Mismatch Negativity and Loudness Dependence of Auditory Evoked Potentials among Patients with Major Depressive Disorder, Bipolar II Disorder, and Bipolar I Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. MMN and Procedure
2.3. LDAEP and Procedure
2.4. Statistical Analysis
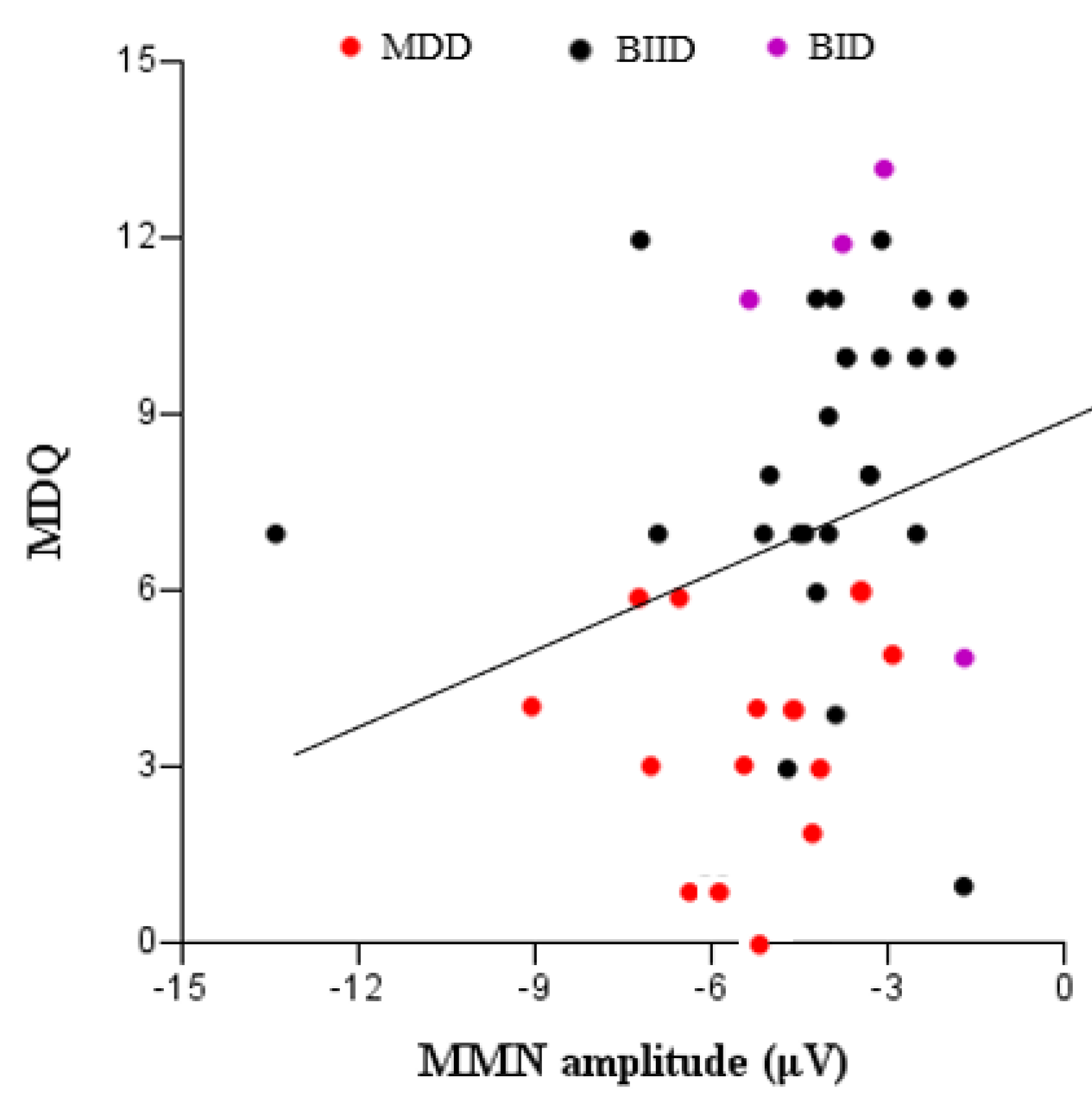
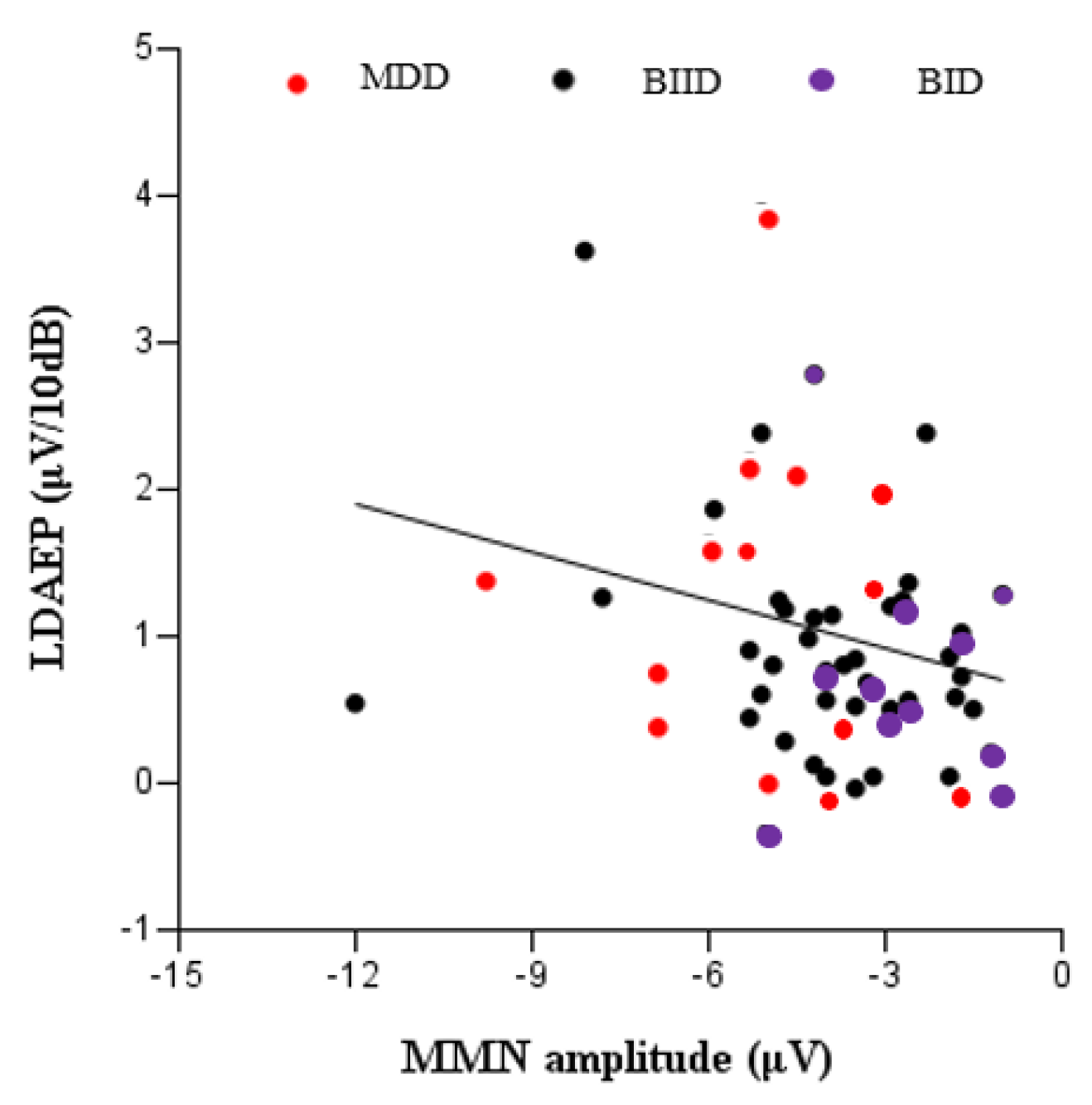
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuellar, A.K.; Johnson, S.L.; Winters, R. Distinctions between bipolar and unipolar depression. Clin. Psychol. Rev. 2005, 25, 307–339. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.J.; Boudreau, V.G.; Yatham, L.N. Neuropsychological functioning in euthymic bipolar disorder: A meta-analysis. Acta Psychiatr. Scand. 2007, 116, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.R.; Miskowiak, K.; Vale, A.M.; Köhler, C.A.; Kjærstad, H.L.; Stubbs, B.; Kessing, L.V.; Vieta, E.; Maes, M.; Goldstein, B.I.; et al. Cognitive Impairment in Euthymic Pediatric Bipolar Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Gallagher, P.; Hughes, J.H.; Watson, S.; Gray, J.M.; Ferrier, I.N.; Young, A.H. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br. J. Psychiatry 2005, 186, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Alain, C.; Woods, D.L.; Knight, R.T. A distributed cortical network for auditory sensory memory in humans. Brain Res. 1998, 812, 23–37. [Google Scholar] [CrossRef]
- Näätänen, R.; Gaillard, A.; Mäntysalo, S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978, 42, 313–329. [Google Scholar] [CrossRef]
- Näätänen, R.; Kujala, T.; Escera, C.; Baldeweg, T.; Kreegipuu, K.; Carlson, S.; Ponton, C. The mismatch negativity (MMN) – A unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012, 123, 424–458. [Google Scholar] [CrossRef]
- Bonetti, L.; Haumann, N.; Brattico, E.; Kliuchko, M.; Vuust, P.; Särkämö, T.; Näätänen, R. Auditory sensory memory and working memory skills: Association between frontal MMN and performance scores. Brain Res. 2018, 1700, 86–98. [Google Scholar] [CrossRef]
- Alho, K.; Woods, D.; Algazi, A.; Knight, R.; Näätänen, R. Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 353–362. [Google Scholar] [CrossRef]
- Donaldson, K.R.; Novak, K.D.; Foti, D.; Marder, M.; Perlman, G.; Kotov, R.; Mohanty, A. Associations of mismatch negativity with psychotic symptoms and functioning transdiagnostically across psychotic disorders. J. Abnorm. Psychol. 2020, 129, 570–580. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.J.; Lee, H.Y.; Lee, H.S.; Kim, S.; Shim, S.H. Mismatch Negativity Indices as a Prognostic Factor for Remission in Schizophrenia. Clin. Psychopharmacol. Neurosci. 2020, 18, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeon, H.; Jang, K.-I.; Kim, Y.-W.; Im, C.-H.; Lee, S.-H. Mismatch Negativity and Cortical Thickness in Patients with Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2018, 45, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hermens, D.F.; Chitty, K.M.; Kaur, M. Mismatch negativity in bipolar disorder: A neurophysiological biomarker of intermediate effect? Schizophr. Res. 2018, 191, 132–139. [Google Scholar] [CrossRef]
- Erickson, M.A.; Ruffle, A.; Gold, J.M. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biol. Psychiatry 2016, 79, 980–987. [Google Scholar] [CrossRef]
- Chitty, K.M.; Lagopoulos, J.; Lee, R.S.; Hickie, I.B.; Hermens, D.F. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. Eur. Neuropsychopharmacol. 2013, 23, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Juckel, G. Identifying psychiatric patients with serotonergic dysfunctions by event-related potentials. World J. Biol. Psychiatry 2000, 1, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Gallinat, J.; Juckel, G. Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J. Affect. Disord. 2001, 62, 93–100. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, X.; Li, L. Metabotropic glutamate receptors subtype 5 are necessary for the enhancement of auditory evoked potentials in the lateral nucleus of the amygdala by tetanic stimulation of the auditory thalamus. Neuroscience 2008, 152, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Park, Y.-M.; Lee, S.-H.; Shim, M. Prediction of Long-Term Treatment Response to Selective Serotonin Reuptake Inhibitors (SSRIs) Using Scalp and Source Loudness Dependence of Auditory Evoked Potentials (LDAEP) Analysis in Patients with Major Depressive Disorder. Int. J. Mol. Sci. 2015, 16, 6251–6265. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, Y.-M. The association between suicidality and serotonergic dysfunction in depressed patients. J. Affect. Disord. 2013, 148, 72–76. [Google Scholar] [CrossRef]
- Park, Y.-M.; Lee, S.-H.; Kim, S.; Bae, S.-M. The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M. Relationship between Childhood Maltreatment, Suicidality, and Bipolarity: A Retrospective Study. Psychiatry Investig. 2017, 14, 136–140. [Google Scholar] [CrossRef]
- Kähkönen, S.; Mäkinen, V.; Jääskeläinen, I.P.; Pennanen, S.; Liesivuori, J.; Ahveninen, J. Serotonergic modulation of mismatch negativity. Psychiatry Res. Neuroimaging 2005, 138, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, S.; Jaaskelainen, I.P.; Pennanen, S.; Liesivuori, J.; Ahveninen, J. Acute Tryptophan Depletion Decreases Intensity Dependence of Auditory Evoked Magnetic N1/P2 Dipole Source Activity. Psychopharmacology 2002, 164, 221–227. [Google Scholar] [PubMed]
- Wienberg, M.; Glenthoj, B.Y.; Jensen, K.S.; Oranje, B. A single high dose of escitalopram increases mismatch negativity without affecting processing negativity or P300 amplitude in healthy volunteers. J. Psychopharmacol. 2009, 24, 1183–1192. [Google Scholar] [CrossRef]
- Jin, M.J.; Jung, W.; Hyun, M.H.; Lee, S.-H. Effect of behavioral inhibition system and childhood emotional neglect on serotonergic activity, negative affect, and rejection sensitivity in non-clinical adults. PLoS ONE 2018, 13, e0207746. [Google Scholar] [CrossRef]
- Näätänen, R.; Sussman, E.S.; Salisbury, D.; Shafer, V.L. Mismatch Negativity (MMN) as an Index of Cognitive Dysfunction. Brain Topogr. 2014, 27, 451–466. [Google Scholar] [CrossRef]
- Menkes, M.W.; Armstrong, K.; Blackford, J.U.; Heckers, S.; Woodward, N.D. Neuropsychological Functioning in Early and Chronic Stages of Schizophrenia and Psychotic Bipolar Disorder. Schizophr. Res. 2019, 206, 413–419. [Google Scholar] [CrossRef]
- Andersson, S.; Barder, H.E.; Hellvin, T.; Løvdahl, H.; Malt, U.F. Neuropsychological and electrophysiological indices of neurocognitive dysfunction in bipolar II disorder. Bipolar Disord. 2008, 10, 888–899. [Google Scholar] [CrossRef]
- Takei, Y.; Kumano, S.; Maki, Y.; Hattori, S.; Kawakubo, Y.; Kasai, K.; Fukuda, M.; Mikuni, M. Preattentive dysfunction in bipolar disorder: A MEG study using auditory mismatch negativity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 903–912. [Google Scholar] [CrossRef]
- Jahshan, C.; Wynn, J.K.; Mathis, K.I.; Altshuler, L.L.; Glahn, D.C.; Green, M.F. Cross-diagnostic comparison of duration mismatch negativity and P3a in bipolar disorder and schizophrenia. Bipolar Disord. 2012, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Vollono, C.; Scaloni, L.; Buccelletti, F.; Camardese, G. Abnormality of Auditory Mismatch Negativity in Depression and Its Dependence on Stimulus Intensity. Clin. EEG Neurosci. 2015, 47, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Wei, D.; Wu, X.; Fu, Q.; Xu, F.; Wang, H.; Ye, M.; Ma, W.; Yang, L.; et al. Neurophysiological handover from MMN to P3a in first-episode and recurrent major depression. J. Affect. Disord. 2015, 174, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Naismith, S.L.; Mowszowski, L.; Ward, P.; Diamond, K.; Paradise, M.B.; Kaur, M.; Lewis, S.J.G.; Hickie, I.B.; Hermens, D.F. Reduced temporal mismatch negativity in late-life depression: An event-related potential index of cognitive deficit and functional disability? J. Affect. Disord. 2012, 138, 71–78. [Google Scholar] [CrossRef]
- Bissonnette, J.N.; Francis, A.M.; Hull, K.M.; Leckey, J.; Pimer, L.; Berrigan, L.I.; Fisher, D.J. MMN-Indexed Auditory Change Detection in Major Depressive Disorder. Clin. EEG Neurosci. 2020, 51, 365–372. [Google Scholar] [CrossRef]
- Kim, S.; Baek, J.H.; Shim, S.-H.; Kwon, Y.J.; Lee, H.Y.; Yoo, J.H.; Kim, J.S. Mismatch negativity indices and functional outcomes in unipolar and bipolar depression. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Mu, Z.; Chang, Y.; Xu, J.; Pang, X.; Zhang, H.; Liu, X.; Zheng, Y.; Liu, X.; Liu, X.; Wan, Y. Pre-attentive dysfunction of musical processing in major depressive disorder: A mismatch negativity study. J. Affect. Disord. 2016, 194, 50–56. [Google Scholar] [CrossRef]
- Kenemans, J.L.; Kähkönen, S. How Human Electrophysiology Informs Psychopharmacology: From Bottom-up Driven Processing to Top-Down Control. Neuropsychopharmacology 2010, 36, 26–51. [Google Scholar] [CrossRef]
- Umbricht, D.; Koller, R.; Vollenweider, F.X.; Schmid, L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol. Psychiatry 2002, 51, 400–406. [Google Scholar] [CrossRef]
- Kreitschmann-Andermahr, I.; Rosburg, T.; Demme, U.; Gaser, E.; Nowak, H.; Sauer, H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Cogn. Brain Res. 2001, 12, 109–116. [Google Scholar] [CrossRef]
- Teichert, T. Loudness- and time-dependence of auditory evoked potentials is blunted by the NMDA channel blocker MK-801. Psychiatry Res. 2017, 256, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.; Croft, R.J.; Guille, V.; Scholes, K.; O’Neill, B.V.; Phan, K.L.; Nathan, P.J.; Scholes-Balog, K.E. Acute dopamine and/or serotonin depletion does not modulate mismatch negativity (MMN) in healthy human participants. Psychopharmacology 2009, 208, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Ahveninen, J. Tryptophan Depletion Effects on EEG and MEG Responses Suggest Serotonergic Modulation of Auditory Involuntary Attention in Humans. Neuroimage 2002, 16, 1052–1061. [Google Scholar] [CrossRef]
- Kaur, M.; Battisti, R.A.; Ward, P.; Ahmed, A.; Hickie, I.B.; Hermens, D.F. MMN/P3a deficits in first episode psychosis: Comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr. Res. 2011, 130, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Park, Y.-M.; Lee, S.-H. Serotonergic Dysfunction in Patients with Bipolar Disorder Assessed by the Loudness Dependence of the Auditory Evoked Potential. Psychiatry Investig. 2012, 9, 298–306. [Google Scholar] [CrossRef]
- Min, J.-A.; Lee, S.-H.; Chae, J.-H.; Lee, C.-U.; Park, Y.-M.; Bae, S.-M. Clinical characteristics associated with different strengths of loudness dependence of auditory evoked potentials (LDAEP) in major depressive disorder. Psychiatry Res. 2012, 200, 374–381. [Google Scholar] [CrossRef]
- Linka, T.; Sartory, G.; Bender, S.; Gastpar, M.; Müller, B.W. The intensity dependence of auditory ERP components in unmedicated patients with major depression and healthy controls. An analysis of group differences. J. Affect. Disord. 2007, 103, 139–145. [Google Scholar] [CrossRef]
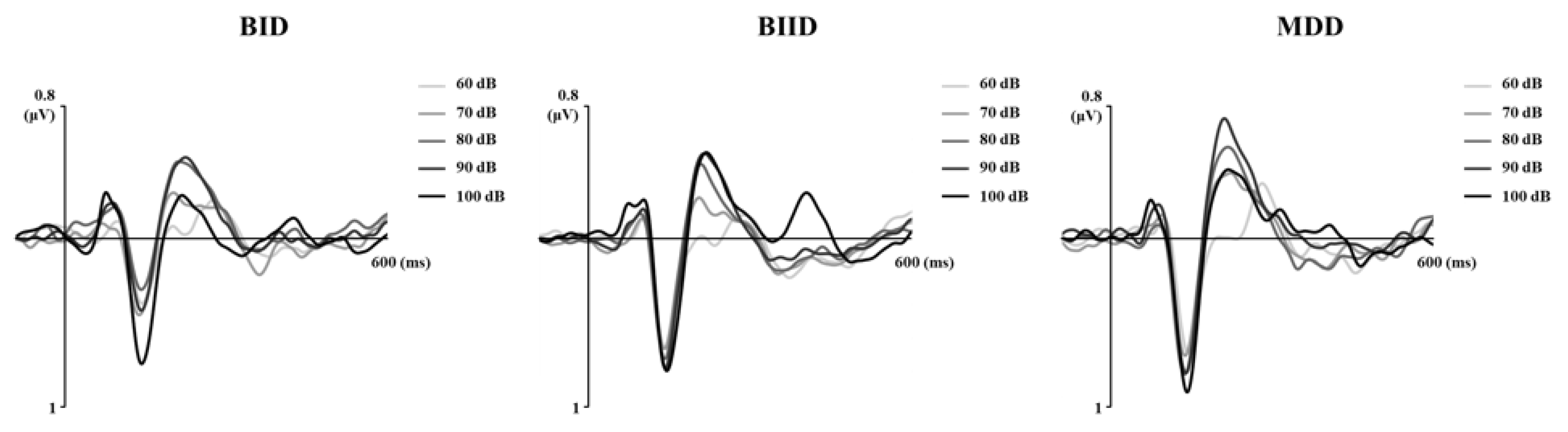
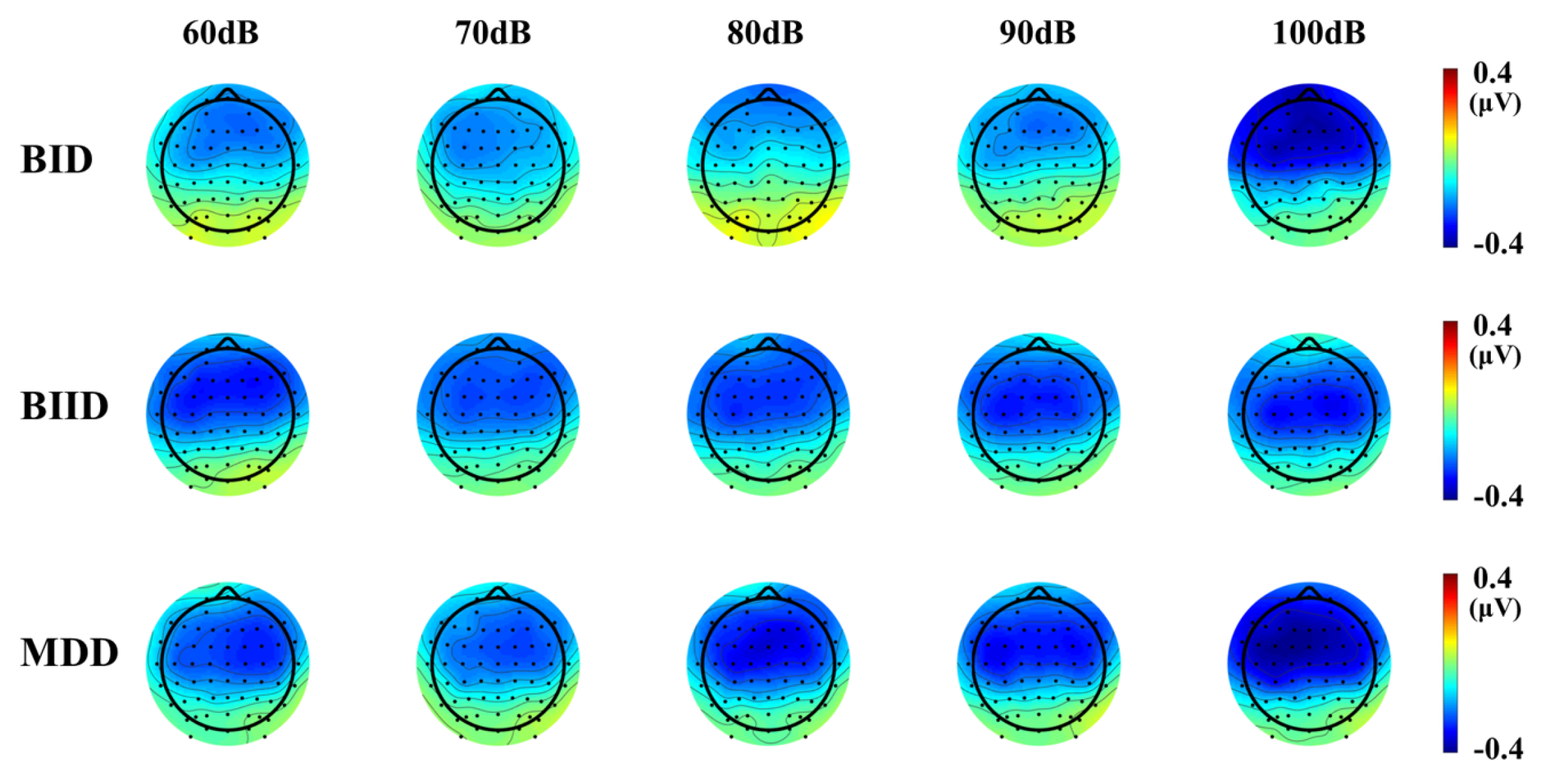
| Variable | MDD (n = 17) | BIID (n = 27) | BID (n = 11) | F | p | Post Hoc (Tukey) |
|---|---|---|---|---|---|---|
| a Age, years | 40.94 ± 17.81 | 33.00 ± 15.49 | 39.64 ± 10.44 | 3.67 | 0.16 | |
| b Sex, males/females | 13/4 | 19/8 | 5/6 | n/a | 0.23 | |
| a LDAEP (µV/10dB), Cz | 1.25 ± 0.96 | 1.02 ± 0.85 | 0.86 ± 0.85 | 1.32 | 0.52 | |
| c MMN latency, F3 | 227.35 ± 17.16 | 226.96 ± 20.55 | 243.00 ± 25.49 | 2.60 | 0.084 | |
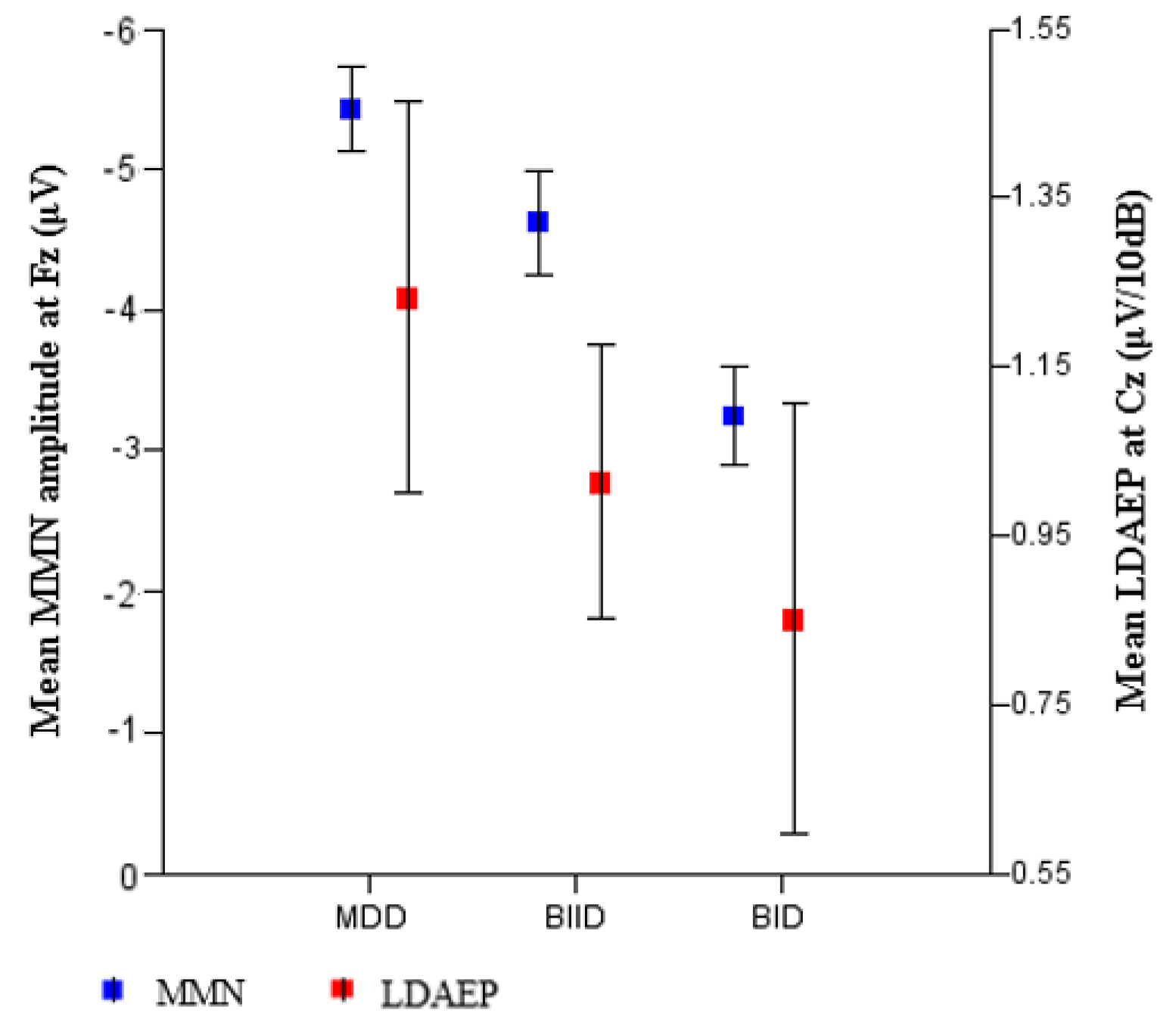
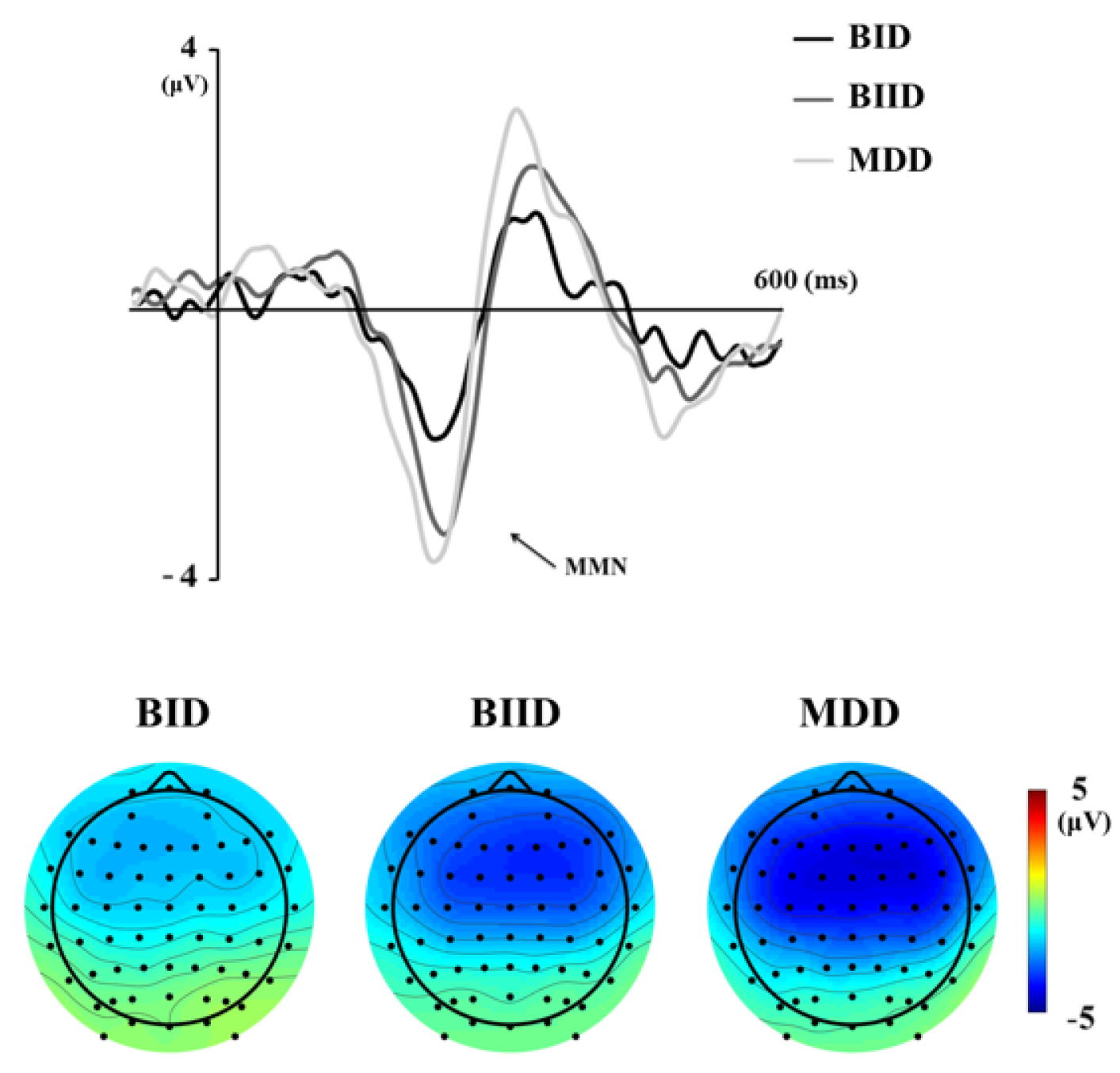
| a MMN amplitude, F3 | −4.92 ± 1.77 | −4.16 ± 2.34 | −2.87 ± 1.44 | 8.54 | 0.014 * | MDD > BID |
| a MMN latency, Fz | 227.65 ± 17.37 | 230.59 ± 18.16 | 241.18 ± 25.99 | 1.89 | 0.39 | |
| c MMN amplitude, Fz | −5.37 ± 1.59 | −4.51 ± 2.41 | −3.16 ± 1.60 | 3.93 | 0.026 * | MDD > BID |
| cMMN latency, F4 | 226.06 ± 17.88 | 229.37 ± 18.03 | 235.09 ± 24.64 | 0.72 | 0.49 | |
| a MMN amplitude, F4 | −5.17 ± 1.57 | −4.33 ± 2.31 | −3.13 ± 1.54 | 9.03 | 0.011 * | MDD > BID |
| c BDI | 27.18 ± 14.38 | 27.28 ± 14.56 | 26.25 ± 13.53 | 0.008 | 0.992 | |
| a K-CTQ | 47.07 ± 21.33 | 44.32 ± 17.83 | 30.25 ± 20.66 | 0.96 | 0.62 | |
| a Emotional abuse | 10.07 ± 6.83 | 10.00 ± 5.10 | 6.67 ± 0.58 | 0.71 | 0.70 | |
| a Physical abuse | 8.86 ± 5.67 | 9.38 ± 5.13 | 8.33 ± 3.06 | 0.24 | 0.89 | |
| a Sexual abuse | 5.14 ± 0.54 | 6.17 ± 2.01 | 6.00 ± 1.73 | 4.28 | 0.12 | |
| c Emotional neglect | 14.29 ± 6.68 | 12.38 ± 5.47 | 9.67 ± 3.22 | 0.95 | 0.40 | |
| a Physical neglect | 8.71 ± 4.25 | 8.25 ± 3.14 | 9.67 ± 5.69 | 0.11 | 0.95 |
| Variables | Coefficient | SE | Wald | df | p-Value | Odd Ratio | 95% Lower CI | 95% Upper CI |
|---|---|---|---|---|---|---|---|---|
| Age | 0.011 | 0.03 | 0.124 | 1 | 0.724 | 1.011 | 0.953 | 1.072 |
| Gender | 1.417 | 0.923 | 2.355 | 1 | 0.125 | 4.126 | 0.675 | 25.208 |
| MMN amplitude | 0.776 | 0.318 | 5.973 | 1 | 0.015* | 2.173 | 1.166 | 4.049 |
| LDAEP groups | 0.031 | 0.898 | 0.001 | 1 | 0.973 | 1.031 | 0.177 | 5.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.R.; Park, Y.-M. Mismatch Negativity and Loudness Dependence of Auditory Evoked Potentials among Patients with Major Depressive Disorder, Bipolar II Disorder, and Bipolar I Disorder. Brain Sci. 2020, 10, 789. https://doi.org/10.3390/brainsci10110789
Kim YR, Park Y-M. Mismatch Negativity and Loudness Dependence of Auditory Evoked Potentials among Patients with Major Depressive Disorder, Bipolar II Disorder, and Bipolar I Disorder. Brain Sciences. 2020; 10(11):789. https://doi.org/10.3390/brainsci10110789
Chicago/Turabian StyleKim, Yang Rae, and Young-Min Park. 2020. "Mismatch Negativity and Loudness Dependence of Auditory Evoked Potentials among Patients with Major Depressive Disorder, Bipolar II Disorder, and Bipolar I Disorder" Brain Sciences 10, no. 11: 789. https://doi.org/10.3390/brainsci10110789
APA StyleKim, Y. R., & Park, Y.-M. (2020). Mismatch Negativity and Loudness Dependence of Auditory Evoked Potentials among Patients with Major Depressive Disorder, Bipolar II Disorder, and Bipolar I Disorder. Brain Sciences, 10(11), 789. https://doi.org/10.3390/brainsci10110789





