B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974?
Abstract
:1. Background on the Tumor Microenvironment in Malignant Lymphomas
2. Immune Cells of the Tumor Environment
3. T Cell and B Cell Subpopulation Types and Their Interplay in Lymphoma
4. Therapeutic Targets for T or B Cell Subpopulations in Lymphoma
5. Extracellular Components in the Lymphoma Microenvironment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qian, L.; Tomuleasa, C.; Florian, I.-A.; Shen, J.; Florian, I.-S.; Zdrenghea, M.; Dima, D. Advances in the treatment of newly diagnosed primary central nervous system lymphomas. Blood Res. 2017, 52, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gafencu, G.-A.; Selicean, S.E.; Petrushev, B.; Cucuianu, A.; Dima, D.; Frinc, I.; Irimie, A.; Pileczki, V.; Berindan-Neagoe, I.; Berce, C.; et al. Clinicopathological analysis of a case series of peripheral T-cell lymphomas, not otherwise specified, of lymphoepithelioid variant (Lennert’s lymphoma). A Central European single-center study. Hum. Pathol. 2016, 53, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Zaharie, F.; Pop, L.-A.; Petrushev, B.; Jurj, A.; Muresan, M.-S.; Eniu, D.; Fetica, B.; Petkov, B.; Pasca, S.; Piciu, D.; et al. Next-generation sequencing-based characterization of the invasion by anatomical contiguity in a primary osseous diffuse large B-cell lymphoma. Correlation between the genetic profile of the malignancy and the clinical outcome of the patient. Histol. Histopathol. 2019, 34, 663–670. [Google Scholar]
- Desmirean, M.; Deak, D.; Rus, I.; Dima, D.; Iluta, S.; Preda, A.; Moldovan, T.; Roman, A.; Tomuleasa, C.; Petrushev, B. Paraneoplastic hypereosinophilia in a patient with peripheral T cell lymphoma, not otherwise specified. Med. Pharm Rep. 2019, 92, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Chetty, M.; Abayomi, E.; Tomuleasa, C.; Fromm, J.R. Use of flow cytometry in the phenotypic diagnosis of hodgkin’s lymphoma. Cytometry 2019, 96, 116–127. [Google Scholar] [CrossRef]
- Bennani, N.N.; Ansell, S.M. Tumor Microenvironment in T-Cell Lymphomas. Cancer Treat. Res. 2019, 176, 69–82. [Google Scholar]
- Tanase, A.; Tomuleasa, C.; Marculescu, A.; Bardas, A.; Colita, A.; Orban, C.; Ciurea, S.O. Haploidentical Donors: Can Faster Transplantation Be Life-Saving for Patients with Advanced Disease? Acta Haematol. 2016, 135, 211–216. [Google Scholar] [CrossRef]
- Brammer, J.E.; Khouri, I.; Gaballa, S.; Anderlini, P.; Tomuleasa, C.; Ahmed, S.; Ledesma, C.; Hosing, C.; Champlin, R.E.; Ciurea, S.O. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning. Biol. Blood Marrow Transpl. 2016, 22, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Tomuleasa, C.; Fuji, S.; Berce, C.; Onaciu, A.; Chira, S.; Petrushev, B.; Micu, W.-T.; Moisoiu, V.; Osan, C.; Constantinescu, C.; et al. Chimeric Antigen Receptor T-Cells for the Treatment of B-Cell Acute Lymphoblastic Leukemia. Front. Immunol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Bagacean, C.; Tomuleasa, C.; Tempescul, A.; Grewal, R.; Brooks, W.H.; Berthou, C.; Renaudineau, Y. Apoptotic resistance in chronic lymphocytic leukemia and therapeutic perspectives. Crit. Rev. Clin. Lab. Sci. 2019, 56, 321–332. [Google Scholar] [CrossRef]
- Colita, A.; Colita, A.; Bumbea, H.; Croitoru, A.; Orban, C.; Lipan, L.E.; Craciun, O.-G.; Soare, D.; Ghimici, C.; Manolache, R.; et al. LEAM vs. BEAM vs. CLV Conditioning Regimen for Autologous Stem Cell Transplantation in Malignant Lymphomas. Retrospective Comparison of Toxicity and Efficacy on 222 Patients in the First 100 Days After Transplant, On Behalf of the Romanian Society for Bone Marrow Transplantation. Front. Oncol. 2019, 9, 892. [Google Scholar] [PubMed]
- Liu, Y.; Sattarzadeh, A.; Diepstra, A.; Visser, L.; van den Berg, A. The microenvironment in classical Hodgkin lymphoma: An actively shaped and essential tumor component. Semin. Cancer Biol. 2014, 24, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Utano, K.; Katsuki, S.; Matsuda, T.; Mitsuzaki, K.; Fujita, T.; Nemoto, D.; Nagata, K.; Lefor, A.K.; Togashi, K. Colon Capsule Endoscopy versus CT Colonography in Patients with Large Non-Polypoid Tumours: A Multicentre Prospective Comparative Study (4CN Study). Digestion 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Ke, X.-Y. The four types of Tregs in malignant lymphomas. J. Hematol. Oncol. 2011, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-Z.; Novak, A.J.; Stenson, M.J.; Witzig, T.E.; Ansell, S.M. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 2006, 107, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Jiang, Q.; Zhao, S.; Zhao, J.; Ye, X.; Qian, Q. Prognostic value of tissue-infiltrating immune cells in tumor microenvironment of follicular lymphoma: A meta-analysis. Int. Immunopharmacol. 2020, 85, 106684. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Seishima, M. Regulation of the induction and function of cytotoxic T lymphocytes by natural killer T cell. J. Biomed. Biotechnol. 2010, 2010, 641757. [Google Scholar] [CrossRef] [Green Version]
- Menter, T.; Tzankov, A.; Zucca, E.; Kimby, E.; Vanazzi, A.; Østenstad, B.; Mey, U.J.; Rauch, D.; Wahlin, B.; Hitz, F.; et al. Prognostic implications of the microenvironment in follicular lymphoma under rituximab and rituximab+lenalidomide therapy. A translational study of the sakk35/10 trial. Hematol. Oncol. 2019, 37, 149–151. [Google Scholar] [CrossRef]
- Carreras, J.; Lopez-Guillermo, A.; Roncador, G.; Villamor, N.; Colomo, L.; Martinez, A.; Hamoudi, R.; Howat, W.J.; Montserrat, E.; Campo, E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J. Clin. Oncol. 2009, 27, 1470–1476. [Google Scholar] [CrossRef]
- Simon, S.; Labarriere, N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology 2017, 7, e1364828. [Google Scholar] [CrossRef]
- Scala, E.; Carbonari, M.; del Porto, P.; Cibati, M.; Tedesco, T.; Mazzone, A.M.; Paganelli, R.; Fiorilli, M. Lymphocyte activation gene-3 (LAG-3, expression and IFN-gamma production are variably coregulated in different human T lymphocyte subpopulations. J. Immunol. 1998, 161, 489–493. [Google Scholar] [PubMed]
- Hemon, P.; Jean-Louis, F.; Ramgolam, K.; Brignone, C.; Viguier, M.; Bachelez, H.; Triebel, F.; Charron, D.; Aoudjit, F.; Al-Daccak, R.; et al. MHC class II engagement by its ligand LAG-3 (CD223), contributes to melanoma resistance to apoptosis. J. Immunol. 2011, 186, 5173–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, T.; Xuan, Q.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 124–133. [Google Scholar] [CrossRef]
- Moon, E.K.; Ranganathan, R.; Eruslanov, E.; Kim, S.; Newick, K.; O’Brien, S.; Lo, A.; Liu, X.; Zhao, Y.; Albelda, S.M. Blockade of Programmed Death 1 Augments the Ability of Human T Cells Engineered to Target NY-ESO-1 to Control Tumor Growth after Adoptive Transfer. Clin. Cancer Res. 2016, 22, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccaluga, P.P.; Agostinelli, C.; Califano, A.; Carbone, A.; Fantoni, L.; Ferrari, S.; Gazzola, A.; Gloghini, A.; Righi, S.; Rossi, M.; et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res. 2007, 67, 10703–10710. [Google Scholar] [CrossRef] [Green Version]
- Dobay, M.P.; Lemonnier, F.; Missiaglia, E.; Bastard, C.; Vallois, D.; Jais, J.-P.; Scourzic, L.; Dupuy, A.; Fataccioli, V.; Pujals, A.; et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica 2017, 102, e148–e151. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Vinuesa, C.G. Multiple checkpoints keep follicular helper T cells under control to prevent autoimmunity. Cell Mol. Immunol. 2010, 7, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Menter, T.; Tzankov, A. Lymphomas and Their Microenvironment: A Multifaceted Relationship. Pathobiology 2019, 86, 225–236. [Google Scholar] [CrossRef]
- Iqbal, J.; Weisenburger, D.D.; Greiner, T.C.; Vose, J.M.; McKeithan, T.; Kucuk, C.; Geng, H.; Deffenbacher, K.; Smith, L.; Dybkaer, K.; et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef]
- De Leval, L.; Rickman, D.S.; Thielen, C.; Reynies, A.; de Huang, Y.-L.; Delsol, G.; Lamant, L.; Leroy, K.; Brière, J.; Molina, T.; et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL, and follicular helper T (TFH, cells). Blood 2007, 109, 4952–4963. [Google Scholar] [CrossRef]
- Huang, Y.; Moreau, A.; Dupuis, J.; Streubel, B.; Petit, B.; le Gouill, S.; Martin-Garcia, N.; Copie-Bergman, C.; Gaillard, F.; Qubaja, M.; et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH), and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 2009, 33, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-I.; Chang, S.-T.; Lin, M.-Y.; Hsieh, Y.-C.; Chu, P.-Y.; Chen, C.-J.; Lin, K.-J.; Jung, Y.-C.; Hwang, W.-S.; Huang, W.-T.; et al. Angioimmunoblastic T-cell lymphoma in Taiwan shows a frequent gain of ITK gene. Int. J. Clin. Exp. Pathol. 2014, 7, 6097–6107. [Google Scholar] [PubMed]
- Quah, B.J.C.; Barlow, V.P.; McPhun, V.; Matthaei, K.I.; Hulett, M.D.; Parish, C.R. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 4259–4264. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, M.J.; Julius, M.H. Two classes of bystander B cell response: Activation requirements reflect those of B cells in general. J. Immunol. 1983, 131, 581–586. [Google Scholar]
- Duffield, A.S.; Ascierto, M.L.; Anders, R.A.; Taube, J.M.; Meeker, A.K.; Chen, S.; McMiller, T.L.; Phillips, N.A.; Xu, H.; Ogurtsova, A.; et al. Th17 immune microenvironment in Epstein-Barr virus–negative Hodgkin lymphoma: Implications for immunotherapy. Blood Adv. 2017, 1, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willenbrock, K.; Roers, A.; Blöhbaum, B.; Rajewsky, K. Hansmann ML. CD8 (+, T cells in Hodgkin’s disease tumor tissue are a polyclonal population with limited clonal expansion but little evidence of selection by antigen. Am. J. Pathol. 2000, 157, 171–175. [Google Scholar] [CrossRef]
- Wobser, M.; Reinartz, T.; Roth, S.; Goebeler, M.; Rosenwald, A.; Geissinger, E. Cutaneous CD8+ Cytotoxic T-Cell Lymphoma Infiltrates: Clinicopathological Correlation and Outcome of 35 Cases. Oncol. Ther. 2016, 4, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Mukai, H.Y.; Hasegawa, Y.; Kojima, H.; Okoshi, Y.; Takei, N.; Yamashita, Y.; Nagasawa, T.; Mori, N. Nodal CD8 Positive Cytotoxic T-Cell Lymphoma: A Distinct Clinicopathological Entity. Mod. Pathol. 2002, 15, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wan, J.; Xia, R.; Huang, Z.; Ni, J.; Yang, M. Functional role of regulatory T cells in B cell lymphoma and related mechanisms. Int. J. Clin. Exp. Pathol. 2015, 8, 9133–9139. [Google Scholar]
- Lundberg, J.; Berglund, D.; Molin, D.; Kinch, A. Intratumoral expression of FoxP3-positive regulatory T-cells in T-cell lymphoma: No correlation with survival. Upsala J. Med. Sci. 2019, 124, 105–110. [Google Scholar] [CrossRef]
- Tzankov, A.; Meier, C.; Hirschmann, P.; Went, P.; Pileri, S.A.; Dirnhofer, S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 2008, 93, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, J.; Canioni, D.; Renand, A.; Marafioti, T.; Paterson, J.C.; Martin-Garcia, N.; Gaulard, P.; Delfau, M.-H.; Hermine, O.; Macintyre, E.; et al. Regulatory T-Cell Depletion in Angioimmunoblastic T-Cell Lymphoma. Am. J. Pathol. 2010, 177, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P.; Clear, A.; Owen, A.; Iqbal, S.; Lee, A.; Matthews, J.; Wilson, A.; Calaminici, M.; Gribben, J.G. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 2013, 122, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Takada, R.; Watanabe, R.; Okamoto, S.; Ikeda, Y. T-helper (Th)1/Th2 imbalance in patients with previously untreated B-cell diffuse large cell lymphoma. Cancer Immunol. Immunother. 2001, 50, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Mitsdoerffer, M.; Lee, Y.; Jager, A.; Kim, H.-J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Yu, S.; Liu, Y.; Yin, C.; Ye, J.; Liu, Z.; Ma, D.; Ji, C. Aberrant Circulating Th17 Cells in Patients with B-Cell Non-Hodgkin’s Lymphoma. PLoS ONE 2016, 11, e0148044. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Xu, X.; Zhu, Z.; Yang, L.; Du, H.; Xia, Z.; Yuan, Z.; Xiong, H.; Du, Q.; Wei, Y.; et al. Increased interleukin-17A levels promote rituximab resistance by suppressing p53 expression and predict an unfavorable prognosis in patients with diffuse large B cell lymphoma. Int. J. Oncol. 2018, 52, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Dalai, S.K.; Mirshahidi, S.; Morrot, A.; Zavala, F.; Sadegh-Nasseri, S. Anergy in Memory CD4+ T Cells Is Induced by B Cells. J. Immunol. 2008, 181, 3221–3231. [Google Scholar] [CrossRef] [Green Version]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, in press. [Google Scholar] [CrossRef]
- Mansour, A.T.; Shandiz, A.E.; Zimmerman, M.K.; Roth, T.D.; Zhou, J. Concomitant lymphoplasmacytic lymphoma and plasma cell myeloma, a diagnostic challenge. Am. J. Blood. Res. 2017, 7, 10–17. [Google Scholar]
- Swaminathan, S.; Müschen, M. Follicular lymphoma: Too many reminders for a memory B cell. J. Clin. Investig. 2014, 124, 5095–5098. [Google Scholar] [CrossRef] [Green Version]
- Bornkamm, G.W. Epstein-Barr virus and the pathogenesis of Burkitt’s lymphoma: More questions than answers. Int. J. Cancer 2009, 124, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Formica, A.M.; Nakao, Y.; Ichikawa, D.; Shinton, S.A.; Brill-Dashoff, J.; Smith, M.R.; Morse, H.C.; Hardy, R.R. Early Generated B-1–Derived B Cells Have the Capacity to Progress to Become Mantle Cell Lymphoma–Like Neoplasia in Aged Mice. J. Immunol. 2018, 201, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Mauri, C.; Menon, M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, M.; Minard-Colin, V.; Matsushita, T.; Tedder, T.F. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Investig. 2011, 121, 4268–4280. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-Z.; Novak, A.J.; Ziesmer, S.C.; Witzig, T.E.; Ansell, S.M. Attenuation of CD8+ T-Cell Function by CD4 + CD25 + Regulatory T Cells in B-Cell Non-Hodgkin’s Lymphoma. Cancer Res. 2006, 66, 10145–10152. [Google Scholar] [CrossRef] [Green Version]
- Pangault, C.; Amé-Thomas, P.; Ruminy, P.; Rossille, D.; Caron, G.; Baia, M.; de Vos, J.; Roussel, M.; Monvoisin, C.; Lamy, T.; et al. Follicular lymphoma cell niche: Identification of a preeminent IL-4-dependent TFH–B cell axis. Leukemia 2010, 24, 2080–2089. [Google Scholar] [CrossRef]
- Alikhan, M.; Song, J.Y.; Sohani, A.R.; Moroch, J.; Plonquet, A.; Duffield, A.S.; Borowitz, M.J.; Jiang, L.; Bueso-Ramos, C.; Inamdar, K.; et al. Peripheral T-cell lymphomas of follicular helper T-cell type frequently display an aberrant CD3−/dimCD4+ population by flow cytometry: An important clue to the diagnosis of a Hodgkin lymphoma mimic. Mod. Pathol. 2016, 29, 1173–1182. [Google Scholar] [CrossRef]
- Galand, C.; Donnou, S.; Crozet, L.; Brunet, S.; Touitou, V.; Ouakrim, H.; Fridman, W.H.; Sautès-Fridman, C.; Fisson, S. Th17 Cells Are Involved in the Local Control of Tumor Progression in Primary Intraocular Lymphoma. PLoS ONE 2011, 6, e24622. [Google Scholar] [CrossRef] [PubMed]
- Jonjić, N.; Seili Bekafigo, I.; Fučkar Čupić, D.; Lučin, K.; Duletić Načinović, A.; Valković, T. Rapid Fatal Acute Peripheral T-Cell Lymphoma Associated with IgG Plasma Cell Leukemia and IgA Hypergammaglobulinemia. Appl. Immunohistochem. Mol. Morphol. 2016, 24, e89–e93. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Rosenwald, A.; Staudt, L.M. Lymphoid Malignancies: The dark side of B-cell differentiation. Nat. Rev. Immunol. 2002, 2, 920–933. [Google Scholar] [CrossRef]
- Gravelle, P.; Do, C.; Franchet, C.; Mueller, S.; Oberic, L.; Ysebaert, L.; Larocca, L.M.; Hohaus, S.; Calmels, M.-N.; Frenois, F.-X.; et al. Impaired functional responses in follicular lymphoma CD8+ TIM-3+ T lymphocytes following TCR engagement. OncoImmunology 2016, 5, e1224044. [Google Scholar] [CrossRef] [Green Version]
- Jurj, A.; Pop, L.; Petrushev, B.; Pasca, S.; Dima, D.; Frinc, I.; Deak, D.; Desmirean, M.; Trifa, A.; Fetica, B.; et al. Exosome-carried microRNA-based signature as a cellular trigger for the evolution of chronic lymphocytic leukemia into Richter syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 501–515. [Google Scholar] [CrossRef]
- Geng, Z.; Xiao, Y.; Zhu, X.-J.; Ye, C.; Zhou, J.-F. Anti-PD-1 therapy for clinical treatment of lymphoma: A single-arm meta-analysis. Oncotarget 2018, in press. [Google Scholar] [CrossRef] [Green Version]
- Xu-Monette, Z.Y.; Zhou, J.; Young, K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Patel, S.S.; Weirather, J.L.; Lipschitz, M.; Lako, A.; Chen, P.-H.; Griffin, G.K.; Armand, P.; Shipp, M.A.; Rodig, S.J. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4- positive T-cells that are PD-1-negative. Blood 2019, 23, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.; van Laethem, F.; Pobezinsky, L.; Guinter, T.; Sharrow, S.O.; Adams, A.; Granger, L.; Kruhlak, M.; Lindsten, T.; Thompson, C.B.; et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood 2012, 119, 5155–5163. [Google Scholar] [CrossRef]
- Leone, R.D.; Lo, Y.-C.; Powell, J.D. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015, 13, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, T.; Song, Z.; Li, L.; Zhang, X.; Liu, J.; Liu, X.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Tumor CD73/A2aR adenosine immunosuppressive axis and tumor-infiltrating lymphocytes in diffuse large B-cell lymphoma: Correlations with clinicopathological characteristics and clinical outcome. Int. J. Cancer 2019, 145, 1414–1422. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223), as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, M.; Liu, Y.; Wang, L.; Tang, Y. Expression of TIM-3 and LAG-3 in extranodal NK/T cell lymphoma, nasal type. Histol. Histopathol. 2017, 33, 307–315. [Google Scholar] [PubMed]
- Gandhi, M.K.; Lambley, E.; Duraiswamy, J.; Dua, U.; Smith, C.; Elliott, S.; Gill, D.; Marlton, P.; Seymour, J.; Khanna, R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen–specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 2006, 108, 2280–2289. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Tomkowicz, B.; Walsh, E.; Cotty, A.; Verona, R.; Sabins, N.; Kaplan, F.; Santulli-Marotto, S.; Chin, C.-N.; Mooney, J.; Lingham, R.B.; et al. TIM-3 Suppresses Anti-CD3/CD28-Induced TCR Activation and IL-2 Expression through the NFAT Signaling Pathway. PLoS ONE 2015, 10, e0140694. [Google Scholar] [CrossRef] [Green Version]
- Horlad, H.; Ohnishi, K.; Ma, C.; Fujiwara, Y.; Niino, D.; Ohshima, K.; Jinushi, M.; Matsuoka, M.; Takeya, M.; Komohara, Y. TIM-3 expression in lymphoma cells predicts chemoresistance in patients with adult T-cell leukemia/lymphoma. Oncol. Lett. 2016, 12, 1519–1524. [Google Scholar] [CrossRef] [Green Version]
- MacGregor, H.L.; Ohashi, P.S. Molecular Pathways: Evaluating the Potential for B7-H4 as an Immunoregulatory Target. Clin. Cancer Res. 2017, 23, 2934–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lee, Y.; Li, Y.; Jiang, Y.; Lu, H.; Zang, W.; Zhao, X.; Liu, L.; Chen, Y.; Tan, H.; et al. Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8+ T Cells. Immunity 2018, 48, C773–C786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, F.; Heng, X.; Zhang, H.; Su, Q.; Zhang, B.; Chen, Y.; Zhang, Z.; Du, Y.; Wang, L. Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol. Immunother. 2017, 66, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front. Oncol. 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Chapoval, A.I.; Flies, D.B.; Zhu, G.; Hirano, F.; Wang, S.; Lau, J.S.; Dong, H.; Tamada, K.; Flies, A.S.; et al. B7-H3 Enhances Tumor Immunity In Vivo by Costimulating Rapid Clonal Expansion of Antigen-Specific CD8+ Cytolytic T Cells. J. Immunol. 2004, 173, 5445–5450. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Yu, L.; Hu, J.; Zhang, Z.; Wang, H.; Lu, D.; Tang, X.; Huang, J.; Zhong, K.; Wang, Z.; et al. Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Transl. Oncol. 2020, 13, 100770. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, J.; Dong, F.; Zhu, M.; Wan, W.; Li, H.; Wu, F.; Yan, X.; Ke, X. B7-H3 silencing inhibits tumor progression of mantle cell lymphoma and enhances chemosensitivity. Int. J. Oncol. 2015, 46, 2562–2572. [Google Scholar] [CrossRef] [Green Version]
- Assal, A.; Kaner, J.; Pendurti, G.; Zang, X. Emerging targets in cancer immunotherapy: Beyond CTLA-4 and PD-1. Immunotherapy 2015, 7, 1169–1186. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.; Lan, X.; Meng, Y.; Guo, X.; Guo, Y.; Zhao, L.; Chen, X.; Liu, A. BTLA marks a less cytotoxic T-cell subset in diffuse large B-cell lymphoma with high expression of checkpoints. Exp. Hematol. 2018, 60, C47–C56. [Google Scholar] [CrossRef]
- M’Hidi, H.; Thibult, M.-L.; Chetaille, B.; Rey, F.; Bouadallah, R.; Nicollas, R.; Olive, D.; Xerri, L. High Expression of the Inhibitory Receptor BTLA in T-Follicular Helper Cells and in B-Cell Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia. Am. J. Clin. Pathol. 2009, 132, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Mintz, M.A.; Felce, J.H.; Chou, M.Y.; Mayya, V.; Xu, Y.; Shui, J.-W.; An, J.; Li, Z.; Marson, A.; Okada, T.; et al. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell-Extrinsic Suppressor in Lymphomagenesis. Immunity 2019, 51, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Josefsson, S.E.; Beiske, K.; Blaker, Y.N.; Førsund, M.S.; Holte, H.; Østenstad, B.; Kimby, E.; Köksal, H.; Wälchli, S.; Bai, B.; et al. TIGIT and PD-1 Mark Intratumoral T Cells with Reduced Effector Function in B-cell Non-Hodgkin Lymphoma. Cancer Immunol. Res. 2019, 7, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, S.E.; Huse, K.; Kolstad, A.; Beiske, K.; Pende, D.; Steen, C.B.; Inderberg, E.M.; Lingjærde, O.C.; Østenstad, B.; Smeland, E.B.; et al. T Cells Expressing Checkpoint Receptor TIGIT Are Enriched in Follicular Lymphoma Tumors and Characterized by Reversible Suppression of T-cell Receptor Signaling. Clin. Cancer Res. 2018, 24, 870–881. [Google Scholar] [CrossRef] [Green Version]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef] [Green Version]
- Tagliamento, M.; Bironzo, P.; Novello, S. New emerging targets in cancer immunotherapy: The role of VISTA. ESMO Open 2020, 4, e000683. [Google Scholar] [CrossRef] [PubMed]
- Musielak, B.; Kocik, J.; Skalniak, L.; Magiera-Mularz, K.; Sala, D.; Czub, M.; Stec, M.; Siedlar, M.; Holak, T.A.; Plewka, J. CA-170—A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules 2019, 24, 2804. [Google Scholar] [CrossRef] [Green Version]
- Albring, J.C.; Sandau, M.M.; Rapaport, A.S.; Edelson, B.T.; Satpathy, A.; Mashayekhi, M.; Lathrop, S.K.; Hsieh, C.-S.; Stelljes, M.; Colonna, M.; et al. Targeting of B and T lymphocyte associated (BTLA, prevents graft-versus-host disease without global immunosuppression. J. Exp. Med. 2010, 207, 2551–2559. [Google Scholar] [CrossRef]
- Ansell, S.M.; Hurvitz, S.A.; Koenig, P.A.; LaPlant, B.R.; Kabat, B.F.; Fernando, D.; Habermann, T.M.; Inwards, D.J.; Verma, M.; Yamada, R.; et al. Phase I Study of Ipilimumab, an Anti-CTLA-4 Monoclonal Antibody, in Patients with Relapsed and Refractory B-Cell Non-Hodgkin Lymphoma. Clin. Cancer Res. 2009, 15, 6446–6453. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.R.; Peinado, P.; Fuentes-Antrás, J.; Pérez-Segura, P.; Pandiella, A.; Amir, E.; Ocaña, A. Prognostic Value of Lymphocyte-Activation Gene 3 (LAG3), in Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 1040. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jacobs, R.; Ghosh, N. Checkpoint Inhibitors Hodgkin Lymphoma and Non-Hodgkin Lymphoma. Curr. Hematol. Malig. Rep. 2018, 13, 543–554. [Google Scholar] [CrossRef]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Inozume, T.; Sato, S. Increased Soluble CD226 in Sera of Patients with Cutaneous T-Cell Lymphoma Mediates Cytotoxic Activity against Tumor Cells via CD155. J. Investig. Dermatol. 2017, 137, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Friedlaender, A.; Addeo, A.; Banna, G. New emerging targets in cancer immunotherapy: The role of TIM3. ESMO Open 2019, 4, e000497. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-Z.; Grote, D.M.; Ziesmer, S.C.; Niki, T.; Hirashima, M.; Novak, A.J.; Witzig, T.E.; Ansell, S.M. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J. Clin. Investig. 2012, 122, 1271–1282. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, C.; Chiorean, R.; Irimie, A.; Chira, S.; Tomuleasa, C.; Neagoe, E.; Paradiso, A.; Achimas-Cadariu, P.; Lazar, V.; Berindan-Neagoe, I. Novel insight into triple-negative breast cancers, the emerging role of angiogenesis, and antiangiogenic therapy. Expert Rev. Mol. Med. 2016, 18, e18. [Google Scholar] [CrossRef]
- Carmeliet, P.; Baes, M. Metabolism and therapeutic angiogenesis. N. Engl. J. Med. 2008, 358, 2511–2512. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Montero, A.J.; Vogel, C. Fighting fire with fire: Rekindling the bevacizumab debate. N. Engl. J. Med. 2012, 366, 374–375. [Google Scholar] [CrossRef]
- Zerbini, G.; Lorenzi, M.; Palini, A. Tumor angiogenesis. N. Engl. J. Med. 2008, 359, 763. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Faurobert, E.; Bouin, A.-P.; Albiges-Rizo, C. Microenvironment, tumor cell plasticity, and cancer. Curr. Opin. Oncol. 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell. Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Marchi, E.; O’Connor, O.A. The rapidly changing landscape in mature T-cell lymphoma (MTCL), biology and management. CA Cancer J. Clin. 2020, 70, 47–70. [Google Scholar] [CrossRef] [Green Version]
- Witalis, M.; Chang, J.; Zhong, M.-C.; Bouklouch, Y.; Panneton, V.; Li, J.; Buch, T.; Kim, S.J.; Kim, W.S.; Ko, Y.H.; et al. Progression of AITL-like tumors in mice is driven by Tfh signature proteins and T-B cross talk. Blood Adv. 2020, 4, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.J.; Allchin, R.L.; Fox, C.P.; Wagner, S.D. Follicular helper T-cells: Expanding roles in T-cell lymphoma and targets for treatment. Br. J. Haematol. 2014, 166, 326–335. [Google Scholar] [CrossRef]
- Jain, S.; Chen, J.; Nicolae, A.; Wang, H.; Shin, D.-M.; Adkins, E.B.; Sproule, T.J.; Leeth, C.M.; Sakai, T.; Kovalchuk, A.L.; et al. IL-21-driven neoplasms in SJL mice mimic some key features of human angioimmunoblastic T-cell lymphoma. Am. J. Pathol. 2015, 185, 3102–3114. [Google Scholar] [CrossRef] [Green Version]
- Ham, J.S.; Park, H.Y.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Elevated serum interleukin-10 level and M2 macrophage infiltration are associated with poor survival in angioimmunoblastic T-cell lymphoma. Oncotarget 2017, 8, 76231–76240. [Google Scholar] [CrossRef] [Green Version]
- Petrushev, B.; Tomuleasa, C.; Suşman, S.; Sorişău, O.; Aldea, M.; Kacsó, G.; Buigă, R.; Irimie, A. The axis of evil in the fight against cancer. Rom. J. Intern. Med. 2011, 49, 319–325. [Google Scholar]
- Tripodo, C.; Gri, G.; Piccaluga, P.P.; Frossi, B.; Guarnotta, C.; Piconese, S.; Franco, G.; Vetri, V.; Pucillo, C.E.; Florena, A.M.; et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am. J. Pathol. 2010, 177, 792–802. [Google Scholar] [CrossRef]
- Gaulard, P.; de Leval, L. The microenvironment in T-cell lymphomas: Emerging themes. Semin. Cancer Biol. 2014, 24, 49–60. [Google Scholar] [CrossRef]
- Jurj, A.; Zanoaga, O.; Braicu, C.; Lazar, V.; Tomuleasa, C.; Irimie, A.; Berindan-Neagoe, I. A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells. Cancers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Gulei, D.; Irimie, A.I.; Cojocneanu-Petric, R.; Schultze, J.L.; Berindan-Neagoe, I. Exosomes-Small Players, Big Sound. Bioconjug. Chem. 2018, 29, 635–648. [Google Scholar] [CrossRef]
- Qian, L.; Dima, D.; Berce, C.; Liu, Y.; Rus, I.; Raduly, L.-Z.; Liu, Y.; Petrushev, B.; Berindan-Neagoe, I.; Irimie, A.; et al. Protein dysregulation in graft versus host disease. Oncotarget 2018, 9, 1483. [Google Scholar] [CrossRef] [Green Version]
- Zaharie, F.; Muresan, M.S.; Petrushev, B.; Berce, C.; Gafencu, G.-A.; Selicean, S.; Jurj, A.; Cojocneanu-Petric, R.; Lisencu, C.-I.; Pop, L.-A.; et al. Exosome-Carried microRNA-375 Inhibits Cell Progression and Dissemination via Bcl-2 Blocking in Colon Cancer. J. Gastrointestin. Liver Dis. 2015, 24, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef]
- Gulei, D.; Berindan-Neagoe, I. Activation of Necroptosis by Engineered Self Tumor-Derived Exosomes Loaded with CRISPR/Cas9. Mol. Ther. Nucleic Acids 2019, 17, 448–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulei, D.; Petrut, B.; Tigu, A.B.; Onaciu, A.; Fischer-Fodor, E.; Atanasov, A.G.; Ionescu, C.; Berindan-Neagoe, I. Exosomes at a glance—Common nominators for cancer hallmarks and novel diagnosis tools. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 564–577. [Google Scholar] [CrossRef]
- Navarro-Tableros, V.; Gomez, Y.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles: New Players in Lymphomas. Int. J. Mol. Sci. 2018, 20, 41. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Piontek, K.B.; Kumbhari, V.; Ishida, M.; Selaru, F.M. Isolation and Profiling of MicroRNA-containing Exosomes from Human Bile. J. Vis. Exp. 2016, in press. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateau, S.; et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014, 60, 896–907. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Piontek, K.; Sakaguchi, M.; Selaru, F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018, 67, 940–954. [Google Scholar] [CrossRef]
- Li, L.; Piontek, K.; Ishida, M.; Fausther, M.; Dranoff, J.A.; Fu, R.; Mezey, E.; Gould, S.J.; Fordjour, F.K.; Meltzer, S.J.; et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017, 65, 501–514. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Poon, I.K.H. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017, 27, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.P.; Spary, L.K.; Sanders, A.J.; Chowdhury, R.; Jiang, W.G.; Steadman, R.; Wymant, J.; Jones, A.T.; Kynaston, H.; Mason, M.D.; et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015, 34, 290–302. [Google Scholar] [CrossRef]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yu, S.; Zinn, K.; Wang, J.; Zhang, L.; Jia, Y.; Kappes, J.C.; Barnes, S.; Kimberly, R.P.; Grizzle, W.E.; et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006, 176, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell. Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Nilsson, R.J.A.; Karachaliou, N.; Berenguer, J.; Gimenez-Capitan, A.; Schellen, P.; Teixido, C.; Tannous, J.; Kuiper, J.L.; Drees, E.; Grabowska, M.; et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 2016, 7, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.-J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.-F.; et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Zimta, A.-A.; Tomuleasa, C.; Sahnoune, I.; Calin, G.A.; Berindan-Neagoe, I. Long Non-coding RNAs in Myeloid Malignancies. Front. Oncol. 2019, 9, 1048. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Manček-Keber, M.; Lainšček, D.; Benčina, M.; Chen, J.G.; Romih, R.; Hunter, Z.R.; Treon, S.P.; Jerala, R. Extracellular vesicle-mediated transfer of constitutively active MyD88L265P engages MyD88wt and activates signaling. Blood 2018, 131, 1720–1729. [Google Scholar] [CrossRef]
- Higuchi, H.; Yamakawa, N.; Imadome, K.-I.; Yahata, T.; Kotaki, R.; Ogata, J.; Kakizaki, M.; Fujita, K.; Lu, J.; Yokoyama, K.; et al. Role of exosomes as a proinflammatory mediator in the development of EBV-associated lymphoma. Blood 2018, 131, 2552–2567. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Philip, P.S.; Attoub, S.; Khan, G. Epstein-Barr virus-infected cells release Fas ligand in exosomal fractions and induce apoptosis in recipient cells via the extrinsic pathway. J. Gen. Virol. 2015, 96, 3646–3659. [Google Scholar] [CrossRef]
- Kumar, D.; Xu, M.L. Microenvironment Cell Contribution to Lymphoma Immunity. Front. Oncol. 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell. Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, M.H. Opening the gateway to tumors. Nat. Med. 2008, 14, 13–14. [Google Scholar] [CrossRef]
- Buckanovich, R.J.; Facciabene, A.; Kim, S.; Benencia, F.; Sasaroli, D.; Balint, K.; Katsaros, D.; O’Brien-Jenkins, A.; Gimotty, P.A.; Coukos, G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008, 14, 28–36. [Google Scholar] [CrossRef]
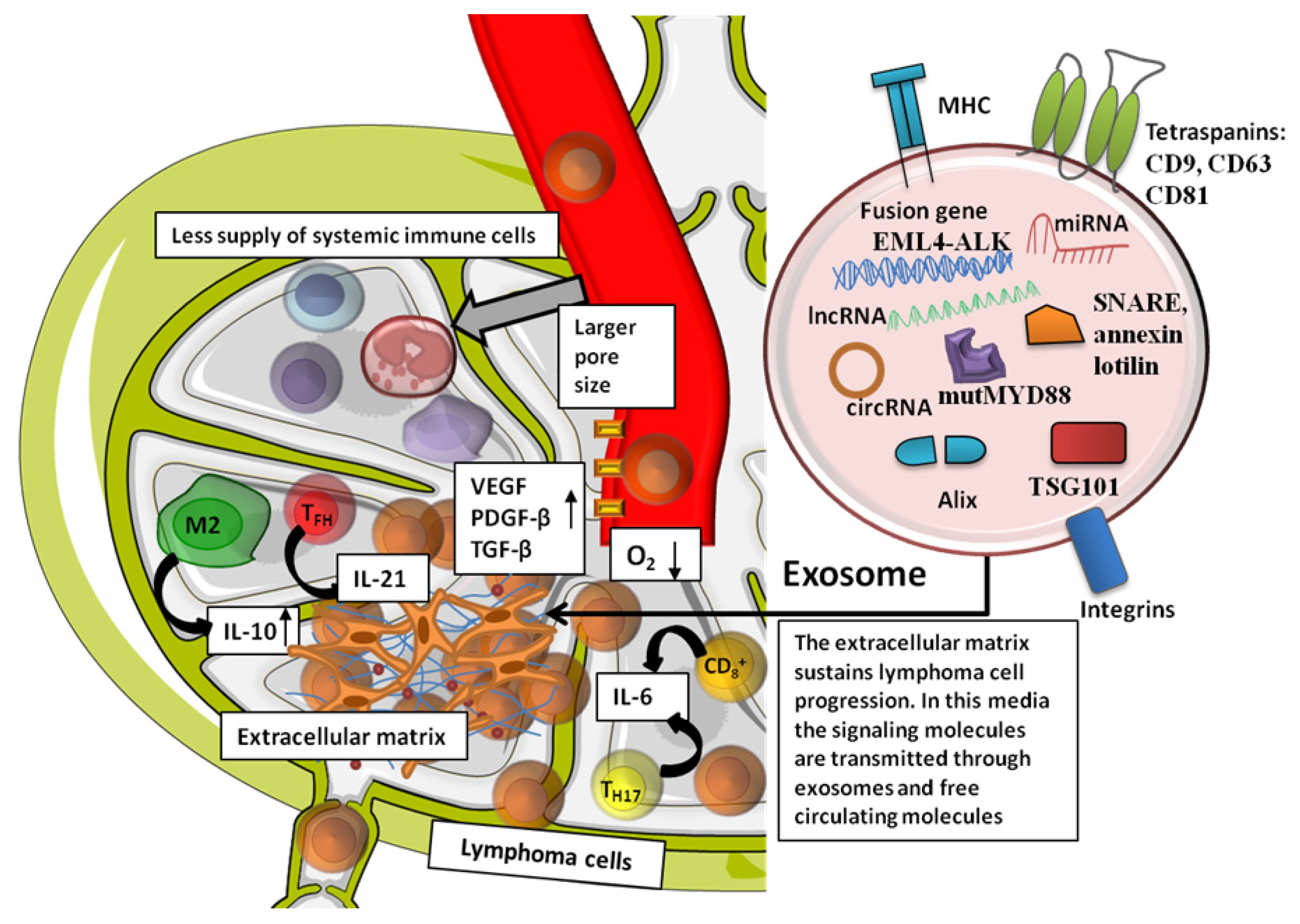
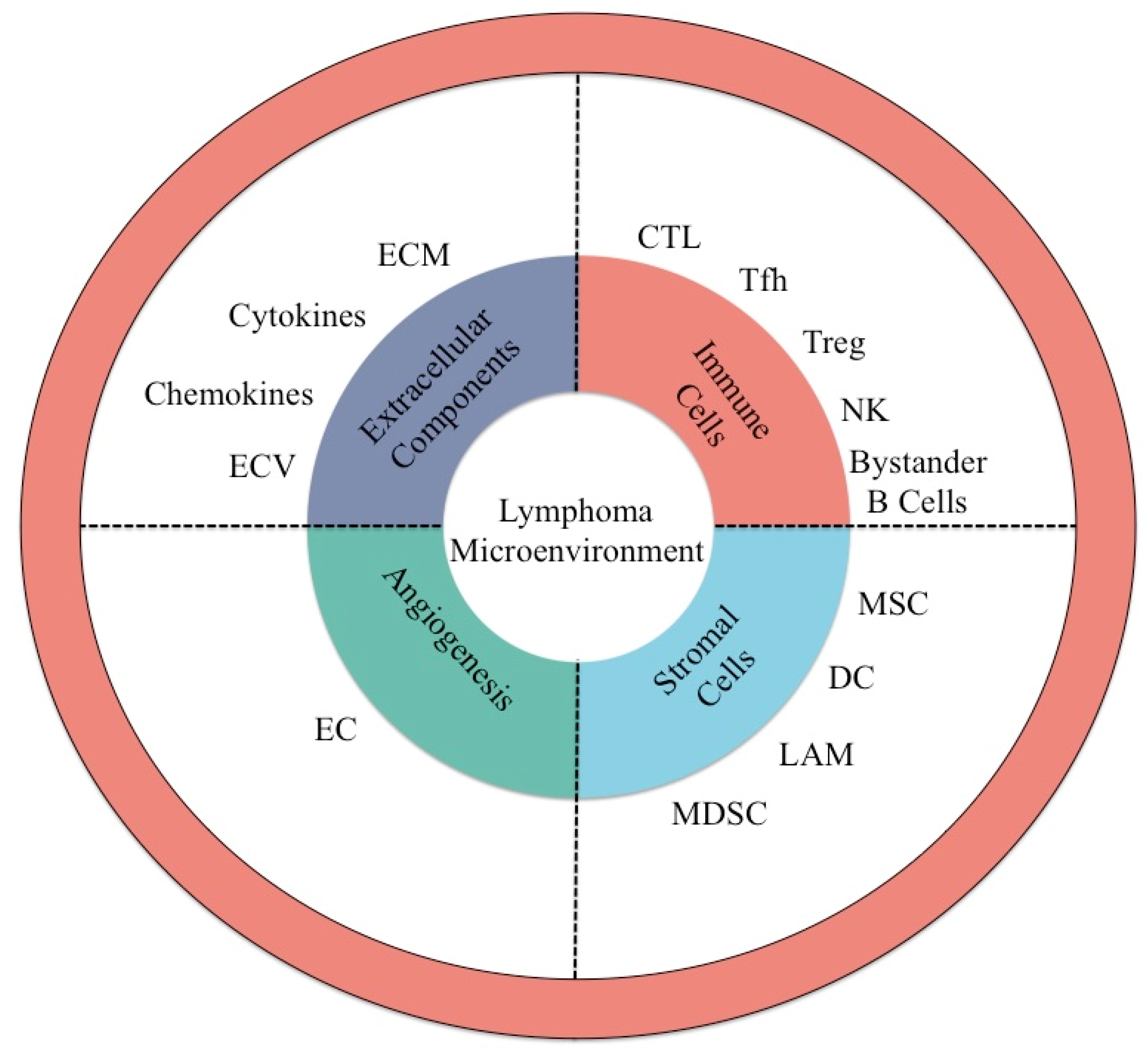
| Cell Type | Activity | Target | Type of Lymphoma | Reference |
|---|---|---|---|---|
| Suppressor Treg | Pro-tumoral | Suppression of CD8+ T cells | Non-Hodgkin’s lymphoma, peripheral T-cell lymphoma, anaplastic large cell lymphoma, Hodgkin’s lymphoma | [14] |
| Malignant Treg | Pro-tumoral | Inhibition of CD8+ activity | T-cell lymphoma | [14] |
| Direct tumor-killing Treg | Anti-tumoral | Are a source of malignant cells | Cutaneous T-cell lymphoma, follicular lymphoma, diffuse T-cell lymphoma, extranodal NK/T-cell lymphoma | [14,42] |
| Incompetent Treg | Anti-tumoral | No effect | Angioimmunoblastic T-cell lymphoma | [14,43] |
| Cytotoxic T cell | Anti-tumoral, Pro-tumoral in CD8+ lymphomas | Inhibition of malignant cells; inhibited by Tregs | Epstein-Barr virus (EBV)-positive Hodgkin’s lymphoma, CD8+ cytotoxic T-cell lymphoma, nodal cytotoxic T-cell lymphoma, Hodgkin’s lymphoma, follicular lymphoma, B-cell non-Hodgkin’s lymphoma | [36,37,38,39,58,64] |
| Follicular helper T cell | Anti-tumoral, Pro-tumoral in follicular T-cell lymphomas | B-cell maturation | Peripheral T-cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), nodular lymphocyte predominant Hodgkin’s lymphoma | [59,60,65] |
| T-helper cell type 1 (Th1) | Anti-tumoral | Activation of CD8+ T cells | EBV-negative classical Hodgkin’s lymphoma (CHL), abundant in classical Hodgkin’s lymphoma, B-cell non-Hodgkin’s lymphoma (NHL), abundant in complete remission of diffuse B-cell large cell lymphoma | [36,44,45] |
| T-helper cell type 2 | Pro-tumoral | Inhibition of Th1 | Depleted in classical Hodgkin’s lymphoma, B-cell non-Hodgkin’s lymphoma (NHL), abundant in untreated B-cell diffuse large cell lymphoma | [44,45] |
| T-helper cell type 17 | Anti-tumoral (mostly), pro-tumoral through rituximab resistance | Contradictory conversion with Tregs, stimulates B-cell proliferation and antibody production | EBV-negative classical Hodgkin’s lymphoma (CHL), B-cell lymphoma, rituximab-resistant B-cell lymphoma | [36,46,47,48,61] |
| B2 follicular B cells | Pro-tumoral | Memory CD4(+) T cells | Probably present in mature B-cell lymphoma | [49] |
| Immunoglobulin A (IgA)+ plasma cell | Pro-tumoral | Activation of CD8+ T cells, antigen-presenting cells | Concomitant lymphoplasmacytic lymphoma and plasma cell myeloma, peripheral T-cell lymphoma associated with IgG plasma cell leukemia and IgA hypergammaglobulinemia | [50,51,62] |
| IgG1+/IgM memory B cells | Anti-tumoral general, pro-tumoral in case of malignant accumulation | Co-operation with CD8+ T cells | Malignant follicular lymphoma (oncogenic role of memory B cells with BCL2: immunoglobulin heavy chain (IgHV) translocation), Burkitt’s lymphoma (EBV+), mantle cell lymphoma | [50,52,63] |
| Regulatory B cells | Pro-tumoral | Suppression of cytotoxic T cells and Th1 cells; activation of malignant T cells | Non-Hodgkin’s lymphoma | [55,57] |
| Marker | Cell | Drug | Effect | Clinical Effect in Lymphoma Patients | Status | Reference |
|---|---|---|---|---|---|---|
| A2AR | CD4+ and CD8+ T cells, Tregs in response to stress | Monoclonal antibodies (mAbs): SCH58261, SYN115, ZM241365, and FSPTP | It downregulates T-helper cells and cytotoxic T cell response, synergy with cytotoxic T-lymphocyte associated 4 (CTLA-4) and PD-1 |
| Phase I | [72,73] |
| B7-H3 | IFN-γ-producing T cells | Potential therapeutic benefits of monoclonal antibodies (mAbs) targeting this biomarker | It increases T-cell reactivity |
| Preclinical data | [85,87,96] |
| BTLA | T follicular helper cell differentiation, Th1 helper cell | Soluble herpes virus entry mediator (HVEM) ectodomain protein (solHVEM) through CART cells or bispecific antibody delivery could restore HVEM– B and T lymphocyte attenuator (BTLA), resulting in apoptosis and tumor growth delay in B-cell lymphomas | It binds to the HVEM receptor, downregulates CD8+ T-cell cytotoxicity in diffuse large B-cell lymphoma, suppresses minor histocompatibility antigen-specific CD8+ T cell, downregulates B-cell response |
| Preclinical results | [88,89,90,97] |
| CTLA-4 | Intracellular vesicles in FOXP3+ Treg cells or activated T cells | Ipilimumab, tremelimumab | It prevents conventional T-cell activation, it is highly expressed on Tregs and stimulates their activity |
| Phase I clinical trial | [68,69,70,98] |
| LAG-3 | Activated CD8+ T cell, T-helper cells | mAbs: BMS-986016, LAG525, MK-4280, and IMP321 (APC activator) | Its overactivation in tumor microenvironment causes T-cell exhaustion, especially in collaboration with PD-1 activation |
| Phase I-II clinical trial in solid tumors, only preclinical data for lymphoma | [74,75,76,77,99] |
| PD-1 | CD4+ T cell | Nivolumab, pembrolizumab | It causes T-cell anergy |
| Phase I and II clinical trial | [66,100] |
| TIGIT | Activated CD4+, CD8+ T cells, T follicular helper (TFH) cells, Tregs | mAbs: MTIG7192A, BMS-986207, OMP-313M32, MK-7684, AB154, CGEN-15137, and CASC-TIGIT | TIGIT+ Tregs are more potent, CD8+ T cells have a much weaker response, PD-1 and TIGIT have a synergic effect |
| Phase I in solid tumors | [92,93,99,101,102] |
| TIM3 | IFN-γ-producing CD4+ and CD8+ T cells, Tregs | mAbs: Sym023, INCAGN02390, LY3321367 ± LY3300054, Sym021 ± Sym023, MBG453 ± PDR001, BGB-A425 + tislelizumab, TSR-022 ± TSR-042, TSR-022 + TSR-042 + chemo; bispecific mAbs: RO7121661, LY3415244 | Its overexpression on T lymphocytes is specific for CD8+ T-cell exhaustion. Single expression shows weak exhaustion, while co-expression with PD-1 has a more pronounced effect |
| Phase I-II clinical trial in solid tumors, only Sym023 is in Phase I clinical trial for lymphoma | [99,103,104] |
| VISTA | Highly expressed on tumor-infiltrating leukocytes | CA-170 V-domain Ig suppressor of T cell activation (VISTA) proteins | Suppressed proliferation of T cells, but not B cells |
| Phase I and II clinical trial | [94,96] |
| VTCN1 (B7-H4/B7- S1) | Activated minor population of T cells, most B cells (defined as B220+), macrophages | Potential therapeutic benefits of mAbs targeting this biomarker | It decreases IFN-γ production by T cells, downregulates cytotoxic T-cell response, decreases T-cell proliferation, synergy with PD-1 and CTLA-4 |
| Preclinical studies in solid tumors | [81,82,83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmirean, M.; Rauch, S.; Jurj, A.; Pasca, S.; Iluta, S.; Teodorescu, P.; Berce, C.; Zimta, A.-A.; Turcas, C.; Tigu, A.-B.; et al. B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974? J. Clin. Med. 2020, 9, 3412. https://doi.org/10.3390/jcm9113412
Desmirean M, Rauch S, Jurj A, Pasca S, Iluta S, Teodorescu P, Berce C, Zimta A-A, Turcas C, Tigu A-B, et al. B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974? Journal of Clinical Medicine. 2020; 9(11):3412. https://doi.org/10.3390/jcm9113412
Chicago/Turabian StyleDesmirean, Minodora, Sebastian Rauch, Ancuta Jurj, Sergiu Pasca, Sabina Iluta, Patric Teodorescu, Cristian Berce, Alina-Andreea Zimta, Cristina Turcas, Adrian-Bogdan Tigu, and et al. 2020. "B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974?" Journal of Clinical Medicine 9, no. 11: 3412. https://doi.org/10.3390/jcm9113412
APA StyleDesmirean, M., Rauch, S., Jurj, A., Pasca, S., Iluta, S., Teodorescu, P., Berce, C., Zimta, A.-A., Turcas, C., Tigu, A.-B., Moldovan, C., Paris, I., Steinheber, J., Richlitzki, C., Constantinescu, C., Sigurjonsson, O. E., Dima, D., Petrushev, B., & Tomuleasa, C. (2020). B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974? Journal of Clinical Medicine, 9(11), 3412. https://doi.org/10.3390/jcm9113412








