Combining Ability and Heterosis for Agronomic Traits, Husk and Cob Pigment Concentration of Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Experiment
2.3. Data Collection
2.4. Sample Preparation and Extraction
2.4.1. Determination of Total Anthocyanin Content (TAC)
2.4.2. Determination of Total Phenolic Content (TPC)
2.4.3. Determination of Antioxidant Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Variance
3.2. General Combining Ability Effects
3.3. Specific Combining Ability Effects and Heterosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Locations | Soil Type | pH (1:1 H2O) | EC 1:5 H2O (dS/m) | Organic Matter (%) | Total Nitrogen (%) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | Exchangeable Calcium (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Khon Kaen | Sandy loam | 6.34 | 0.06 | 0.99 | 0.06 | 730.8 | 730.8 | 478 |
| Uthai Thani | Clay loam | 5.56 | 0.07 | 1.6 | 0.12 | 85.8 | 85.8 | 1860 |
References
- Wu, F.; Guclu, H. Global maize trade and food security: Implications from a social network model. Risk Anal. 2013, 33, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Odunitan-Wayas, F.A.; Kolanisi, U.; Chimonyo, M.; Siwela, M. Effect of provitamin A biofortified maize inclusion on quality of meat from indigenous chickens. J. Appl. Poult. Res. 2016, 25, 581–590. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Scott, M.P.; Lertrat, K. Genotypic variability in anthocyanins, total phenolics, and antioxidant activity among diverse waxy corn germplasm. Euphytica 2015, 203, 237–248. [Google Scholar] [CrossRef]
- Simla, S.; Boontang, S.; Harakotr, B. Anthocyanin content, total phenolic content, and antiradical capacity in different ear components of purple waxy corn at two maturation stages. Aust. J. Crop Sci. 2016, 10, 675–682. [Google Scholar] [CrossRef]
- Li, C.Y.; Kim, H.W.; Won, S.R.; Min, H.K.; Park, K.J.; Park, J.Y.; Ahn, M.S.; Rhee, H.I. Corn husk as a potential source of anthocyanins. J. Cent. South Univ. T 2008, 56, 11413–11416. [Google Scholar] [CrossRef]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic variation in anthocyanins, phenolic compounds, and antioxidant activity in cob and husk of purple field corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Gonzalez da Mejia, E.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef]
- Herrera-Sotero, M.Y.; Cruz-Hernández, C.D.; Trujillo-Carretero, C.; Rodríguez-Dorantes, M.; García-Galindo, H.S.; Chávez-Servia, J.L.; Oliart-Ros, R.M.; Guzmán-Gerónimo, R.I. Antioxidant and antiproliferative activity of blue corn and tortilla from native maize. Chem. Cent. J. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17–29. [Google Scholar] [CrossRef]
- Aviram, M.; Kaplan, M.; Rosenblat, M.; Fuhrman, B. Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handb. Exp. Pharmacol. 2005, 170, 263–300. [Google Scholar]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010, 123, 77–84. [Google Scholar] [CrossRef]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. F 2017, 16, 234–246. [Google Scholar] [CrossRef]
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W.; Wannanon, P.; Tong-Un, T.; Suriharn, B.; Lertrat, K. Preventive effect of Zea mays L. (purple waxy corn) on experimental diabetic cataract. Biol. Med. Res. Int. 2014, 4, 1–8. [Google Scholar]
- Westfall, A. Evaluation of the Efficacy of Anthocyanins as Biologically Active Ingredients in Lipstick Formulations. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Kapcum, C.; Uriyapongson, J. Effects of storage conditions on phytochemical and stability of purple corn cob extract powder. Food. Sci. Technol. Campinas. 2018, 2061, 301–305. [Google Scholar] [CrossRef]
- Wachirapakorn, C.; Pilachai, K.; Wanapat, M.; Pakdee, P.; Cherdthong, A. Effect of ground corn cobs as a fiber source in total mixed ration on feed intake, milk yield and milk composition in tropical lactating crossbred Holstein cows. Anim. Nutr. 2016, 2, 334–338. [Google Scholar] [CrossRef]
- Chen, M.; Xia, L.; Xue, P. Enzymatic hydrolysis of corn cob and ethanol production from cellulosic hydrolysate. Int. Biodeterior. Biodegrad. 2007, 59, 85–89. [Google Scholar] [CrossRef]
- Ji, Z.; Lin, H.; Chen, Y.F.; Dong, Y.B.; Imran, M. Corn cob modified by lauric acid and ethanediol for emulsified oil adsorption. J. Cent. South Univ. 2015, 22, 2096–2105. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Silva, D.W.; Mendes, R.F.; Mendes, L.M. Use of maize cop for production of particleboard. Cienc. Agrotecnol. 2006, 37, 330–337. [Google Scholar] [CrossRef]
- Sprague, G.F.; Tatum, L.A. General vs. specific combining ability in single crosses of corn. J. Am. Soc. Agron. 1942, 34, 923–932. [Google Scholar] [CrossRef]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Baker, R.J. Issues in diallel analysis. Crop Sci. 1978, 18, 533–536. [Google Scholar] [CrossRef]
- Zhang, X.; Yong, H.; Zhou, Z.; Zhang, C.; Lu, M.; Sun, Q.; Zhang, X.; Li, M.; Zhang, D.; Weng, J.; et al. Heterosis and combining ability of seven maize germplasm populations. Euphytica 2017, 213, 1–11. [Google Scholar] [CrossRef]
- Al-falahy, M.A.H. Estimation combining ability, heterosis and some genetic parameters across four environments using full diallel cross method. Int. J. Pure. Appl. Sci. Technol. 2015, 26, 34–44. [Google Scholar]
- Adebayo, M.A.; Menkir, A.; Blay, E.; Gracen, V.; Danquah, E. Combining ability and heterosis of elite drought-tolerant maize inbred lines evaluated in diverse environments of lowland tropics. Euphytica 2017, 213, 1–12. [Google Scholar] [CrossRef]
- Musila, R.N.; Diallo, A.O.; Makumbi, D.; Njoroge, K. Combining ability of early-maturing quality protein maize inbred lines adapted to Eastern Africa. Field Crops Res. 2010, 119, 231–237. [Google Scholar] [CrossRef]
- Guerrero, C.G.; Robles, M.A.G.; Ortega, J.G.L.; Castillo, I.O.; Vázquez, C.V.; Carrillo, M.G.; Resendez, A.M.; Torres, A.G. Combining ability and heterosis in corn breeding lines to forage and grain. Am. J. Plant Sci. 2014, 5, 845–856. [Google Scholar] [CrossRef]
- Sibiya, J.; Tongoona, P.; Derera, J. Combining ability and GGE biplot analyses for resistance to northern leaf blight in tropical and subtropical elite maize inbred lines. Euphytica 2013, 19, 245–257. [Google Scholar] [CrossRef]
- Mwimali, M.; Derera, J.; Tongoona, P.; Mugo, S.; Gichuru, L. Combining ability for stem borer resistance and heterotic orientation of maize inbred lines using CIMMYT single cross testers under Busseola fusca infestation. Euphytica 2016, 208, 323–335. [Google Scholar] [CrossRef]
- Lorenz, A.J.; Coors, J.G.; de Leon, N.; Wolfrum, E.J.; Hames, B.R.; Sluiter, A.D.; Weimer, P.J. Characterization, genetic variation, and combining ability of maize traits relevant to the production of cellulosic ethanol. Crop Sci. 2009, 49, 85–98. [Google Scholar] [CrossRef]
- Mahan, A.L.; Murray, S.C.; Rooney, L.W.; Crosby, K.M. Combining ability for total phenols and secondary traits in a diverse set of colored (red, blue, and purple) maize. Crop Sci. 2013, 53, 1248–1255. [Google Scholar] [CrossRef]
- Li, R.; Xiao, L.H.; Wang, J.; Lu, Y.L.; Rong, T.Z.; Pan, G.T.; Wu, Y.Q.; Tang, Q.; Lan, H.; Cao, M.J. Combining ability and parent-offspring correlation of maize (Zea may L.) grain β-carotene content with a complete diallel. J. Integr. Agric. 2013, 12, 19–26. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Filho, J.B.M. Testers and combining ability. In Quantitative Genetics in Maize Breeding; Handbook of Plant Breeding; Springer: New York, NY, USA, 2010; Volume 6. [Google Scholar]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, G.; Gu, Z.; Han, Y.; Chen, Z. Optimization extraction of anthocyanins from purple corn (Zea mays L.) cob using tristimulus colorimetry. Eur. Food Res. Technol. 2008, 227, 409–415. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Hu, Q.P.; Xu, J.G. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedure for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Rodríguez, F.; Alvarado, G.; Pacheco, A.; Burgueño, J.; Crossa, J. AGD-R (Analysis of Genetic Designs with R for Windows) Version 5.0. International Maize and Wheat Improvement Center (CIMMYT), México. 2015. Available online: https://data.cimmyt.org/dataset.xhtml?persistentId=hdl:11529/10202 (accessed on 5 December 2019).
- Khampas, S.; Lertrat, K.; Lomthaisong, K.; Simla, S.; Suriharn, B. Effect of location, genotype and their interactions for anthocyanins and antioxidant activities of purple waxy corn cob. Turk. J. Field Crops 2015, 20, 15–23. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Simmonds, N.W. Principles of Crop Improvement; Longman: London, UK, 1979. [Google Scholar]
- Ketthaisong, D.; Suriharn, B.; Tangwongchai, R.; Lertrat, K. Combining ability analysis in complete diallel cross of waxy corn (Zea mays var. ceratina) for characteristics starch pasting viscosity. Sci. Hort. 2014, 175, 229–235. [Google Scholar]
- Abera, W.; Hussein, S.; Derera, J.; Worku, M. Heterosis and combining ability of elite maize inbred lines under northern corn leaf blight disease prone environments of the mid-altitude tropics. Euphytica 2016, 208, 291–400. [Google Scholar] [CrossRef]
- Sun, J.; Gao, J.; Yu, X.; Liu, J.; Su, Z.; Feng, Y.; Wang, D. Combining ability of sixteen USA maize inbred lines and their outbreeding prospects in china. Agronomy 2018, 8, 281. [Google Scholar] [CrossRef]
- Landi, P.; Cane, M.A.; Frascaroli, E. Responses to divergent selection for cob color in maize. Euphytica 2008, 164, 645–658. [Google Scholar] [CrossRef]
- Zhang, F.; Peterson, T. Comparisons of maize pericarp color1 alleles reveal paralogous gene recombination and an organ-specific enhancer region. Plant Cell 2005, 17, 903–914. [Google Scholar] [CrossRef]
- Goettel, W.; Messing, J. Divergence of gene regulation through chromosomal rearrangements. BMC Genom. 2010, 11, 678. [Google Scholar] [CrossRef] [PubMed]
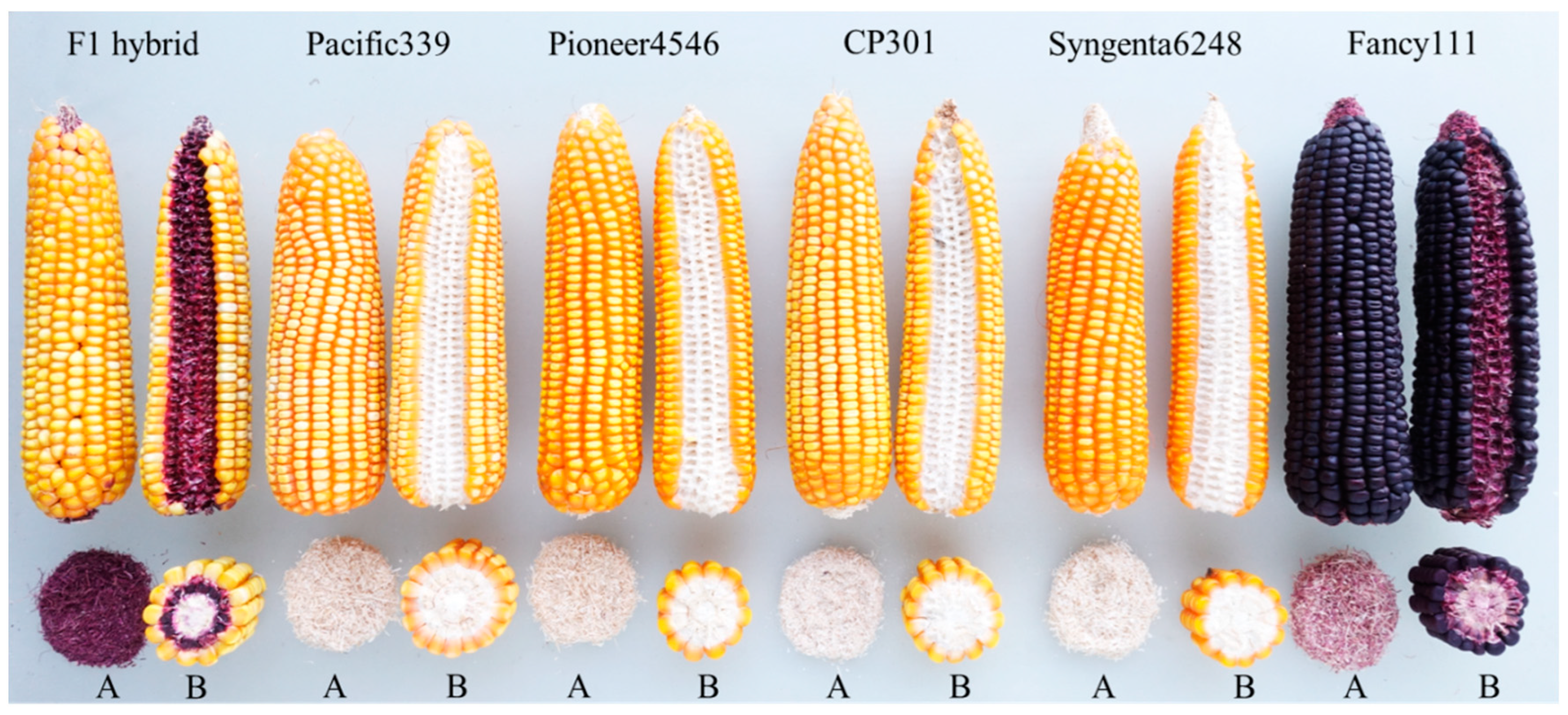
| No. | Lines | Kernel Color | Husk Color | Cob Color |
|---|---|---|---|---|
| Female | ||||
| 1 | NakhonSawan1 | Orange | Green | White |
| 2 | NakhonSawan2 | Orange | Green | White |
| 3 | Takfa1 | Orange | Green | White |
| 4 | Takfa2 | Orange | Green | White |
| 5 | Takfa3 | Orange | Green | White |
| Male | ||||
| 1 | KKU–PFC1 | Purple | Purple | Purple |
| 2 | KKU–PFC2 | White | Purple | Purple |
| 3 | KKU–PFC3 | Yellow | Purple | Purple |
| 4 | KKU–PFC4 | Purple | Purple | Purple |
| 5 | KKU–PFC5 | White | Purple | Purple |
| Source of Variation | df | Anthesis Day (day) | Plant Height (cm) | Ear Height (cm) | Husk Mass (kg ha−1) | Cob Mass (kg ha−1) | TAY | Grain Yield | |
|---|---|---|---|---|---|---|---|---|---|
| Husk | Cob | kg ha−1 | |||||||
| Location (L) | 1 | 1098.9 ** | 147,951 ** | 102,998 ** | 44,161 ** | 436,476 ** | 12.2 ** | 51.5 ** | 181,288,043 ** |
| Hybrid | 24 | 9.2 ** | 597 ** | 143 ** | 35,840 ** | 13,226 ** | 27.7 ** | 23.1 ** | 2,850,862 ** |
| Hybrid × L | 24 | 0.5 ns | 329 ** | 89 ** | 24,363 ** | 7686 ns | 3.4 ** | 4.4 ** | 888,633 ** |
| GCA female | 4 | 40.9 ** | 1512 ** | 245 ** | 91,596 ** | 18,082 ** | 92.7 ** | 28.5 ** | 5,786,196 ** |
| GCA male | 4 | 5.8 ** | 1571 ** | 210 ** | 34,406 ** | 5055 ns | 5.6 ** | 51.1 ** | 751,590 ** |
| SCA | 16 | 2.2 ** | 125 ns | 101 ** | 22,259 ** | 14,055 ** | 17.0 ** | 14.7 ** | 2,641,847 ** |
| GCA female × L | 4 | 1.1 ns | 1326 ** | 178 ** | 5176 ns | 20,163 ** | 2.9 ** | 0.3 ns | 1,034,618 ** |
| GCA male × L | 4 | 0.0 ns | 88 ns | 176 ** | 10,143 ns | 6298 ns | 3.5 ** | 17.4 ** | 97,905 ** |
| SCA × L | 16 | 6.9 ns | 2246 ns | 710 ns | 523,438 ** | 78,611 ns | 55.0 ** | 34.9 ** | 1,049,818 ** |
| Error | 96 | 0.8 | 128 | 37 | 5486 | 4882 | 0.2 | 0.2 | 14,090 |
| % SS GCA female | 73.9 | 42.2 | 28.6 | 42.6 | 22.8 | 55.8 | 20.6 | 33.8 | |
| % SS GCA male | 10.5 | 43.8 | 24.4 | 16 | 6.4 | 3.4 | 37 | 4.4 | |
| % SS SCA | 15.6 | 14 | 47 | 41.4 | 70.8 | 40.8 | 42.4 | 61.8 | |
| Source of Variation | df | Husk | Cob | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TAC (mg CGE/100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/100 g DW) | TAC (mg CGE/100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/100 g DW) | ||
| Location (L) | 1 | 272,450 ** | 1,077,998 ** | 205.5 ** | 108.5 ** | 290,542 ** | 857,825 ** | 2.5 ns | 2.1 ** |
| Hybrid | 24 | 288,280 ** | 549,025 ** | 415.2 ** | 43.9 ** | 315,603 ** | 547,021 ** | 368.2 ** | 34.2 ** |
| Hybrid × L | 24 | 51,424 ** | 142,574 ** | 53.4 ** | 12.3 ** | 49,328 ** | 217,542 ** | 83.7 ** | 10.5 ** |
| GCA female | 4 | 884,742 ** | 1,349,445 ** | 927.6 ** | 134.5 ** | 433,796 ** | 773,513 ** | 360.3 ** | 46.4 ** |
| GCA male | 4 | 90,326 ** | 483,590 ** | 395.2 ** | 43.3 ** | 719,558 ** | 1,024,086 ** | 766.9 ** | 49.3 ** |
| SCA | 16 | 188,653 ** | 365,279 ** | 292.1 ** | 21.4 ** | 185,065 ** | 371,132 ** | 270.5 ** | 27.3 ** |
| GCA female × L | 4 | 41,053 ** | 144,920 ** | 37.5 ** | 22.7 ** | 3388 ** | 154,720 ** | 48.7 ** | 8.0 ** |
| GCA male × L | 4 | 75,216 ** | 146,508 ** | 77.0 ** | 17.9 ** | 192,258 ** | 695,223 ** | 271.9 ** | 26.3 ** |
| SCA × L | 16 | 769,089 ** | 2,256,069 ** | 823.8 ** | 133.9 ** | 401,291 ** | 1,821,247 ** | 726.4 ** | 115.9 ** |
| Error | 96 | 680 | 525 | 1.0 | 0.1 | 606 | 1629 | 1.1 | 0.0 |
| % SS GCA female | 51.2 | 40.9 | 37.2 | 51.1 | 22.9 | 23.6 | 16.3 | 22.6 | |
| % SS GCA male | 5.2 | 14.7 | 15.9 | 16.4 | 38.0 | 31.2 | 34.7 | 24.1 | |
| % SS SCA | 43.6 | 44.4 | 46.9 | 32.5 | 39.1 | 45.2 | 49.0 | 53.3 | |
| Parental Lines | Anthesis Day (day) | Plant Height (cm) | Ear Height (cm) | Husk Mass (kg ha−1) | Cob Mass (kg ha−1) | TAY | Grain Yield (kg ha−1) | |
|---|---|---|---|---|---|---|---|---|
| Husk | Cob | |||||||
| NakhonSawan1 | −0.5 * | −11.2 ** | −4.4 ** | −74.7 ** | −37.7 ** | −0.8 * | 0.5 * | −405.8 * |
| NakhonSawan2 | −0.7 * | 5.4 * | 3.1 ** | 7.1 | −5.7 | −0.7 | 0.7 * | 107.4 |
| Takfa1 | −1.2 ** | −2.8 | −1.1 | −23.4 | 2.4 | −0.5 | −0.8 * | 228.3 * |
| Takfa2 | 0.7 * | 5.5 * | 1.0 | 14.6 | 28.0 ** | −1.1 * | 0.9 ** | −484.9 ** |
| Takfa3 | 1.7 ** | 3.1 | 1.4 * | 76.3 ** | 13.0 * | 3.1 ** | −1.3 ** | 555.0 ** |
| KKU–PFC1 | −0.6 ** | 0.0 | −1.4 | −35.4 ** | −4.2 | 0.1 | −0.3 | 165.5 ** |
| KKU–PFC2 | −0.1 | −11.8 ** | −3.8 ** | −36.3 ** | −1.2 | −0.6 ** | −0.2 | −203.5 ** |
| KKU–PFC3 | 0.0 | 6.8 ** | 2.0 * | 16.4 * | −18.9 ** | 0.2 * | 0.5 | 68.8 |
| KKU–PFC4 | 0.1 | 4.7 * | 2.8 ** | 38.1 ** | 13.6 ** | 0.5 ** | 1.8 ** | 99.0 * |
| KKU–PFC5 | 0.6 ** | 0.3 | 0.4 | 17.1 * | 10.6 * | −0.2 * | −1.8 ** | −129.9 * |
| SE Female | 0.5 | 3.2 | 1.3 | 24.7 | 11.0 | 0.8 | 0.4 | 196.4 |
| SE Male | 0.2 | 3.2 | 1.2 | 15.1 | 5.8 | 0.2 | 0.6 | 70.8 |
| Parental Lines | Husk | Cob | ||||||
|---|---|---|---|---|---|---|---|---|
| TAC | TPC | DPPH | TEAC | TAC | TPC | DPPH | TEAC | |
| mg CGE/100 g DW | mg GAE/100 g DW | % | mmol TE/100 g DW | mg CGE/100 g DW | mg GAE/100 g DW | % | mmol TE/100 g DW | |
| NakhonSawan1 | −43.1 | −78 | −1 | −1.1 * | 84.7 * | 166.7 ** | 2.6* | 0 |
| NakhonSawan2 | −86.5 * | −98.5 * | −3.5 * | −0.6 | 91.0 * | −83.0 * | 3.1 ** | 0.2 |
| Takfa1 | −38.7 | 11.7 | 0.7 | −0.3 | −100.9 * | −77.7 * | −3.9 ** | −0.7 * |
| Takfa2 | −131.5 * | −191.8 ** | −5.3 ** | −1.7 * | 83.8 * | 173.6 ** | 1.9 * | 1.9 ** |
| Takfa3 | 299.7 ** | 356.7 ** | 9.0 ** | 3.7 ** | −158.6 ** | −179.6 ** | −3.7 ** | −1.4 ** |
| KKU–PFC1 | −51.1 ** | −36.5 | −0.8 | −0.2 | −33.7 | 21.7 | −1.3 | 0.4 |
| KKU–PFC2 | 60.3 ** | 119.5 ** | 3.1 * | 1.3 ** | −25.3 | −1.7 | −0.8 | 0.5 |
| KKU–PFC3 | 57.5 ** | 143.9 ** | 4.2 ** | 1.2 ** | 62.4 | −10.7 | 2 | 0.6 * |
| KKU–PFC4 | −44.7 * | −148.3 ** | −4.6 ** | −1.4 ** | 210.7 ** | 256.0 ** | 7.0 ** | 0.8 * |
| KKU–PFC5 | −22 | −78.6 * | −1.9 * | −0.8 * | −214.1 ** | −265.4 ** | −6.9 ** | −2.3 ** |
| SE Female | 76.8 | 94.8 | 2.5 | 0.9 | 53.8 | 71.8 | 1.5 | 0.6 |
| SE Male | 24.5 | 56.8 | 1.6 | 0.5 | 69.3 | 82.6 | 2.3 | 0.6 |
| Lines/Hybrids | Anthesis Day (day) | Plant Height (cm) | Ear Height (cm) | Husk Mass (kg ha−1) | Cob Mass (kg ha−1) | TAY | Grain Yield (kg ha−1) | |
|---|---|---|---|---|---|---|---|---|
| Husk | Cob | |||||||
| NakhonSawan1 | 53 | 135 | 60 | 624 | 617 | 0 | 0 | 2634 |
| NakhonSawan2 | 54 | 147 | 66 | 665 | 621 | 0 | 0 | 2930 |
| Takfa1 | 53 | 135 | 60 | 669 | 655 | 0 | 0 | 2916 |
| Takfa2 | 55 | 140 | 62 | 648 | 669 | 0 | 0 | 2205 |
| Takfa3 | 57 | 142 | 65 | 595 | 680 | 0 | 1 | 1597 |
| KKU–PFC1 | 43 | 174 | 78 | 427 | 515 | 8 | 9 | 2693 |
| KKU–PFC2 | 43 | 157 | 75 | 394 | 498 | 5 | 5 | 2544 |
| KKU–PFC3 | 45 | 174 | 80 | 468 | 554 | 5 | 4 | 2590 |
| KKU–PFC4 | 46 | 174 | 83 | 480 | 547 | 4 | 4 | 2677 |
| KKU–PFC5 | 46 | 172 | 77 | 610 | 578 | 6 | 2 | 2630 |
| NakhonSawan1 × KKU–PFC1 | 53 | 184 | 82 | 799 | 780 | 4 | 5 | 4347 |
| NakhonSawan1 × KKU–PFC2 | 54 | 173 | 81 | 712 | 763 | 5 | 4 | 3899 |
| NakhonSawan1 × KKU–PFC3 | 53 | 189 | 89 | 758 | 750 | 5 | 5 | 3802 |
| NakhonSawan1 × KKU–PFC4 | 53 | 190 | 91 | 819 | 771 | 1 | 5 | 4667 |
| NakhonSawan1 × KKU–PFC5 | 54 | 180 | 82 | 750 | 852 | 2 | 4 | 2996 |
| NakhonSawan2 × KKU–PFC1 | 53 | 200 | 90 | 815 | 814 | 3 | 2 | 4352 |
| NakhonSawan2 × KKU–PFC2 | 53 | 182 | 83 | 852 | 871 | 3 | 6 | 4441 |
| NakhonSawan2 × KKU–PFC3 | 53 | 208 | 98 | 805 | 843 | 4 | 6 | 4665 |
| NakhonSawan2 × KKU–PFC4 | 53 | 204 | 95 | 930 | 792 | 5 | 8 | 4627 |
| NakhonSawan2 × KKU–PFC5 | 54 | 204 | 95 | 844 | 757 | 2 | 2 | 4192 |
| Takfa1 × KKU–PFC1 | 52 | 197 | 89 | 856 | 779 | 5 | 2 | 4975 |
| Takfa1 × KKU–PFC2 | 52 | 176 | 86 | 764 | 789 | 4 | 1 | 4684 |
| Takfa1 × KKU–PFC3 | 54 | 193 | 82 | 861 | 797 | 3 | 3 | 5292 |
| Takfa1 × KKU–PFC4 | 53 | 197 | 91 | 805 | 859 | 3 | 7 | 4001 |
| Takfa1 × KKU–PFC5 | 53 | 195 | 92 | 809 | 893 | 4 | 1 | 3930 |
| Takfa2 × KKU–PFC1 | 54 | 198 | 88 | 766 | 868 | 2 | 6 | 4285 |
| Takfa2 × KKU–PFC2 | 55 | 189 | 94 | 870 | 860 | 3 | 5 | 3262 |
| Takfa2 × KKU–PFC3 | 54 | 213 | 91 | 945 | 844 | 4 | 6 | 3976 |
| Takfa2 × KKU–PFC4 | 54 | 199 | 88 | 839 | 904 | 5 | 4 | 2921 |
| Takfa2 × KKU–PFC5 | 56 | 201 | 90 | 864 | 769 | 2 | 1 | 4871 |
| Takfa3 × KKU–PFC1 | 55 | 193 | 90 | 799 | 844 | 5 | 2 | 4608 |
| Takfa3 × KKU–PFC2 | 56 | 193 | 84 | 831 | 816 | 7 | 2 | 4437 |
| Takfa3 × KKU–PFC3 | 55 | 203 | 95 | 924 | 777 | 8 | 2 | 4349 |
| Takfa3 × KKU–PFC4 | 56 | 206 | 94 | 1009 | 847 | 7 | 5 | 6019 |
| Takfa3 × KKU–PFC5 | 56 | 193 | 89 | 1029 | 887 | 11 | 2 | 5101 |
| Pacific339 | 62 | 213 | 85 | 1179 | 834 | 0 | 0 | 6442 |
| CP301 | 62 | 191 | 82 | 1097 | 859 | 0 | 0 | 5869 |
| Pioneer4546 | 61 | 195 | 82 | 1117 | 916 | 0 | 0 | 6054 |
| Syngenta6248 | 64 | 193 | 95 | 956 | 988 | 0 | 0 | 6221 |
| Fancy111 | 48 | 174 | 73 | 577 | 944 | 1 | 1 | 3794 |
| Mean | 53 | 184 | 84 | 789 | 775 | 3 | 3 | 4062 |
| LSD (0.05) | 1 | 13 | 7 | 78 | 74 | 1 | 1 | 134 |
| SE | 1 | 3 | 2 | 29 | 19 | 0 | 0 | 188.7 |
| Hybrids | Anthesis Day (day) | Plant Height (cm) | Ear Height (cm) | Husk Mass (kg ha−1) | Cob Mass (kg ha−1) | TAY | Grain Yield (kg ha−1) | |
|---|---|---|---|---|---|---|---|---|
| Husk | Cob | |||||||
| NakhonSawan1 × KKU–PFC1 | −3.4 ** | −3.4 ** | −0.2 ** | −34.6 ** | 58.3 ** | −1.2 ** | 1.6 ** | −816.4 ** |
| NakhonSawan1 × KKU–PFC2 | 4.1 ** | 2.3 ** | 0.3 ** | −22.2 ** | −68.5 ** | −1.3 ** | −1.3 ** | −133.7 * |
| NakhonSawan1 × KKU–PFC3 | 3.3 ** | 3.8 ** | −0.5 ** | −26.8 ** | 59.2 ** | −0.1 | 0.2 | −516.4 ** |
| NakhonSawan1 × KKU–PFC4 | 1.1 * | −0.4 | 0.4 ** | −10.1 * | −91.0 ** | −1.1 ** | −1.5 ** | 1138.3 ** |
| NakhonSawan1 × KKU–PFC5 | −5.0 ** | −2.3 ** | 0.0 | 93.8 ** | 42.0 ** | 3.7 ** | 1.1 ** | 328.2 ** |
| NakhonSawan2 × KKU–PFC1 | 1.0 * | −1.6 ** | 0.4 ** | 66.4 ** | 1.0 | 0.9 ** | 0.5 ** | 239.6 ** |
| NakhonSawan2 × KKU–PFC2 | 0.0 | −1.0 * | 0.1 * | 1.1 | 2.4 | −0.1 | −2.1 ** | −269.3 ** |
| NakhonSawan2 × KKU–PFC3 | 5.4 ** | 2.1 ** | −0.4 ** | 72.3 ** | −40.7 ** | 1.9 ** | −0.5 ** | 233.0 ** |
| NakhonSawan2 × KKU–PFC4 | −2.1 ** | −0.4 | 0.3 ** | −55.7 ** | 23.4 ** | −0.6 ** | 1.8 ** | 256.8 ** |
| NakhonSawan2 × KKU–PFC5 | −4.2 ** | 0.9* | −0.3 ** | −84.1 ** | 13.8 * | −2.2 ** | 0.2 | −460.1 ** |
| Takfa1 × KKU–PFC1 | 1.2* | −0.5 | 0.8 ** | −19.3 * | −19.0 ** | 1.7 ** | −0.5 ** | 160.7 * |
| Takfa1 × KKU–PFC2 | −5.5 ** | −5.8 ** | −0.5 ** | 39.0 ** | 56.5 ** | −0.5 ** | 1.2 ** | 189.1 * |
| Takfa1 × KKU–PFC3 | −3.6 ** | 1.5 ** | −0.5 ** | −18.8 * | −33.1 ** | −0.1 | −1.3 ** | 311.0 ** |
| Takfa1 × KKU–PFC4 | 1.1 * | 7.2 ** | 0.1 * | 50.0 ** | 12.2 * | −0.2 | 0.9 ** | −398.1 ** |
| Takfa1 × KKU–PFC5 | 6.8 ** | −2.4 ** | 0.1 * | −50.8 ** | −16.5 ** | −1.0 ** | −0.2 | −262.8 ** |
| Takfa2 × KKU–PFC1 | −0.7 * | 1.9 ** | −0.3 ** | −25.6 ** | −14.4 * | 0.7 ** | −0.1 | −209.3 * |
| Takfa2 × KKU–PFC2 | 1.6 ** | 4.1 ** | −0.1 * | −60.7 ** | 46.6 ** | 0.0 | 1.1 ** | 141.0 * |
| Takfa2 × KKU–PFC3 | −5.6 ** | −7.7 ** | 1.1 ** | 25.6 ** | −7.8 | −1.1 ** | −0.7 ** | 646.9 ** |
| Takfa2 × KKU–PFC4 | 5.9 ** | −1.2 * | −0.1 * | 71.8 ** | 13.8 * | 0.3 * | 1.1 ** | 44.2 |
| Takfa2 × KKU–PFC5 | −1.2 * | 3.0 ** | −0.6 ** | −11.1 * | −38.2 ** | 0.1 | −1.3 ** | −622.7 ** |
| Takfa3 × KKU–PFC1 | 2.0 ** | 3.6 ** | −0.7 ** | 13.1 * | −25.8 ** | −2.3 ** | −1.5 ** | 625.4 ** |
| Takfa3 × KKU–PFC2 | −0.2 | 0.4 | 0.2 ** | 42.8 ** | −37.0 ** | 1.9 ** | 1.1 ** | 73.0 |
| Takfa3 × KKU–PFC3 | 0.5 | 0.3 | 0.4 ** | −52.4 ** | 22.4 ** | −0.6 ** | 2.4 ** | −674.6 ** |
| Takfa3 × KKU–PFC4 | −6.0 ** | −5.1 ** | −0.7 ** | −55.9 ** | 41.5 ** | 1.5 ** | −2.2 ** | −1041.2 ** |
| Takfa3 × KKU–PFC5 | 3.6 ** | 0.8 * | 0.8 ** | 52.3 ** | −1.1 | −0.6 ** | 0.2 | 1017.4 ** |
| SE | 0.7 | 0.7 | 0.1 | 9.9 | 7.9 | 0.3 | 0.3 | 108.4 |
| Hybrids | Anthesis Day | Plant Height | Ear Height | Husk Mass | Cob Mass | TAY | Grain Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | |
| NakhonSawan1 × KKU–PFC1 | 10.8 * | 0.3 | 18.2 | 5.1 | 23.2 | 7.4 | 53.9 * | 29.3 * | 38.6 * | 24.1 * | −2.5 * | −51.1 * | 8.6 | −45.6 | 63.8 * | 57.9 * |
| NakhonSawan1 × KKU–PFC2 | 12.7 * | 2.2 | 16.3 | 7.7 | 24.6 | 10.5 | 40.4 * | 14.2 * | 38.6 * | 26.4 * | 102.7 * | 1.8 | 33.7 * | −32.9 | 51.1 * | 44.7 * |
| NakhonSawan1 × KKU–PFC3 | 8.6 * | 0.3 | 22.2 | 8.7 | 28.5 | 11.3 | 39.4 * | 21.9 * | 29.5 * | 19.7 | 97.5 * | −0.8 | 121.9 * | 11.5 | 46.1 * | 39.7 * |
| NakhonSawan1 × KKU–PFC4 | 7.1 | −0.3 | 22.3 | 9.1 | 30.7 | 11 | 50.6 * | 32.6 * | 33.8 * | 22.2 * | −30.5 | −65 | 106.6 * | 3.7 | 77.3 * | 70.0 * |
| NakhonSawan1 × KKU–PFC5 | 9.1 * | 1.6 | 16.4 | 3.5 | 22.6 | 7.2 | 21.7 * | 17.1 * | 47.0 * | 41.3 * | −3.4 | −51.5 | 369.2 * | 136.3 * | 14.0 * | 9.2 * |
| NakhonSawan2 × KKU–PFC1 | 8.6 * | −2.5 | 23.8 * | 14.4 | 29.2 | 18 | 50.3 * | 23.8 * | 43.5 * | 31.1 * | −27.5 | −63.7 | −49 | −74.4 | 55.2 * | 47.1 * |
| NakhonSawan2 × KKU–PFC2 | 8.5 * | −2.5 | 18.8 | 14.7 | 18.4 | 10.9 | 61.6 * | 29.3 * | 56.0 * | 40.8 * | 25 | −37.4 | 110.6 * | 5.6 | 62.8 * | 50.4 * |
| NakhonSawan2 × KKU–PFC3 | 7.5 * | −1.6 | 29.2 * | 19.5 | 38.9 | 26.1 | 42.8 * | 23.7 | 43.3 * | 35.8 * | 66.5 * | −16.5 | 201.6 * | 51.3 * | 69.8 * | 58.0 * |
| NakhonSawan2 × KKU–PFC4 | 7.4 * | −0.9 | 26.3 * | 16.4 | 29.3 | 15 | 62.8 * | 39.9 * | 35.9 * | 27.7 * | 208.2 * | 54.8 * | 234.4 * | 67.7 * | 65.8 * | 56.2 * |
| NakhonSawan2 × KKU–PFC5 | 8.8 * | 0.3 | 27.6 * | 18 | 36.5 | 25.5 | 32.8 * | 21.7 * | 26.1 * | 19.0 * | 0.1 | −49.8 | 51.9 | −23.5 | 51.5 * | 41.8 * |
| Takfa1 × KKU–PFC1 | 7.9 * | −2.2 | 28.6 * | 14.4 | 28.6 | 14 | 57.9 * | 28.5 * | 32.7 * | 18.6 * | 35.7 | −32 | −47.5 | −73.7 | 78.6 * | 69.4 * |
| Takfa1 × KKU–PFC2 | 8.8 * | −1.2 | 21.1 * | 11.8 | 29.8 | 17.5 | 45.1 * | 16.0 * | 37.3 * | 21.4 * | 49.6 * | −25 | −44 | −71.9 | 72.3 * | 60.3 * |
| Takfa1 × KKU–PFC3 | 10.1 * | 1.9 | 24.8 * | 10.9 | 14.5 | 0.4 | 51.7 * | 29.9 * | 31.2 * | 21.2 * | 44.4 | −27.6 | 32 | −33.6 | 93.2 * | 81.1 * |
| Takfa1 × KKU–PFC4 | 8.0 * | 0.7 | 28.0 * | 14.2 | 26.8 | 9.6 | 41.0 * | 21.6 * | 43.2 * | 32.0 * | 67.3 * | −16 | 219.6 * | 60.7 * | 44.4 * | 36.2 |
| Takfa1 × KKU–PFC5 | 7.4 | 0 | 27.5 * | 13.2 | 37.2 | 22.4 | 27.1 * | 18.5 * | 45.5 * | 37.0 * | 41 | −29.3 | 39.2 * | −29.6 | 42.8 * | 34.1 |
| Takfa2 × KKU–PFC1 | 10.8 * | −1.5 | 25.6 * | 13.3 | 26.2 | 13 | 44.1 * | 20.2 * | 47.1 * | 30.9 * | −47.8 | −73.9 | 45.7 * | −26.9 | 76.0 * | 62.3 * |
| Takfa2 × KKU–PFC2 | 11.3 * | −0.9 * | 27.2 * | 20.5 | 40.6 | 29.7 | 68.1 * | 35.9 * | 48.1 * | 30.0 * | 23.3 | −38.2 | 103.0 * | 1.8 | 38.1 * | 30.8 |
| Takfa2 × KKU–PFC3 | 8.9 * | −1.2 * | 35.5 * | 22.5 | 28.5 | 14.2 | 72.6 * | 49.5 * | 38.6 * | 27.3 * | 70.6 * | −14.5 | 200.4 * | 50.9 * | 66.8 * | 56.8 * |
| Takfa2 × KKU–PFC4 | 7.2 * | −2.1 | 27.1 * | 15.3 | 21.4 | 6.1 | 49.0 * | 29.1 * | 48.9 * | 34.9 * | 154.8 * | 27.8 | 93.5 * | −2.9 | 20.1 * | 10.7 * |
| Takfa2 × KKU–PFC5 | 10.5 * | 0.9 | 30.0 * | 19 | 28.4 | 16.8 | 38.9 * | 27.7 * | 23.8 * | 15.5 | −16.1 | −58 | 45.7 | −26.7 | 102.6 * | 89.0 * |
| Takfa3 × KKU–PFC1 | 9.6 * | −3.8 | 21.4 | 10.7 | 25.1 | 15.2 | 56.9 * | 35.4 * | 42.9 * | 25.6 * | 18.1 | −40.8 | −44.9 | −70.5 | 113.4 * | 73.9 * |
| Takfa3 × KKU–PFC2 | 11.5 * | −2 | 27.6 * | 21.1 | 20.6 | 12.2 | 70.4 * | 41.9 * | 38.6 * | 20.2 * | 147.8 * | 24.3 | −25.9 | −59 | 111.8 * | 78.5 * |
| Takfa3 × KKU–PFC3 | 8.2 * | −3.2 | 28.3 * | 16.7 | 31.3 | 19.8 | 77.3 * | 59.5 * | 27.0 * | 15.9 | 235.2 * | 68.3 * | −26.8 | −58.4 | 106.4 * | 71.1 * |
| Takfa3 × KKU–PFC4 | 10.3 * | −0.6 | 29.5 * | 17 | 24.2 | 11.2 | 92.9 * | 73.5 * | 38.5 * | 25.4 * | 290.8 * | 96.6 * | 78.8 * | 0.8 | 180.8 * | 128.0 * |
| Takfa3 × KKU–PFC5 | 9.7 * | −1.2 | 21.3 | 10.1 | 22.7 | 13.2 | 73.4 * | 65.9 * | 40.9 * | 30.1 * | 318.9 * | 110.3 * | 48.7 * | −2.8 | 139.4 * | 98.5 * |
| Lines/Hybrids | Husk | Cob | ||||||
|---|---|---|---|---|---|---|---|---|
| TAC (mg CGE/100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/100 g DW) | TAC (mg CGE/100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/100 g DW) | |
| NakhonSawan1 | 4 | 49 | 1 | 5 | 3 | 58 | 7 | 5 |
| NakhonSawan2 | 2 | 41 | 1 | 6 | 2 | 68 | 3 | 4 |
| Takfa1 | 2 | 25 | 2 | 6 | 4 | 54 | 2 | 3 |
| Takfa2 | 2 | 20 | 2 | 5 | 2 | 82 | 2 | 4 |
| Takfa3 | 3 | 75 | 3 | 6 | 82 | 235 | 8 | 5 |
| KKU–PFC1 | 1869 | 2799 | 64 | 22 | 1728 | 2428 | 61 | 19 |
| KKU–PFC2 | 1384 | 1762 | 45 | 18 | 1067 | 1764 | 44 | 16 |
| KKU–PFC3 | 1036 | 1710 | 42 | 17 | 735 | 1531 | 37 | 17 |
| KKU–PFC4 | 737 | 1013 | 31 | 15 | 813 | 1567 | 37 | 15 |
| KKU–PFC5 | 894 | 1284 | 31 | 15 | 381 | 718 | 16 | 7 |
| NakhonSawan1 × KKU–PFC1 | 487 | 843 | 28 | 12 | 577 | 1112 | 26 | 10 |
| NakhonSawan1 × KKU–PFC2 | 765 | 1193 | 36 | 13 | 466 | 1056 | 24 | 9 |
| NakhonSawan1 × KKU–PFC3 | 628 | 1113 | 31 | 12 | 611 | 1095 | 26 | 10 |
| NakhonSawan1 × KKU–PFC4 | 131 | 262 | 9 | 6 | 605 | 1186 | 25 | 8 |
| NakhonSawan1 × KKU–PFC5 | 330 | 554 | 17 | 8 | 479 | 1130 | 27 | 10 |
| NakhonSawan2 × KKU–PFC1 | 353 | 742 | 22 | 10 | 270 | 759 | 16 | 7 |
| NakhonSawan2 × KKU–PFC2 | 390 | 691 | 20 | 11 | 640 | 1165 | 30 | 11 |
| NakhonSawan2 × KKU–PFC3 | 504 | 946 | 24 | 12 | 724 | 648 | 34 | 12 |
| NakhonSawan2 × KKU–PFC4 | 599 | 882 | 26 | 12 | 935 | 1124 | 37 | 11 |
| NakhonSawan2 × KKU–PFC5 | 279 | 601 | 17 | 8 | 200 | 636 | 14 | 7 |
| Takfa1 × KKU–PFC1 | 609 | 1160 | 31 | 13 | 293 | 733 | 17 | 9 |
| Takfa1 × KKU–PFC2 | 525 | 1027 | 30 | 12 | 188 | 632 | 13 | 7 |
| Takfa1 × KKU–PFC3 | 379 | 720 | 31 | 11 | 339 | 897 | 20 | 8 |
| Takfa1 × KKU–PFC4 | 381 | 716 | 14 | 10 | 829 | 1577 | 34 | 14 |
| Takfa1 × KKU–PFC5 | 470 | 790 | 24 | 10 | 160 | 518 | 13 | 6 |
| Takfa2 × KKU–PFC1 | 270 | 360 | 13 | 8 | 720 | 1361 | 33 | 13 |
| Takfa2 × KKU–PFC2 | 373 | 738 | 22 | 11 | 625 | 1129 | 26 | 12 |
| Takfa2 × KKU–PFC3 | 440 | 875 | 23 | 10 | 730 | 1485 | 32 | 13 |
| Takfa2 × KKU–PFC4 | 546 | 878 | 28 | 10 | 473 | 1121 | 25 | 11 |
| Takfa2 × KKU–PFC5 | 271 | 545 | 16 | 9 | 184 | 516 | 10 | 8 |
| Takfa3 × KKU–PFC1 | 583 | 1067 | 28 | 13 | 286 | 889 | 18 | 10 |
| Takfa3 × KKU–PFC2 | 805 | 1303 | 35 | 15 | 268 | 755 | 18 | 11 |
| Takfa3 × KKU–PFC3 | 893 | 1419 | 38 | 17 | 222 | 567 | 14 | 6 |
| Takfa3 × KKU–PFC4 | 677 | 876 | 27 | 12 | 525 | 1018 | 30 | 8 |
| Takfa3 × KKU–PFC5 | 1097 | 1472 | 43 | 17 | 219 | 619 | 18 | 4 |
| Pacific339 | 2 | 165 | 1 | 9 | 8 | 319 | 4 | 1 |
| CP301 | 2 | 69 | 1 | 5 | 5 | 70 | 3 | 1 |
| Pioneer4546 | 3 | 76 | 2 | 6 | 2 | 35 | 2 | 1 |
| Syngenta6248 | 3 | 71 | 1 | 6 | 5 | 54 | 3 | 1 |
| Fancy111 | 157 | 247 | 6 | 8 | 125 | 508 | 13 | 3 |
| Mean | 472 | 779 | 22 | 11 | 413 | 830 | 21 | 8 |
| LSD (0.05) | 55 | 52 | 2 | 1 | 50 | 80 | 2 | 1 |
| SE | 65 | 93 | 2 | 1 | 58 | 87 | 2 | 1 |
| Hybrids | Husk | Cob | ||||||
|---|---|---|---|---|---|---|---|---|
| TAC (mg CGE/100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/ 100 g DW) | TAC (mg CGE/ 100 g DW) | TPC (mg GAE/100 g DW) | DPPH (%) | TEAC (mmol TE/100 g DW) | |
| NakhonSawan1 × KKU–PFC1 | −116.1 ** | −160.2 ** | −5.3 ** | −1.8 ** | 145.8 ** | 279.6 ** | 8.4 ** | 2.6 ** |
| NakhonSawan1 × KKU–PFC2 | −124.2 ** | −93.0 ** | −2.8 ** | −1.4 ** | −139.6 ** | 34.8 | −5.1 ** | −0.2 |
| NakhonSawan1 × KKU–PFC3 | 19.4 | −14.2 | −0.2 | −0.4* | 12.3 | −88.0 ** | 0.1 | −0.3 * |
| NakhonSawan1 × KKU–PFC4 | −87.2 ** | −55.7 * | −2.2 * | 0.4 * | −148.0 ** | −341.0 ** | −8.5 ** | −0.8 ** |
| NakhonSawan1 × KKU–PFC5 | 308.2 ** | 323.1 ** | 10.5 ** | 3.2 ** | 129.5 ** | 114.6 ** | 5.1 ** | −1.3 ** |
| NakhonSawan2 × KKU–PFC1 | 69.6 ** | 86.6 ** | 4.9 * | 1.6 ** | 62.8 ** | −25.7 | 1.5 * | 0.6 * |
| NakhonSawan2 × KKU–PFC2 | −21.1 | 6.0 | 0.9 | −0.4 * | −250.3 ** | −129.1 ** | −8.8 ** | −2.7 ** |
| NakhonSawan2 × KKU–PFC3 | 187.3 ** | 313.6 ** | 6.1 ** | 1.9 ** | −35.6 * | −159.9 ** | −1.4 * | −0.4 |
| NakhonSawan2 × KKU–PFC4 | −59.0 ** | −282.5 ** | −6.6 ** | −1.7 ** | 207.5 ** | 217.1 ** | 9.0 | 1.2 ** |
| NakhonSawan2 × KKU–PFC5 | −176.9 ** | −123.7 ** | −5.2 ** | −1.4 ** | 15.6 | 97.6 ** | −0.3 | 1.3 ** |
| Takfa1 × KKU–PFC1 | 236.7 ** | 280.3 ** | 8.2 ** | 1.9 ** | −56.4 * | −58.1 * | −0.6 | −0.9 ** |
| Takfa1 × KKU–PFC2 | −94.7 ** | −200.6 ** | −4.8 ** | −0.9 ** | 111.6 ** | 300.3 ** | 4.7 ** | 0.7 ** |
| Takfa1 × KKU–PFC3 | −8.1 | 25.0 | 0.6 | −0.1 | −148.3 ** | −238.1 ** | −5.3 ** | −2.2 ** |
| Takfa1 × KKU–PFC4 | −67.5 ** | −61.0 * | −1.5 * | −0.1 | 103.6 ** | 8.5 | 2.2 ** | −0.1 |
| Takfa1 × KKU–PFC5 | −66.4 ** | −43.7 * | −2.5 ** | −0.9 ** | −10.5 | −12.6 | −1.1 * | 2.5 ** |
| Takfa2 × KKU–PFC1 | 102.0 ** | 176.2 ** | 2.6 ** | 0.8 ** | 1.0 | −10.2 | −2.0 ** | 0.4 * |
| Takfa2 × KKU–PFC2 | 21.4 | 30.1 | −1.7 * | 0.1 | 107.6 * | −207.6 ** | 5.5 ** | 2.2 ** |
| Takfa2 × KKU–PFC3 | −151.2 ** | −306.4 ** | 1.0 | −1.5 ** | −85.1 ** | 36.7 | −1.2 * | −1.4 * |
| Takfa2 × KKU–PFC4 | 3.2 | 52.2 * | −1.7* | −0.6 ** | 121.5 ** | 373.0 ** | 4.8 ** | 0.9 ** |
| Takfa2 × KKU–PFC5 | 24.7 | 47.9 * | −0.1 | 1.2 ** | −145.0 ** | −191.8 ** | −7.0 ** | −2.1 ** |
| Takfa3 × KKU–PFC1 | −292.2 ** | −383.0 ** | −10.3 ** | −2.4 ** | −153.1 ** | −185.6 ** | −7.4 ** | −2.8 ** |
| Takfa3 × KKU–PFC2 | 218.6 ** | 257.5 ** | 8.3 ** | 2.5 ** | 170.6 ** | 1.6 | 3.8 ** | 0.1 |
| Takfa3 × KKU–PFC3 | −47.3 * | −17.9 | −7.4 ** | 0.0 | 256.8 ** | 449.2 ** | 7.9 ** | 4.3 ** |
| Takfa3 × KKU–PFC4 | 210.5 ** | 346.9 ** | 12.1 ** | 2.0 ** | −284.6 ** | −257.5 ** | −7.5 ** | −1.2 ** |
| Takfa3 × KKU–PFC5 | −89.5 ** | −203.6 ** | −2.7 ** | −2.1 ** | 10.3 | −7.7 | 3.3 ** | −0.4 * |
| SE | 29.0 | 40.3 | 1.1 | 0.3 | 28.7 | 40.6 | 1.1 | 0.3 |
| Hybrids | Husk | Cob | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAC | TPC | DPPH | TEAC | TAC | TPC | DPPH | TEAC | |||||||||
| MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | MP | HP | |
| NakhonSawan1 × KKU–PFC1 | −48.5 | −74.2 | −40.8 | −69.9 | −12.7 | −55.5 | −14.6 | −47.9 | −31.2 | −65.5 | −8.7 | −53.2 | −22.7 | −56.9 | −11.9 | −45.9 |
| NakhonSawan1 × KKU–PFC2 | 10.3 | −44.7 | 31.9 * | −32.2 | 56.7 * | −19.5 | 16.4 * | −26.3 | −13.0 | −56.4 | 15.8 * | −40.2 | −3.9 | −44.3 | −12.1 | −44.4 |
| NakhonSawan1 × KKU–PFC3 | 21.1 * | −39.2 | 26.6 * | −34.9 | 44.3 * | −25.8 | 13.8 * | −26.4 | 62.4 * | −18.5 | 36.1 * | −29.4 | 18.5 | −29.7 | −1.1 | −38.8 |
| NakhonSawan1 × KKU–PFC4 | −63.2 | −81.5 | −45.8 | −71.4 | −40.0 | −68.8 | −34.5 | −56.4 | 46.0 * | −26.8 | 43.8 * | −25.4 | 16.2 * | −30.8 | −19.4 | −49.3 |
| NakhonSawan1 × KKU–PFC5 | −22.5 | −61.1 | −12.2 | −54.3 | 9.0 | −43.4 | −23.2 | −48.9 | 193.2 * | 47.7 * | 221.4 * | 75.7 * | 130.7 * | 69.5 * | 59.4 * | 29.4 |
| NakhonSawan2 × KKU–PFC1 | −62.7 | −81.3 | −47.0 | −73.2 | −32.5 | −65.5 | −27.6 | −54.1 | −68.2 | −84.1 | −39.0 | −68.7 | −49.7 | −73.7 | −33.4 | −61.3 |
| NakhonSawan2 × KKU–PFC2 | −43.1 | −71.5 | −22.9 | −60.5 | −12.1 | −54.7 | −8.5 | −39.7 | 19.8 * | −40.0 | 26.9 * | −34.1 | 30.0 * | −31.1 | 11.8 | −32.4 |
| NakhonSawan2 × KKU–PFC3 | −4.1 | −52.0 | 7.7 | −44.8 | 12.7 | −41.8 | 5.2 | −29.1 | 98.9 * | −0.3 | −18.9 | −57.6 | 72.9 * | −7.7 | 21.5 | −27.4 |
| NakhonSawan2 × KKU–PFC4 | 59.7 * | −20.0 | 64.6 * | −14.4 | 59.0 * | −17.0 | 14.2 | −20.2 | 128.1 * | 14.3 * | 37.7 * | −28.1 | 88.4 * | 1.2 | 13.6 | −30.1 |
| NakhonSawan2 × KKU–PFC5 | −33.9 | −66.9 | −4.3 | −50.7 | 10.6 | −42.3 | −19.3 | −43.7 | 14.9 | −42.2 | 64.2 * | −9.7 | 49.5 * | −12.2 | 32.6 | −0.8 |
| Takfa1 × KKU–PFC1 | −34.8 | −67.4 | −17.6 | −58.4 | −1.9 | −49.6 | −10.6 | −42.4 | −65.9 | −82.9 | −39.9 | −69.2 | −47.1 | −72.6 | −22.7 | −55.4 |
| Takfa1 × KKU–PFC2 | −23.8 | −61.9 | 15.4 * | −41.5 | 28.9 * | −33.2 | −0.3 | −33.2 | −64.8 | −82.3 | −30.2 | −64.0 | −42.7 | −69.9 | −27.3 | −57.2 |
| Takfa1 × KKU–PFC3 | −24.9 | −62.4 | −16.6 | −57.6 | 43.8 * | −25.2 | −5.9 | −34.9 | −7.9 | −53.7 | 12.6 | −41.7 | 3.2 | −45.4 | −20.9 | −54.4 |
| Takfa1 × KKU–PFC4 | −0.4 * | −50.1 | 27.5 * | −34.9 | −13.2 | −54.3 | −8.0 | −35.7 | 101.9 * | 1.4 * | 92.1 * | −0.6 * | 76.4 * | −6.3 | 54.7 * | −8.5 |
| Takfa1 × KKU–PFC5 | 4.8 | −47.5 | 21.1 | −38.2 | 49.9 * | −21.0 | −7.9 | −34.6 | −11.4 | −55.2 | 35.6 | −26.8 | 35.0 * | −22.9 | 32.3 | −12.0 |
| Takfa2 × KKU–PFC1 | −71.2 | −85.6 | −73.7 | −86.7 | −59.6 | −79.1 | −43.6 | −64.5 | −15.1 | −57.5 | 11.0 | −42.6 | 4.8 | −45.8 | 15.6 | −32.4 |
| Takfa2 × KKU–PFC2 | −45.1 | −72.5 | −16.1 | −57.6 | −4.4 | −50.5 | −9.2 | −41.2 | 16.8 * | −41.5 | 23.0 * | −35.6 | 15.8 * | −39.3 | 19.0 | −27.9 |
| Takfa2 × KKU–PFC3 | −15.6 | −57.8 | 0.9 | −49.0 | 3.0 | −46.5 | −7.5 | −38.6 | 95.6 * | −1.9 | 82.1 * | −4.1 | 65.2 * | −13.0 | 25.1 | −25.8 |
| Takfa2 × KKU–PFC4 | 44.7 * | −27.5 | 64.1 * | −16.4 | 69.5 * | −10.3 | 1.2 | −30.9 | 16.1 | −41.8 | 33.8 | −29.6 | 26.1 * | −33.2 | 18.5 | −27.8 |
| Takfa2 × KKU–PFC5 | −40.7 | −70.3 | −18.9 | −58.8 | −1.1 | −47.6 | −9.3 | −37.2 | 8.5 | −45.5 | 35.6 | −23.7 | 3.2 | −40.0 | 56.4 * | 13.9 |
| Takfa3 × KKU–PFC1 | −37.4 | −68.6 | −25.2 | −61.7 | −13.7 | −55.1 | −2.8 | −38.8 | −67.4 | −82.8 | −30.3 | −62.1 | −47.1 | −70.0 | −17.5 | −49.4 |
| Takfa3 × KKU–PFC2 | 15.1 | −42.3 | 41.9 * | −25.9 | 48.6 * | −21.0 | 29.7 * | −15.2 | −52.7 | −74.5 | −25.2 | −57.4 | −32.5 | −60.0 | 6.0 | −32.6 |
| Takfa3 × KKU–PFC3 | 71.0 * | −14.2 | 58.1 * | −17.2 | 72.0 * | −8.4 | 56.5 * | 4.7 | −47.2 | −70.7 | −35.8 | −63.2 | −34.0 | −59.7 | −41.2 | −63.1 |
| Takfa3 × KKU–PFC4 | 81.3 * | −8.9 | 63.9 * | −12.7 | 61.8 * | −12.0 | 12.9 | −22.0 | 14.1 * | −37.2 | 11.9 * | −35.3 | 33.5 * | −18.4 | −13.1 | −44.1 |
| Takfa3 × KKU–PFC5 | 144.7 * | 22.8 * | 119.7 * | 15.6 * | 159.6 * | 41.1 * | 70.3 * | 17.8 * | 3.0 | −35.7 | 31.3 | −8.9 | 44.9 * | 8.7 * | −30.7 | −43.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Scott, M.P.; Lertrat, K.; Suriharn, B. Combining Ability and Heterosis for Agronomic Traits, Husk and Cob Pigment Concentration of Maize. Agriculture 2020, 10, 510. https://doi.org/10.3390/agriculture10110510
Khamphasan P, Lomthaisong K, Harakotr B, Scott MP, Lertrat K, Suriharn B. Combining Ability and Heterosis for Agronomic Traits, Husk and Cob Pigment Concentration of Maize. Agriculture. 2020; 10(11):510. https://doi.org/10.3390/agriculture10110510
Chicago/Turabian StyleKhamphasan, Ponsawan, Khomsorn Lomthaisong, Bhornchai Harakotr, Marvin Paul Scott, Kamol Lertrat, and Bhalang Suriharn. 2020. "Combining Ability and Heterosis for Agronomic Traits, Husk and Cob Pigment Concentration of Maize" Agriculture 10, no. 11: 510. https://doi.org/10.3390/agriculture10110510
APA StyleKhamphasan, P., Lomthaisong, K., Harakotr, B., Scott, M. P., Lertrat, K., & Suriharn, B. (2020). Combining Ability and Heterosis for Agronomic Traits, Husk and Cob Pigment Concentration of Maize. Agriculture, 10(11), 510. https://doi.org/10.3390/agriculture10110510





