Low Cost MR Compatible Haptic Stimulation with Application to fMRI Neurofeedback
Abstract
:1. Introduction
2. Materials and Methods
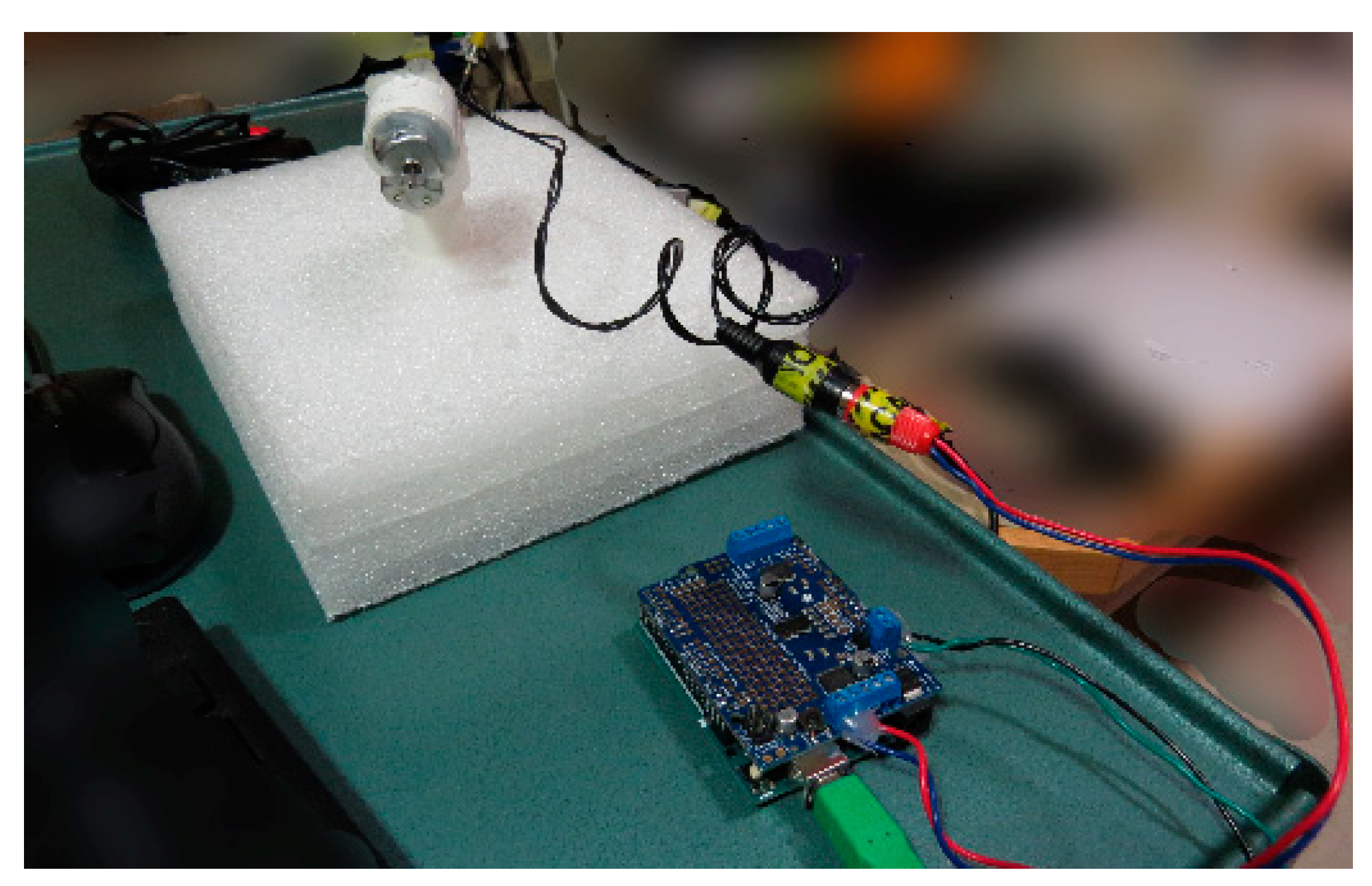
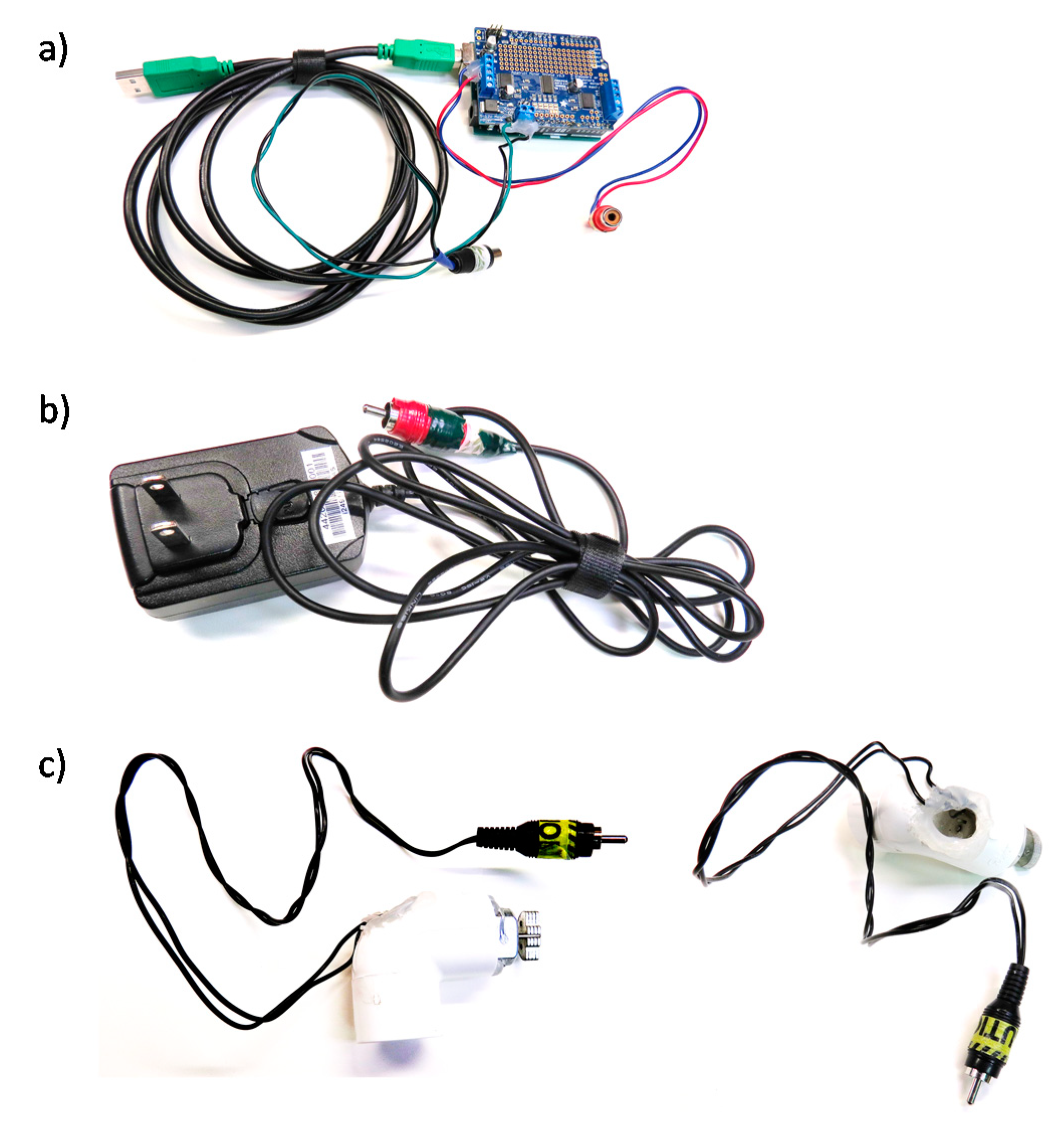
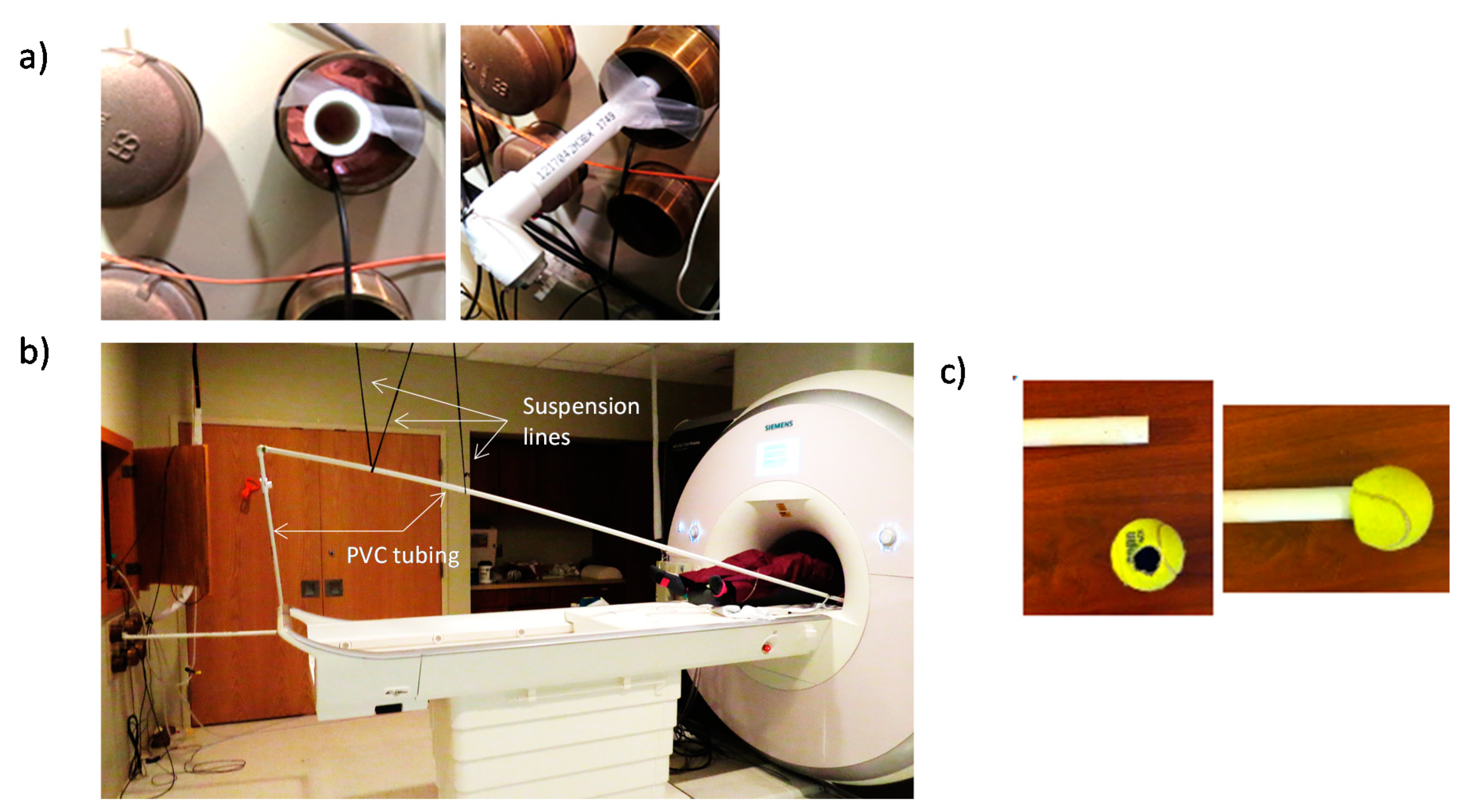
2.1. Haptic Setup for MRI
2.2. Procedure
3. Results
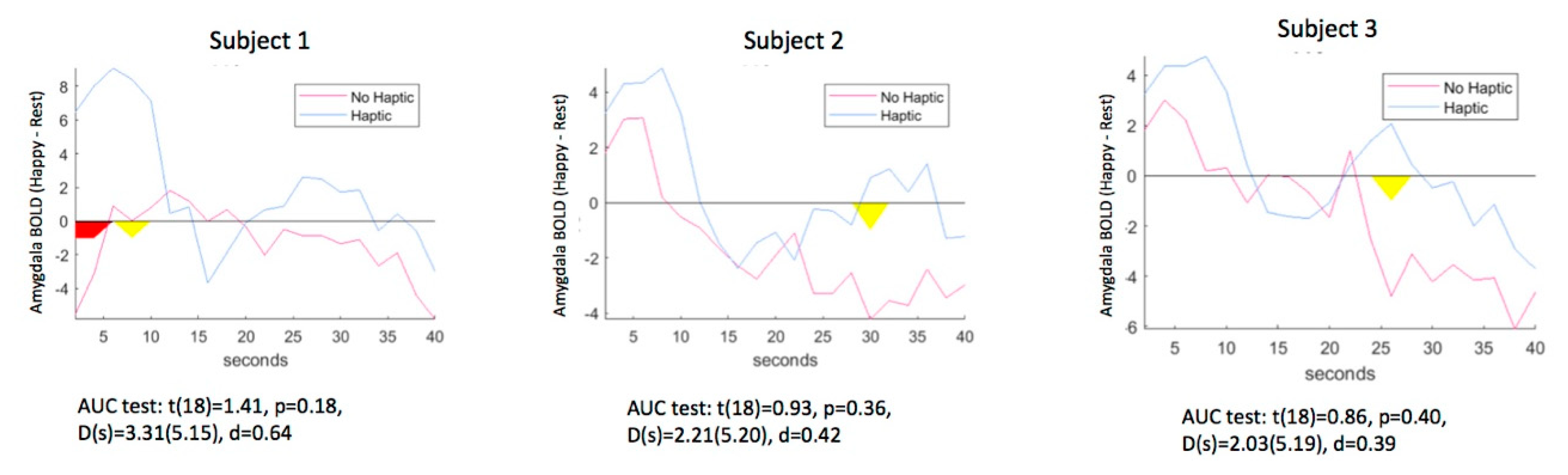
3.1. Q1: Can Neurofeedback Effects Be Detected with Haptic Stimulation? Analysis of Amygdala Reactivity with vs. without Haptic Feedback
3.2. Q2: Does Haptic Stimuliation Occlude Effects of Interest? Resting BOLD Response with vs. without Haptic Stimulation in Neurofeedback Regions for Which Detection of Haptic Stimuliation Would Be Problamatic (Amygdala, Intraparietal Sulcus)
3.3. Q3: Are Effects of Haptic Stimuliation Detecable? Resting BOLD Response with vs. without Haptic Stimulation in Regions Where Detection of Haptic Stimuliation Is Expected (Insula, Somatosensory Cortex)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleury, M.; Lioi, G.; Barillot, C.; Lecuyer, A. A survey on the Use of Haptic Feedback for Brain-Computer Interfaces and Neurofeedback. Front. Neurosci. 2020, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Kosmyna, N.; Maes, P. AttentivU: An EEG-based closed loop biofeedback system for real-time monitoring and improvement of engagement for personalized learning. Sensors 2019, 19, 5200. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I.; Jung, S.Y.; Min, S.; Seol, E.; Seo, S.; Hur, J.W.; Jung, D.; Lee, H.J.; Lee, S.; Kim, G.J.; et al. Visuo-Haptic-Based Multimodal Feedback Virtual Reality Solution to Improve Anxiety Symptoms: A Proof-of-Concept Study. Psychiatry Investig. 2019, 16, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overtoom, E.M.; Horeman, T.; Jansen, F.W.; Dankelman, J.; Schreuder, H.W.R. Haptic Feedback, Force Feedback, and Force-Sensing in Simulation Training for Laparoscopy: A Systematic Overview. J. Surg. Educ. 2019, 76, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Prause, N.; Siegle, G.J.; Deblieck, C.; Wu, A.; Iacoboni, M. EEG to Primary Rewards: Predictive Utility and Malleability by Brain Stimulation. PLoS ONE 2016, 11, e0165646. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Zhu, J.; Goyal, D.; Khatib, O. Haptic fMRI: Reliability and performance of electromagnetic haptic interfaces for motion and force neuroimaging experiments. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3930–3935. [Google Scholar]
- Maddahi, Y.; Zareinia, K.; Tomanek, B.; Sutherland, G.R. Challenges in developing a magnetic resonance-compatible haptic hand-controller for neurosurgical training. Proc. Inst. Mech. Eng. H J. Eng. Med. 2018, 232, 1148–1167. [Google Scholar] [CrossRef]
- Vigaru, B.; Sulzer, J.; Gassert, R. Design and Evaluation of a Cable-Driven fMRI-Compatible Haptic Interface to Investigate Precision Grip Control. IEEE Trans. Haptics 2015, 9, 20–32. [Google Scholar] [CrossRef]
- Kim, J.; Chung, Y.G.; Chung, S.-C.; Bülthoff, H.H.; Kim, S.-P. Neural Categorization of Vibrotactile Frequency in Flutter and Vibration Stimulations: An fMRI Study. IEEE Trans. Haptics 2016, 9, 455–464. [Google Scholar] [CrossRef]
- Puckett, A.M.; Bollmann, S.; Junday, K.; Barth, M.; Cunnington, R. Bayesian population receptive field modeling in human somatosensory cortex. NeuroImage 2020, 208, 116465. [Google Scholar] [CrossRef]
- Malone, P.S.; Eberhardt, S.P.; Wimmer, K.; Sprouse, C.; Klein, R.; Glomb, K.; Scholl, C.A.; Bokeria, L.; Cho, P.; Deco, G.; et al. Neural mechanisms of vibrotactile categorization. Hum. Brain Mapp. 2019, 40, 3078–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-H.; Velenosi, L.A.; Schröder, P.; Ludwig, S.; Blankenburg, F. Decoding vibrotactile choice independent of stimulus order and saccade selection during sequential comparisons. Hum. Brain Mapp. 2018, 40, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prause, N.; Roberts, V.; Legarreta, M.; Cox, L.M.R. Clinical and research concerns with vibratory stimulation: A review and pilot study of common stimulation devices. Sex. Relatsh. Ther. 2012, 27, 17–34. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Chen, L.; Gu, B.; Liu, S.; Xu, M.; Qi, H.; He, F.; Ming, N. A BCI based visual-haptic neurofeedback training improves cortical activations and classification performance during motor imagery. J. Neural Eng. 2019, 16, 066012. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, M.; Gharabaghi, A. Oscillatory entrainment of the motor cortical network during motor imagery is modulated by the feedback modality. NeuroImage 2015, 111, 1–11. [Google Scholar] [CrossRef]
- Rossi-Izquierdo, M.; Ernst, A.; Soto-Varela, A.; Santos-Pérez, S.; Faraldo-García, A.; Sesar-Ignacio, Á.; Basta, D. Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: Reducing the number of falls. Gait Posture 2013, 37, 195–200. [Google Scholar] [CrossRef]
- Basta, D.; Rossi-Izquierdo, M.; Soto-Varela, A.; Greters, M.E.; Bittar, R.S.; Steinhagen-Thiessen, E.; Eckardt, R.; Harada, T.; Goto, F.; Ogawa, K.; et al. Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: A double-blind, placebo-controlled multicenter study. Otol. Neurotol. 2011, 32, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, H.; Moreira, R.; Rodrigues, A.; Santos, C.P. Finding Parameters around the Abdomen for a Vibrotactile System: Healthy and Patients with Parkinson’s Disease. J. Med. Syst. 2018, 42, 232. [Google Scholar] [CrossRef]
- Paszkiel, S. Data Acquisition Methods for Human Brain Activity. In Analysis and Classification of EEG Signals for Brain-Computer Interfaces; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Stoeckel, L.; Garrison, K.; Ghosh, S.; Wighton, P.; Hanlon, C.; Gilman, J.; Greer, S.; Turk-Browne, N.; DeBettencourt, M.; Scheinost, D.; et al. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage Clin. 2014, 5, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.T.; MacPherson, A.; Lifshitz, M.; Roth, R.R.; Raz, A. Neurofeedback with fMRI: A critical systematic review. NeuroImage 2018, 172, 786–807. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, J.; Haller, S.; Scharnowski, F.; Weiskopf, N.; Birbaumer, N.; Blefari, M.; Bruehl, A.; Cohen, L.G.; Decharms, R.; Gassert, R.; et al. Real-time fMRI neurofeedback: Progress and challenges. NeuroImage 2013, 76, 386–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zotev, V.; Krueger, F.; Phillips, R.; Alvarez, R.P.; Simmons, W.K.; Bellgowan, P.; Drevets, W.C.; Bodurka, J. Self-Regulation of Amygdala Activation Using Real-Time fMRI Neurofeedback. PLoS ONE 2011, 6, e24522. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Real-Time fMRI Neurofeedback Training of Amygdala Activity in Patients with Major Depressive Disorder. PLoS ONE 2014, 9, e88785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.D.; Siegle, G.J.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am. J. Psychiatry 2017, 174, 748–755. [Google Scholar] [CrossRef]
- Young, K.D.; Misaki, M.; Harmer, C.J.; Victor, T.; Zotev, V.; Phillips, R.; Siegle, G.J.; Drevets, W.C.; Bodurka, J. Real-Time fMRI Amygdala Neurofeedback Changes Positive Information Processing in Major Depressive Disorder. Biol. Psychiatry 2017, 82, 578–586. [Google Scholar] [CrossRef]
- Hwang, J.; Hwang, W. Vibration Perception and Excitatory Direction for Haptic Devices. J. Intell. Manuf. 2011, 22, 17–27. [Google Scholar] [CrossRef]
- Watanabe, H.; Ujike, H. Comfort in observing stereoscopic images reduced by vibration stimuli. Health 2012, 4, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Hiraba, H.; Inoue, M.; Gora, K.; Sato, T.; Nishimura, S.; Yamaoka, M.; Kumakura, A.; Ono, S.; Wakasa, H.; Nakayama, E.; et al. Facial Vibrotactile Stimulation Activates the Parasympathetic Nervous System: Study of Salivary Secretion, Heart Rate, Pupillary Reflex, and Functional Near-Infrared Spectroscopy Activity. BioMed Res. Int. 2014, 2014, 910812. [Google Scholar] [CrossRef]
| Subject 1 | Subject 2 | Subject 3 | Overall Mean | |
|---|---|---|---|---|
| Baseline | −0.41 | −0.10 | −0.18 | −0.23 |
| Visual Run 1 | 0.26 | 0.58 | 0.03 | 0.29 |
| Haptic Run 1 | 0.33 | 0.51 | 0.22 | 0.35 |
| Visual Run 2 | 0.55 | 0.76 | 0.17 | 0.49 |
| Haptic Run 2 | 0.61 | 0.99 | 0.40 | 0.67 |
| Mean Visual All Runs | 0.41 | 0.67 | 0.10 | 0.39 |
| Mean Haptic All Runs | 0.47 | 0.75 | 0.31 | 0.51 |
| % Signal Change Vibration On—Off | ||||||
|---|---|---|---|---|---|---|
| Left Amygdala | Left Intraparietal Sulcus | Left Insula | Right Insula | Left Somatosensory Cortex | Right Somatosensory Cortex | |
| Subject 1 | 0.01 | 0.02 | 0.34 | 0.19 | 0.30 | 0.29 |
| Subject 2 | −0.01 | −0.01 | 0.28 | 0.27 | 0.36 | 0.36 |
| Subject 3 | 0.01 | −0.01 | 0.19 | 0.20 | 0.26 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.D.; Prause, N.; Lazzaro, S.; Siegle, G.J. Low Cost MR Compatible Haptic Stimulation with Application to fMRI Neurofeedback. Brain Sci. 2020, 10, 790. https://doi.org/10.3390/brainsci10110790
Young KD, Prause N, Lazzaro S, Siegle GJ. Low Cost MR Compatible Haptic Stimulation with Application to fMRI Neurofeedback. Brain Sciences. 2020; 10(11):790. https://doi.org/10.3390/brainsci10110790
Chicago/Turabian StyleYoung, Kymberly D., Nicole Prause, Sarah Lazzaro, and Greg J. Siegle. 2020. "Low Cost MR Compatible Haptic Stimulation with Application to fMRI Neurofeedback" Brain Sciences 10, no. 11: 790. https://doi.org/10.3390/brainsci10110790
APA StyleYoung, K. D., Prause, N., Lazzaro, S., & Siegle, G. J. (2020). Low Cost MR Compatible Haptic Stimulation with Application to fMRI Neurofeedback. Brain Sciences, 10(11), 790. https://doi.org/10.3390/brainsci10110790




