnRH antagonist. Reproduction 1999, 117, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Goodhand, K.L.; Broadbent, P.J.; Hutchinson, J.S.M.; Watt, R.G.; Staines, M.E.; Higgins, L.C. In vivo oocyte recovery and in vitro embryo production in cattle pre-treated with FSH, progestogen and estradiol. Theriogenology 2000, 45, 355. [Google Scholar] [CrossRef]
- Labrecque, R.; Vigneault, C.; Blondin, P.; Sirard, M.A. Gene Expression Analysis of Bovine Oocytes with High Developmental Competence Obtained From FSH-Stimulated Animals. Mol. Reprod. Dev. 2013, 80, 428–440. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Maylem, E.R.S.; Tilwani, R.C.; Yanagawa, Y.; Katagiri, S.; Atabay, E.C.; Atabay, E.P. Effects of follicle-stimulating hormone followed by gonadotropin-releasing hormone on embryo production by ovum pick-up and in vitro fertilization in the river buffalo (Bubalus bubalis). Anim. Sci. J. 2019, 90, 690–695. [Google Scholar] [CrossRef]
- De Carvalho, J.G.S.; de Carvalho, N.A.T.; Bayeux, B.M.; Watanabe, Y.F.; Watanabe, O.Y.; Mingoti, R.D.; Baruselli, P.S. Superstimulation prior to the ovum pick-up improves the in vitro embryo production in nulliparous, primiparous and multiparous buffalo (Bubalus bubalis) donors. Theriogenology 2019, 138, 164–168. [Google Scholar] [CrossRef]
- Neglia, G.; Gasparrini, B.; Caracciolo di Brienza, V.; Di Palo, R.; Campanile, G.; Antonio Presicce, G.; Zicarelli, L. Bovine and buffalo in vitro embryo production using oocytes derived from abattoir ovaries or collected by transvaginal follicle aspiration. Theriogenology 2003, 59, 1123–1130. [Google Scholar] [CrossRef]
- Galli, C.; Duchi, R.; Colleoni, S.; Lagutina, I.; Lazzari, G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology 2014, 81, 138–151. [Google Scholar] [CrossRef]
- De Roover, R.; Feugang, J.; Bols, P.; Genicot, G.; Hanzen, C. Effects of Ovum Pick-up Frequency and FSH Stimulation: A Retrospective Study on Seven Years of Beef Cattle In Vitro Embryo Production. Reprod. Domest. Anim. 2008, 43, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nivet, A.L.; Bunel, A.; Labrecque, R.; Belanger, J.; Vigneault, C.; Blondin, P.; Sirard, M.A. FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction 2012, 143, 165–171. [Google Scholar] [CrossRef]
- Gasparrini, B. Effects of reproductive season on embryo development in the buffalo. Reprod. Fertil. Dev. 2019, 31, 68–81. [Google Scholar] [CrossRef]
- Brum, D.S.; Leivas, F.G.; Silva, C.A.M.; Rubin, M.I.B.; Rauber, L.P.; Fialho, S.S.; Pilla, L.F.C.; Bernardi, M.L. The effects of number of oocytes and the volume of maturation medium in bovine in vitro embryo production. Anim. Reprod. Sci. 2005, 2, 70–73. [Google Scholar]
- O’Doherty, E.M.; Wade, M.G.; Hill, J.L.; Boland, M.P. Effects of cul-turing bovine oocytes either singly or in groups on development toblastocysts. Theriogenology 1997, 48, 161–169. [Google Scholar] [CrossRef]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine inter-actions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef]
- Parrish, J.J.; Susko-Parrish, J.L.; Leibfried-Rutledge, M.L.; Critser, E.S.; Eyestone, W.H.; First, N.L. Bovine in vitro fertilization withfrozen–thawed semen. Theriogenology 1986, 25, 591–600. [Google Scholar] [CrossRef]
- Tervit, H.R.; Whittingham, D.G.; Rowson, L.E. Successful culture in vitro of sheep and cattle ova. J. Reprod. Fertil. 1972, 30, 493–497. [Google Scholar] [CrossRef]
- Presicce, G.A.; Senatore, E.M.; De Santis, G.; Stecco, R.; Terzano, G.M.; Borghese, A.; De Mauro, G.J. Hormonal stimulation and oocyte maturational competence in prepuberal Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology 2002, 15, 1877–1884. [Google Scholar] [CrossRef]
- Boni, R.; Roviello, S.; Zicarelli, L. Repeated ovum pick-up in Italian Mediterranean buffalo cows. Theriogenology 1996, 46, 899–909. [Google Scholar] [CrossRef]
- Raghu, H.M.; Nandi, S.; Reddy, S.M. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod. Fertil. Dev. 2002, 14, 55–61. [Google Scholar] [CrossRef]
- Yousaf, M.R.; Chohan, K.R. Nuclear morphology, diameter and meiotic competence of buffalo oocytes relative to follicle size. Reprod. Fertil. Dev. 2003, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Boccia, L.; De Rosa, A.; Attanasio, L.; Neglia, G.; Vecchio, D.; Campanile, G.; Zicarelli, L.; Gasparrini, B. Developmental Speed Affects the Cryotolerance of In Vitro Produced Buffalo (Bubalus Bubalis) Embryos. Ital. J. Anim. Sci. 2013, 12, e80. [Google Scholar] [CrossRef]
- Gasparrini, B.; Boccia, L.; De Rosa, A.; Di Palo, R.; Campanile, G.; Zicarelli, L. Chemical activation of buffalo (Bubalus bubalis) oocytes by different methods: Effects of aging on post-parthenogenetic development. Theriogenology 2004, 62, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, B.; De Rosa, A.; Attanasio, L.; Boccia, L.; Di Palo, R.; Campanile, G.; Zicarelli, L. Influence of the duration of in vitro maturation and gamete co-incubation on the efficiency of in vitro embryo development in Italian Mediterranean buffalo (Bubalus bubalis). Anim. Reprod. Sci. 2008, 105, 354–364. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control | FSH3 | FSH6 |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Total follicles n * | 3.4 ± 0.2 A | 5.4 ± 0.5 B | 7.2 ± 0.5 B |
| Large follicles n * % | 0.2 ± 0.1 A 5.8 ± 2.6 a | 0.3 ± 0.1 A,B 5.1 ± 3.4 a | 1.2 ± 0.2 B 17.1 ± 2.5 b |
| Medium follicles n * % | 0.7 ± 0.1 A 21.1 ± 3.3 A | 1.3 ± 0.2 B 24.1 ± 4.4 A | 2.7 ± 0.3 B 37.4 ± 3.3 B |
| Small follicles n * % | 2.5 ± 0.2a 73.1 ± 4.2 A | 3.9 ± 0.4 b 70.8 ± 4.9 A | 3.3 ± 0.4 a,b 45.5 ± 4.0 B |
| Recovery rate % | 54.2 ± 4.6 A,B | 41.6 ± 5.2 A | 59.5 ± 4.1 B |
| COCs n * | 2.3 ± 0.3 A | 2.4 ± 0.4 A | 4.6 ± 0.5 B |
| A + B + C COCs n * (%) | 1.3 ± 0.2 A (56.5 ± 4.9) A,a | 1.7 ± 0.3 A (69.3 ± 6.8) b | 3.5 ± 0.4 B (75.7 ± 4.1) B |
| Cleavage n * (%) | 0.6 ± 0.1 a (34.3 ± 3.8) a | 1.0 ± 0.1 a (43.4 ± 0.2) b | 2.1 ± 0.2 b (51.5 ± 2.3) b |
| Grade 1,2 BL n * (%) | 0.2 ± 0.1 A (10.3 ± 7.0) | 0.4 ± 0.3 A (18.8 ± 12.0) | 0.9 ± 0.1 B (23.6 ± 2.4) |
| Fast BL n * (%) | 0.1 ± 0.1 a (8.0 ± 6.5) | 0.4 ± 0.2 a,b (16.2 ± 9.5) | 0.7 ± 0.1 b (16.5 ± 2.3) |
| Parameters | Coasting Time | ||
|---|---|---|---|
| C1 | C2 | C3 | |
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Total follicles n * | 6.4 ± 0.7 | 5.2 ± 0.5 | 6.2 ± 0.6 |
| Large follicles n * % | 1.6 ± 0.3 29.3 ± 5.1 | 1.1 ± 0.2 25.0 ± 5.1 | 1.5 ± 0.3 29.1 ± 5.4 |
| Medium follicles n * % | 2.3 ± 0.6 30.8 ± 5.0 | 1.6 ± 0.4 25.6 ± 5.4 | 2.2 ± 0.4 32.3 ± 4.8 |
| Small follicles n * % | 2.5 ± 0.4 39.9 ± 5.3 | 2.5 ± 0.3 49.4 ± 5.1 | 2.5 ± 0.5 38.6 ± 5.1 |
| COCs n * | 3.8 ± 0.6 | 3.5 ± 0.5 | 4.0 ± 0.7 |
| Recovery rate % | 56.0 ± 6.1 | 66.4 ± 6.2 | 63.8 ± 6.7 |
| Grade A + B + C COCs n * (%) | 2.7 ± 0.4 (79.0 ± 5.2) a | 2.7 ± 0.4 (78.9 ± 6.5) a | 2.2 ± 0.4 (58.6 ± 7.3) b |
| Expanded COCs n * (%) | 0.3 ± 0.1 (6.1 ± 3.0) a | 0.5 ± 0.2 (14.7 ± 6.4) a,b | 1.0 ± 0.2 (26.3 ± 3.3) b |
| Total discarded COCs n * (%) | 1.1 ± 0.4 (21.0 ± 5.2) a | 0.8 ± 0.2 (21.1 ± 6.5) a | 1.7 ± 0.4 (41.4 ± 7.3) b |
| Cleavage n * (%) | 2.4 ± 0.2 (73.8 ± 12.4) | 2.0 ± 0.1 (72.7 ± 8.4) | 1.0 ± 0.5 (72.8 ± 9.2) |
| Grade 1,2 BL n * (%) | 1.4 ± 0.3 A (41.8 ± 11.1) B | 1.0 ± 0.1 A,B (35.7 ± 2.9) B | 0.4 ± 0.1 B (11.2 ± 3.8) A |
| Fast BL n * (%) | 1.2 ± 0.3 (34.8 ± 9.9) a | 0.8 ± 0.7 (29.2 ± 1.7) a,b | 0.4 ± 0.7 (11.2 ± 3.8) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovas, G.; Kosior, M.A.; Presicce, G.A.; Russo, M.; Zullo, G.; Albero, G.; Alkan, S.; Gasparrini, B. FSH Stimulation with Short Withdrawal Improves Oocyte Competence in Italian Mediterranean Buffalo (Bubalus bubalis). Animals 2020, 10, 1997. https://doi.org/10.3390/ani10111997
Petrovas G, Kosior MA, Presicce GA, Russo M, Zullo G, Albero G, Alkan S, Gasparrini B. FSH Stimulation with Short Withdrawal Improves Oocyte Competence in Italian Mediterranean Buffalo (Bubalus bubalis). Animals. 2020; 10(11):1997. https://doi.org/10.3390/ani10111997
Chicago/Turabian StylePetrovas, Georgios, Michal Andrzej Kosior, Giorgio Antonio Presicce, Marco Russo, Gianluigi Zullo, Giuseppe Albero, Serhat Alkan, and Bianca Gasparrini. 2020. "FSH Stimulation with Short Withdrawal Improves Oocyte Competence in Italian Mediterranean Buffalo (Bubalus bubalis)" Animals 10, no. 11: 1997. https://doi.org/10.3390/ani10111997
APA StylePetrovas, G., Kosior, M. A., Presicce, G. A., Russo, M., Zullo, G., Albero, G., Alkan, S., & Gasparrini, B. (2020). FSH Stimulation with Short Withdrawal Improves Oocyte Competence in Italian Mediterranean Buffalo (Bubalus bubalis). Animals, 10(11), 1997. https://doi.org/10.3390/ani10111997





