The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mix Proportions: Preparation of Emulsion
- The oil phase: this is a fatty acid ester trimethylolpropane trioleate or TMP oleate, Weichol 3/134W from Industrial Química Lasem (IQL, Castellgalí, Spain), commercially used as environmentally adapted lubricant [41].
- The aqueous phase: this is a solution of 2-aminoethanol (MEA) (supplied by Across, Noisy Le Grans, France) in distilled deionized water to reach and maintain a pH above 9. The ideal pH of water-based MWF is within the range 8.5 to 9.5. At this condition, it prevents corrosion on ferrous metals, minimizes the potential for contact dermatitis, and controls biological growth [42,43].
- The surfactant blend: this is a mixture of a non-ionic surfactant oleyl/cetyl propoxylated alcohol with the trade name Dehypon OCP502 (BASF, Ludwigshafen, Germany) and the different surfactants under study (Kao Chemicals GmbH, Emmerich, Germany) (Table 2) with a 2:3 ratio. Adding a non-ionic surfactant allows closer packing at the interface and it contributes to stabilizing the emulsion. The oleyl/cetyl propoxylated alcohol was selected according to guidelines for formulating microemulsions from the experimental results of the study conducted by Zhao et al. [40], where the hydrocarbon chain length of the non-ionic surfactant should be equal to or greater than the hydrocarbon chain length of the oil fatty acids.
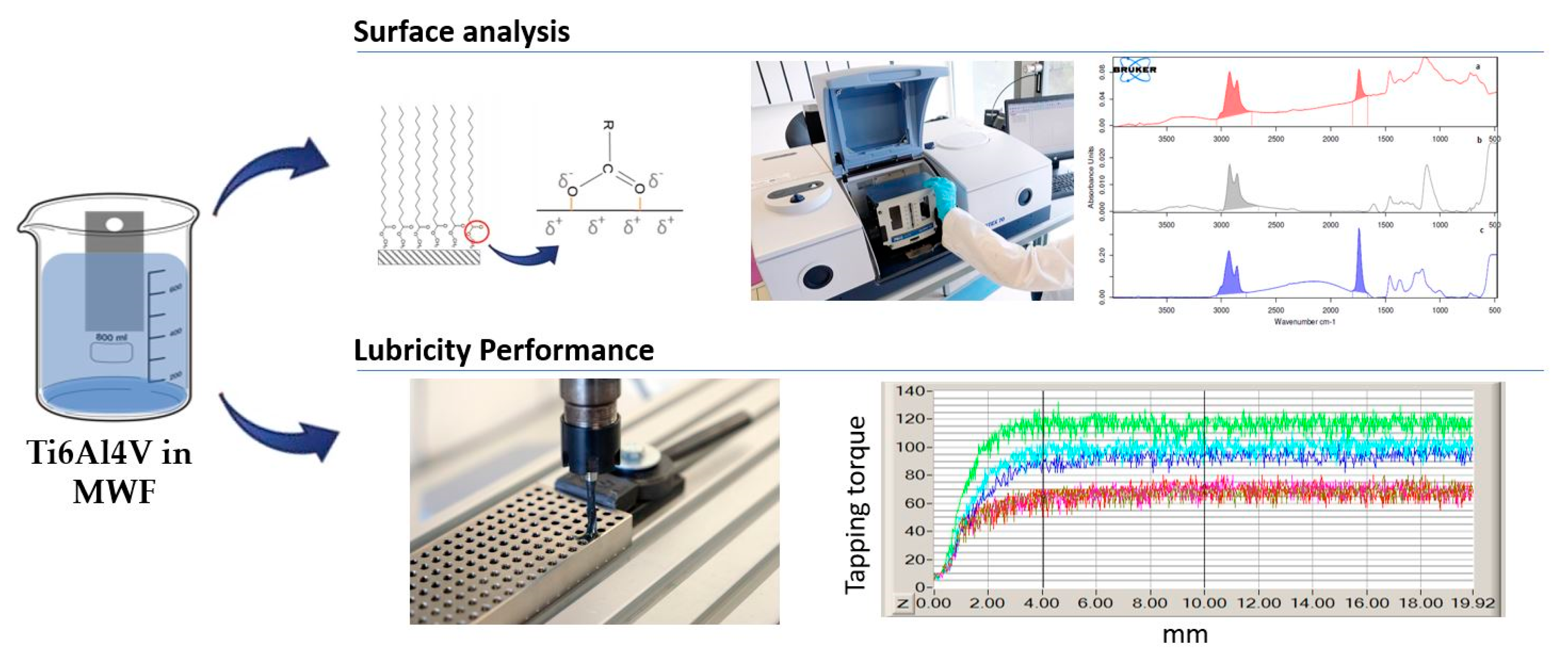
2.2. Determination of Fatty Acid Ester Content on Ti6Al4V Surface
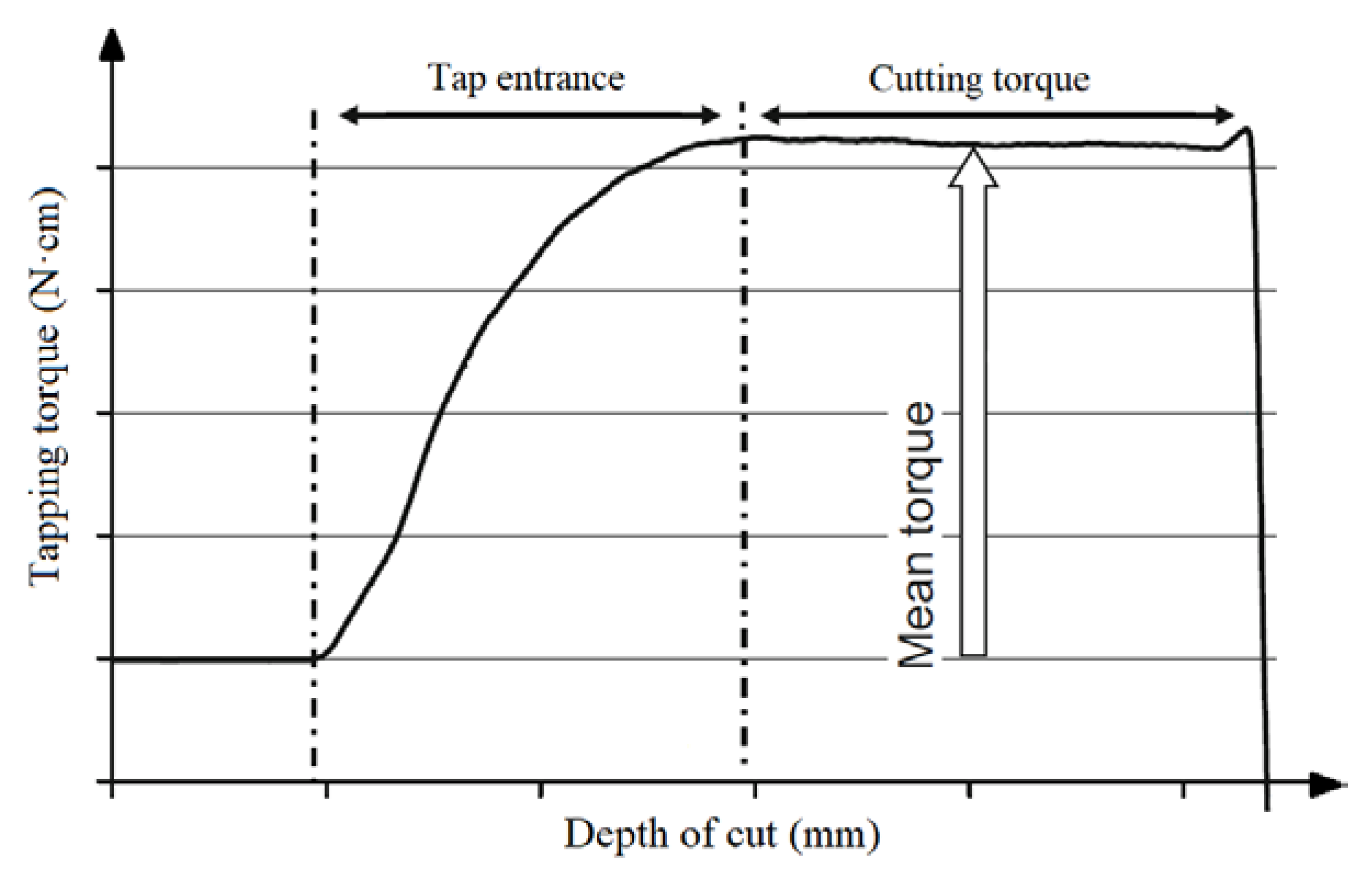
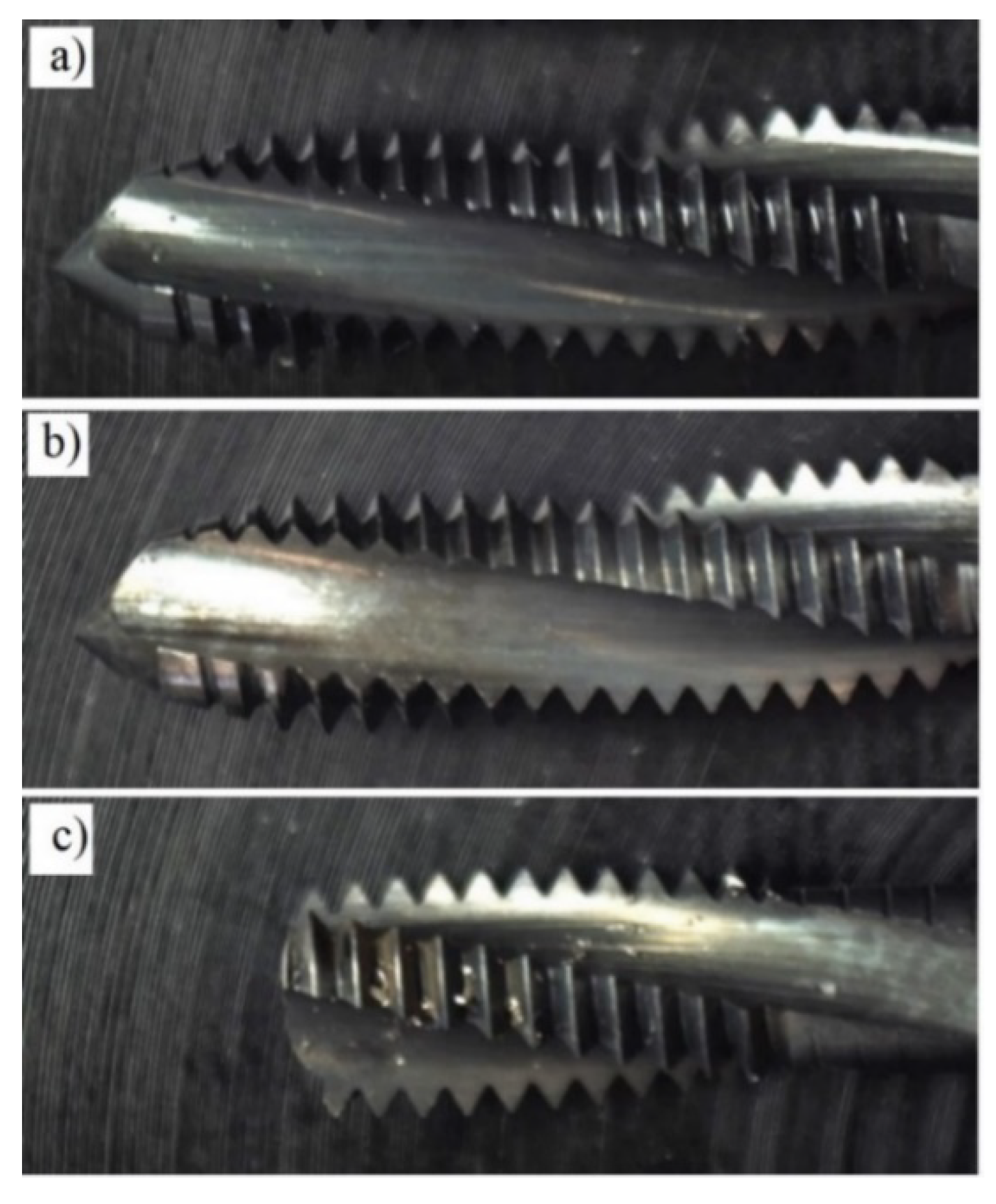
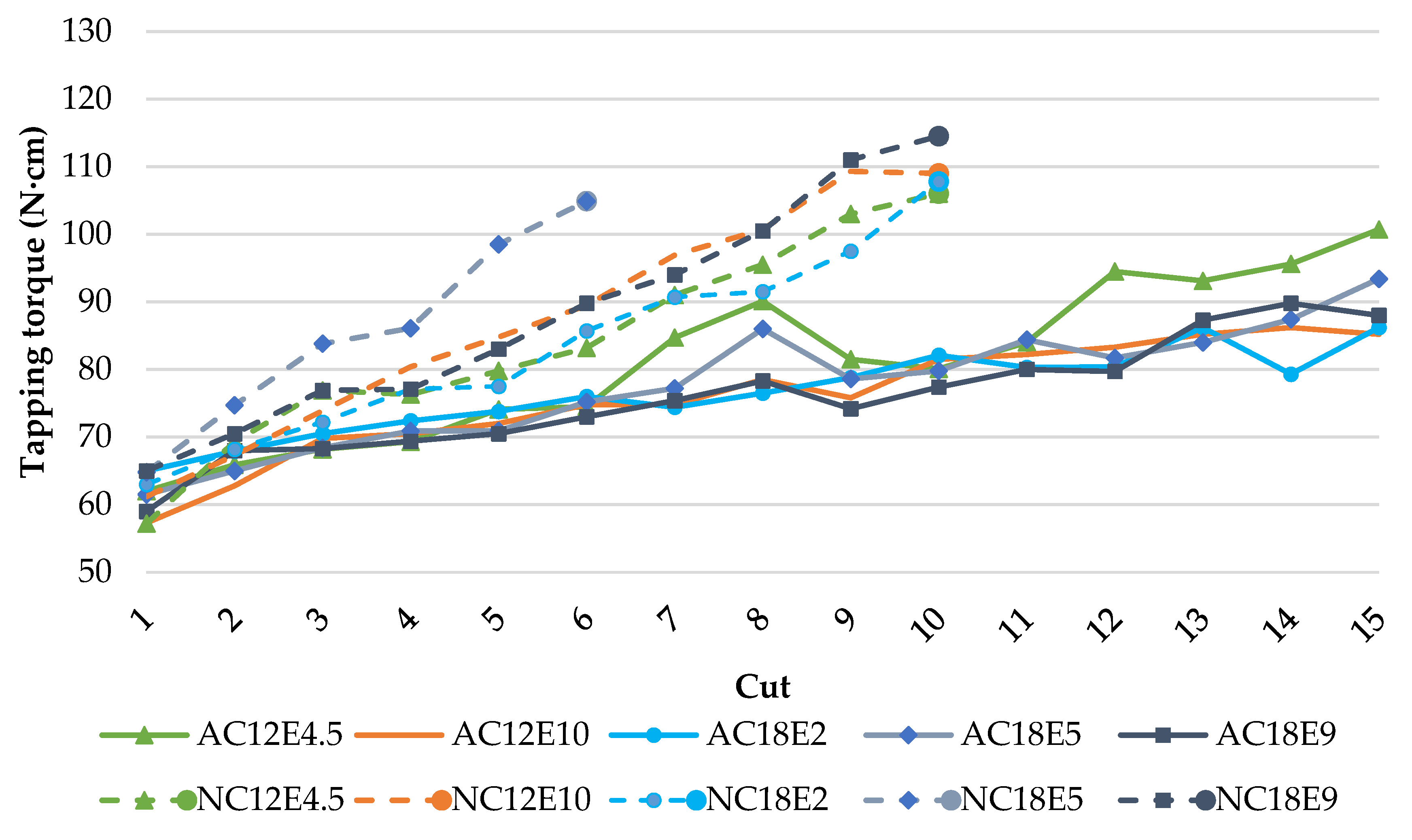
2.3. Tapping Torque Test for Tribological Study
3. Results and Discussion
3.1. Effect of Surfactant Charge on the Lubricity Performance of Emulsions
3.2. Effect of Surfactant’s Hydrocarbon Chain Length on the Tribological Performance of Emulsions
3.3. Effect of Anionic Surfactant’s Ethoxylation Degree on the Tribological Performance of Emulsions
4. Conclusions
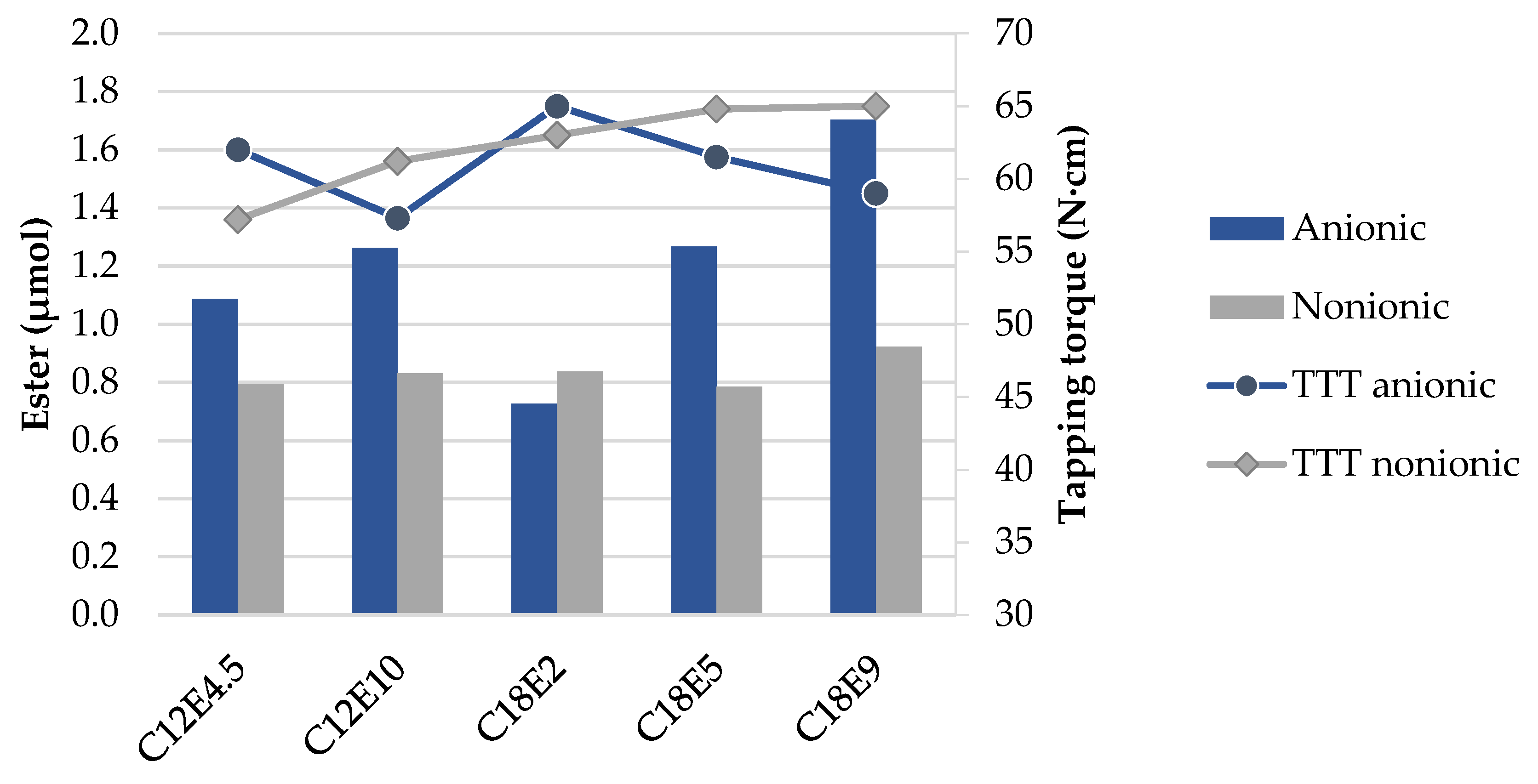
- It was found that, from the surfactants tested, non-ionic surfactants are less promising and their structures have little impact on the adherence of TMP oleate. The application of surfactants bearing an anionic group can be successful, as they not only promote TMP oleate adhesion, but also, they improve the anti-wear.
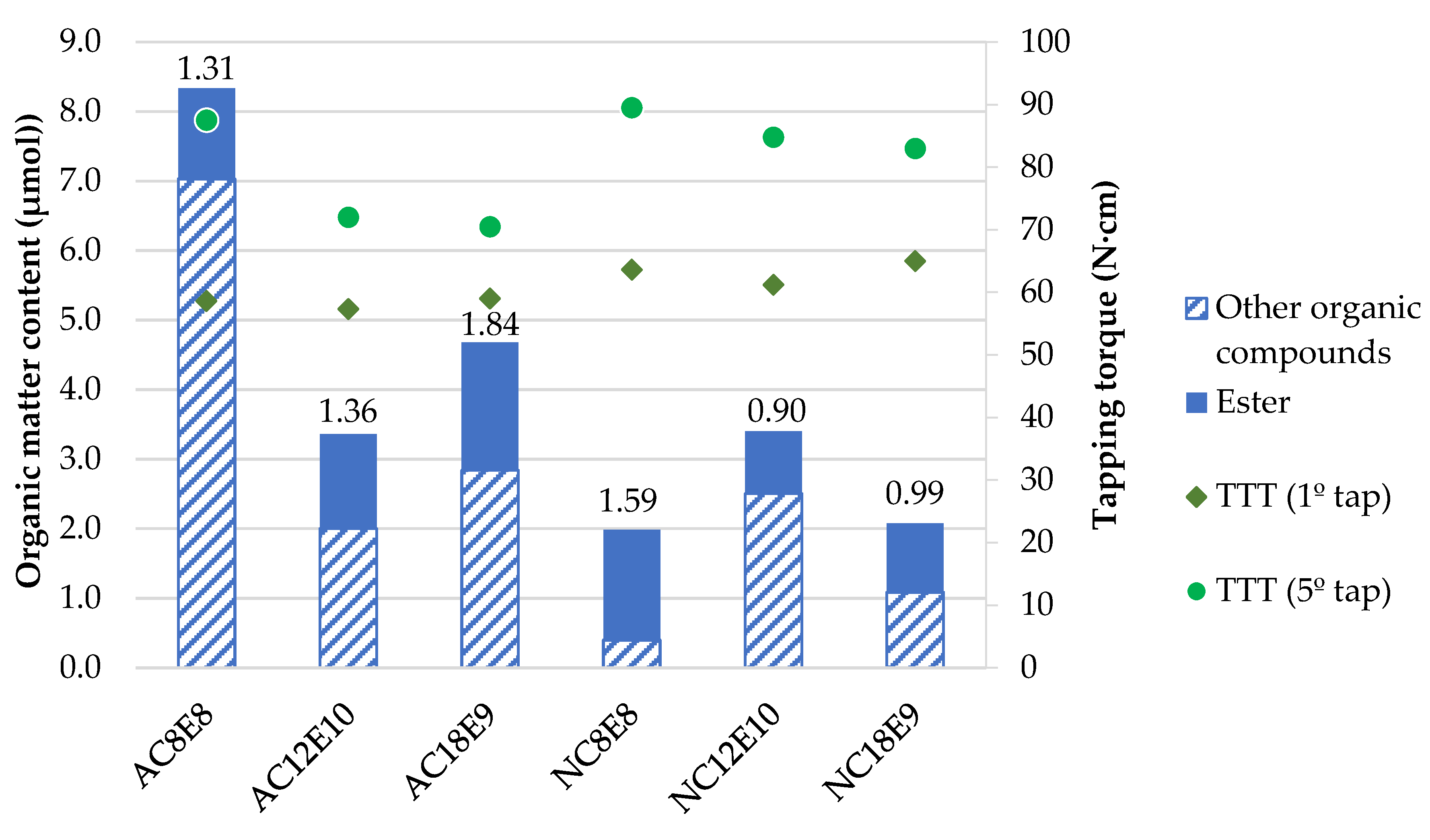
- The data also indicate that the molecular structure of anionic surfactants has a high impact on the amount of ester adhered on the titanium alloy surface, forming a lubricating film that prevents direct metal contact. The more ester is adhered, the lower the tapping torque values are, indicating less wear.
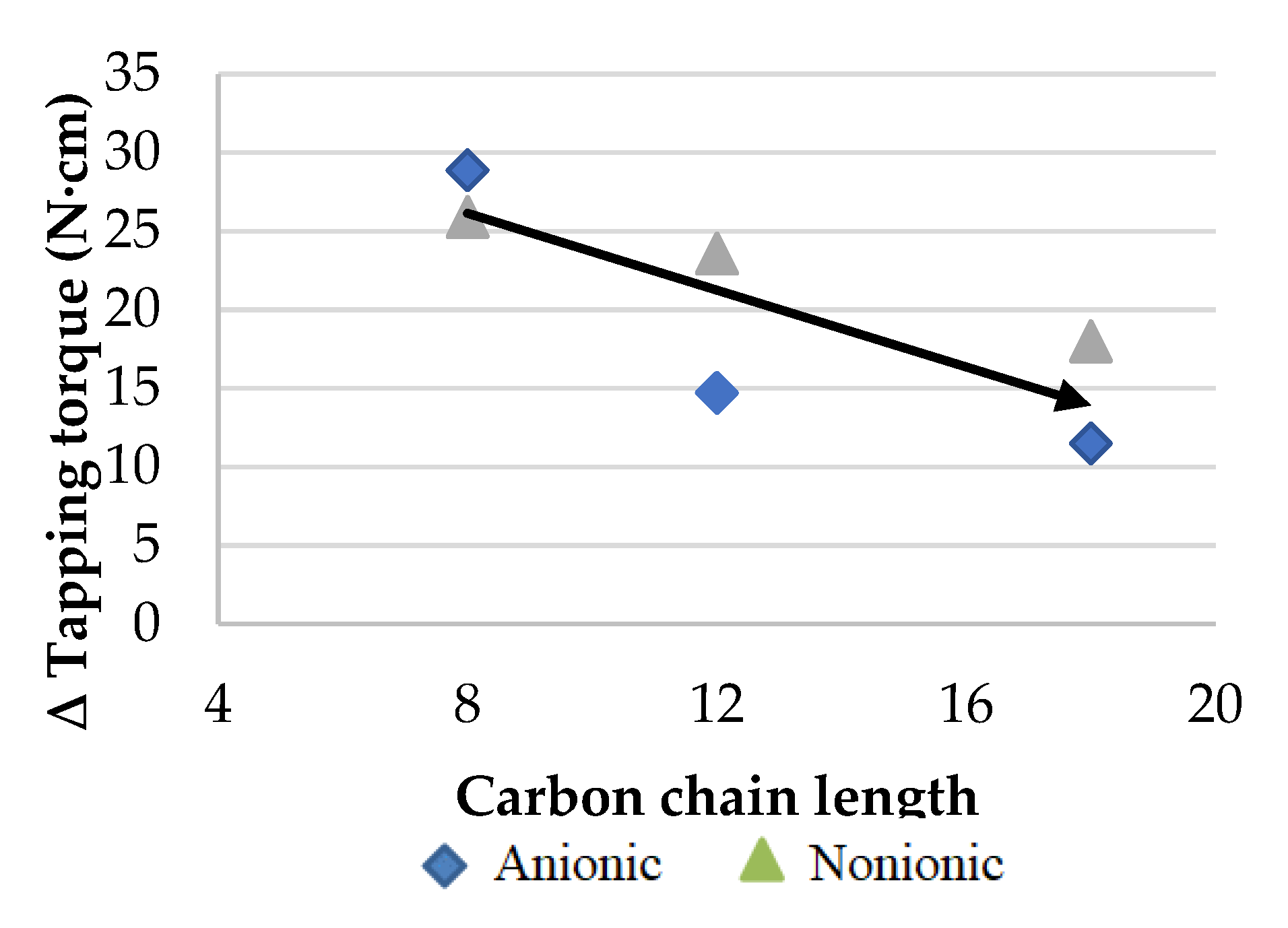
- The concentration of ester increases with the hydrocarbon chain length of the anionic surfactant, as it becomes more lipophilic. However, surfactants with a hydrocarbon chain below eight carbons show high emulsion instability.
- It was apparent that the longer the hydrocarbon chain of the surfactant is, the higher the wear reduction is, regardless of surfactant type, whether it is anionic or non-ionic.
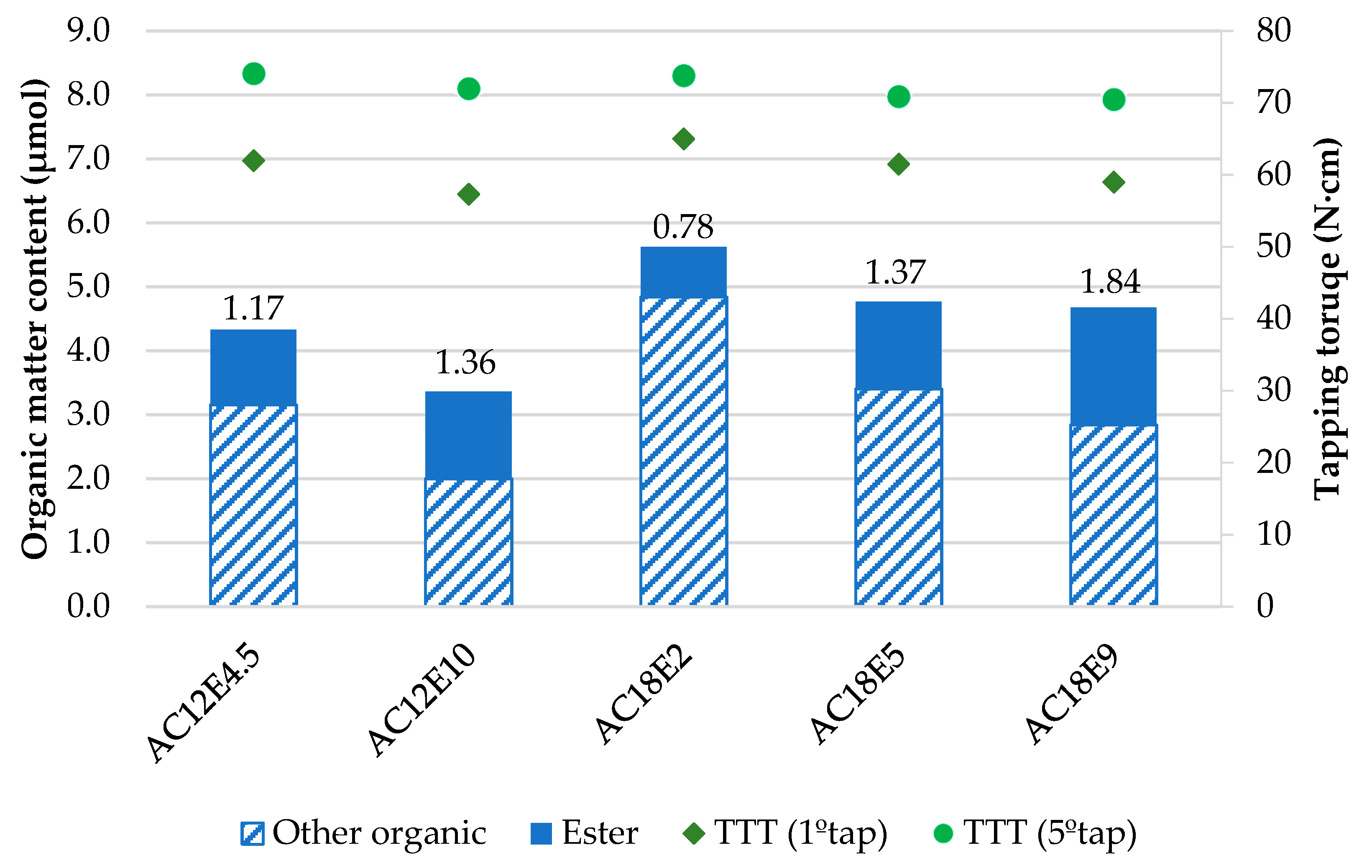
- In the tested anionic surfactants, the higher the number of ethoxylations, the more significant the increase in lubricity observed. Even though there is less surfactant on the surface, due to higher solubility increases, the amount of ester increases, forming a more resistant layer.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amit, P.; Isushil, I.; Yogesh, M.; Manik, N. Machining Challenges in Ti-6Al-4V—A Review. Int. J. Innov. Eng. Technol. 2015, 5, 6–23. [Google Scholar]
- Pervaiz, S.; Deiab, I.; Rashid, A.; Nicolescu, M. Minimal quantity cooling lubrication in turning of Ti6Al4V: Influence on surface roughness, cutting force and tool wear. J. Eng. Manuf. 2015, 1–17. [Google Scholar] [CrossRef]
- Kumar, U.; Senthil, P. A comparative machinability study on titanium alloy Ti-6Al-4V during dry turning by cryogenic treated and untreated condition of uncoated WC inserts. Mater. Today Proc. 2019, 27, 2324–2328. [Google Scholar] [CrossRef]
- Ezugwu, E.O.; Wang, Z.M. Titanium alloys and their machinability. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Pramanik, A.; Littlefair, G. Machining of Titanium Alloy (Ti-6Al-4V)—Theory to Application. Mach. Sci. Technol. 2015, 19, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Elshwain, A.; Redzuan, N.; Yusof, N.M.; Engineering, M. Machinability of Nickel and Titanium Alloys Under of Gas-Based Coolant-Lubricants (Cls)—A Review. Int. J. Res. Eng. Technol. 2013, 2, 690–702. [Google Scholar] [CrossRef]
- Revuru, R.S.; Zhang, J.Z.; Posinasetti, N.R.; Kidd, T. Optimization of titanium alloys turning operation in varied cutting fluid conditions with multiple machining performance characteristics. Int. J. Adv. Manuf. Technol. 2017, 1–13. [Google Scholar] [CrossRef]
- Namb, M. Influence of Coolant in Machinability of Titanium Alloy (Ti-6Al-4V). J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, C.; Dai, Y.; Luo, J. Tribological properties of titanium alloys under lubrication of SEE oil and aqueous solutions. Tribol. Int. 2017, 109, 40–47. [Google Scholar] [CrossRef]
- Pervaiz, S.; Anwar, S.; Qureshi, I.; Ahmed, N. Recent Advances in the Machining of Titanium Alloys using Minimum Quantity Lubrication (MQL) Based Techniques. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 133–145. [Google Scholar] [CrossRef]
- Yumashev, A.; Mikhaylov, A. Development of polymer film coatings with high adhesion to steel alloys and high wear resistance. Polym. Compos. 2020, 41, 2875–2880. [Google Scholar] [CrossRef]
- Nune, M.M.R.; Chaganti, P.K. Development, characterization, and evaluation of novel eco-friendly metal working fluid. Meas. J. Int. Meas. Confed. 2019, 137, 401–416. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Benedicto, E.; Carou, D.; Rubio, E.M. Technical, Economic and Environmental Review of the Lubrication/Cooling Systems Used in Machining Processes. Procedia Eng. 2017, 184, 99–116. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Ram, D.; Ravi Sankar, M. Biodegradation and Hard Machining Performance Comparison of Eco-friendly Cutting Fluid and Mineral Oil Using Flood Cooling and Minimum Quantity Cutting Fluid Techniques. J. Clean. Prod. 2017. [Google Scholar] [CrossRef]
- Talib, N.; Rahim, E.A. Experimental evaluation of physicochemical properties and tapping torque of hexagonal boron nitride in modified jatropha oils-based as sustainable metalworking fluids. J. Clean. Prod. 2018, 171, 743–755. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J. Bio Tribo Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Maria, R.; Saboya, A.; Murilo, F.; De Luna, T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S. Green tribology: Fundamentals and future development. Friction 2013, 1, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Zulkifli, N.W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- DIN. Schmierstoffe—Bearbeitungsmedien für die Umformung und Zerspanung von Werkstoffen—Begriffe; DIN: Berlin, Germany, 2013. [Google Scholar]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J.; Ratoi, M.; Spikes, H.A. The effect of emulsifier concentration on the lubricating properties of oil-in-water emulsions. Tribol. Lett. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Kumar, D.; Daniel, J.; Biswas, S.K. Tribology of steel/steel interaction in oil-in-water emulsion; a rationale for lubricity. J. Colloid Interface Sci. 2010, 345, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cambiella, Á.; Benito, J.M.; Pazos, C.; Coca, J. Interfacial properties of oil-in-water emulsions designed to be used as metalworking fluids. Colloids Surf. A Physicochem. Eng. Asp. 2007, 305, 112–119. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Adsorption of Surface-Active Agents at Interfaces: The Electrical Double Layer. In Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 39–122. [Google Scholar]
- Paswan, B.K.; Mahto, V. Development of environment-friendly oil-in-water emulsion based drilling fluid for shale gas formation using sunflower oil. J. Pet. Sci. Eng. 2020, 191, 107129. [Google Scholar] [CrossRef]
- Pegiadou-Koemtjopoulou, S.; Tsatsaroni, E.; Mylona, M. Pyrimidinium cationic surfactants as emulsifiers for oil-in-water emulsions. J. Surfactants Deterg. 1998, 1, 499–502. [Google Scholar] [CrossRef]
- Sulek, M.; Wasilewski, T.; Zieba, M. Tribological and physical-chemical properties of aqueous solutions of cationic surfactants. Ind. Lubr. Tribol. 2010, 62, 279–284. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, X.; Tan, T. Preparation of a Water-Based Lubricant from Lignocellulosic Biomass and Its Tribological Properties. Ind. Eng. Chem. Res. 2017, 56, 7858–7864. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Clarens, A.F.; Hayes, K.F.; Skerlos, S.J. Design of Hard Water Stable Emulsifier Systems for Petroleum- and Bio-based Semi-synthetic Metalworking Fluids. Environ. Sci. Technol. 2003, 37, 5278–5288. [Google Scholar] [CrossRef]
- Sułek, M.W.; Ogorzałek, M.; Wasilewski, T.; Klimaszewska, E. Alkyl polyglucosides as components of water based lubricants. J. Surfactants Deterg. 2013, 16, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Benito, J.M.; Cambiella, A.; Lobo, A.; Gutiérrez, G.; Coca, J.; Pazos, C. Formulation, characterization and treatment of metalworking oil-in-water emulsions. Clean Technol. Environ. Policy 2010, 12, 31–41. [Google Scholar] [CrossRef]
- Ahmed, N.; Nassar, A.; Zaki, N.; Gharieb, H. Stability and Rheology of Heavy Crude Oil-in-Water Emulsion Stabilized By an Anionic-Nonionic Surfactant Mixture. Pet. Sci. Technol. 1999, 17, 553–576. [Google Scholar] [CrossRef]
- Srikant, R.R.; Ramana, V.S.N.V. Performance evaluation of vegetable emulsifier based green cutting fluid in turning of American Iron and Steel Institute (AISI) 1040 steel—An initiative towards sustainable manufacturing. J. Clean. Prod. 2015, 108, 104–109. [Google Scholar] [CrossRef]
- Sharma, D.; Pandey, K.M. Ecological friendly functional fluids and lubricant techniques in machining processes: A review. Int. J. Hydromechatronics 2018, 1, 182. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Meyer, D.; Huesmann-Cordes, A.; Herrmann, C. Metalworking fluids—Mechanisms and performance. CIRP Ann. Manuf. Technol. 2015, 64, 605–628. [Google Scholar] [CrossRef] [Green Version]
- Joyner, H.S.; Pernell, C.W.; Daubert, C.R. Impact of oil-in-water emulsion composition and preparation method on emulsion physical properties and friction behaviors. Tribol. Lett. 2014, 56, 143–160. [Google Scholar] [CrossRef]
- Shiloach, A.; Blankschtein, D. Predicting Micellar Solution Properties of Binary Surfactant Mixtures. Langmuir 1998, 14, 1618–1636. [Google Scholar] [CrossRef]
- Benedicto, E.; Carou, D.; Rubio, E.M.; Batlle, L. A novel method for the determination of fatty acid esters in aqueous emulsion on Ti6Al4V surface with IRRAS and carbon quantification. Tribol. Int. 2018, 128, 155–160. [Google Scholar] [CrossRef]
- Zhao, F.; Clarens, A.; Murphree, A.; Hayes, K.; Skerlos, S.J. Structural aspects of surfactant selection for the design of vegetable oil semi-synthetic metalworking fluids. Environ. Sci. Technol. 2006, 40, 7930–7937. [Google Scholar] [CrossRef]
- Rahim, E.A.; Sani, A.S.A.; Talib, N. Tribological Interaction of Bio-Based Metalworking Fluids in Machining Process. Lubr. Tribol. Lubr. Addit. 2018. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabagh, A.M. Investigation of oil and emulsion stability of locally prepared metalworking fluids. Ind. Lubr. Tribol. 2012, 64, 346–358. [Google Scholar] [CrossRef]
- Rao, D.N.; Srikant, R.R.; Rao, C.S. Influence of emulsifier content on properties and durability of cutting fluids. J. Braz. Soc. Mech. Sci. Eng. 2007, 29, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Pereira, I.C.; Da Silva, M.B.; Da Cunha, D.F.; Sales, W.F. Analysis of tapping process in three types of cast iron. Int. J. Adv. Manuf. Technol. 2016, 82, 1041–1048. [Google Scholar] [CrossRef]
- Ni, J.; Feng, G.; Meng, Z.; Hong, T.; Chen, Y.; Zheng, X. Reinforced lubrication of vegetable oils with graphene additive in tapping ADC12 aluminum alloy. Int. J. Adv. Manuf. Technol. 2017, 1–10. [Google Scholar] [CrossRef]
- ASTM. ASTM D5619-00: Standard Test Method for Comparing Metal Removal Fluids Using the Tapping Torque Test Machine (Withdrawn 2016); ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Microtap GmbH. The TTT System—A Window into Tribology. Available online: https://www.tapping-torque-test.com/files/4914/6218/6159/TTT-System_a_Window_into_Tribology_KMM-April-2016.pdf (accessed on 10 October 2020).
- Pottirayil, A.; Kailas, S.V.; Biswas, S.K. Lubricity of an oil in water emulsion in metal cutting: The effect of hydrophilic/lypophilic balance of emulsifiers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 323–330. [Google Scholar] [CrossRef]
- Plaza, S.; Margielewski, L.; Celichowski, G.; Wesolowski, R.W.; Stanecka, R. Tribological performance of some polyoxyethylene dithiophosphate derivatives water solutions. Wear 2001, 249, 1077–1089. [Google Scholar] [CrossRef]


| Product | Molar |
|---|---|
| TMP Oleate | 0.0010 |
| Dehypon OCP 502 | 0.0008 |
| Surfactant under study | 0.0012 |
| Charge | Abbreviation | Chain | Ethoxylation Degree (EO) | Chemical Name |
|---|---|---|---|---|
| Anionic | AC8E8 | C8 | 8 EO | Capryleth-9 carboxylic acid |
| AC12E4.5 | C12 | 4.5 EO | Laureth-6 carboxylic acid | |
| AC12E10 | C12 | 10 EO | Laureth-11 carboxylic acid | |
| AC18E2 | C18 | 2 EO | Oleth-3 carboxylic acid | |
| AC18E5 | C18 | 5 EO | Oleth-6 carboxylic acid | |
| AC18E9 | C18 | 9 EO | Oleth-10 carboxylic acid | |
| Non-ionic | NC8E8 | C8 | 8 EO | Octyl alcohol, ethoxylated |
| NC12E4.5 | C12 | 4.5 EO | Lauryl alcohol, ethoxylated | |
| NC12E10 | C12 | 10 EO | Lauryl alcohol, ethoxylated | |
| NC18E2 | C18 | 2 EO | Oleyl alcohol, ethoxylated | |
| NC18E5 | C18 | 5 EO | Oleyl alcohol, ethoxylated | |
| NC18E10 | C18 | 9 EO | Oleyl alcohol, ethoxylated |
| Parameter | Value |
|---|---|
| Spindle speed (rpm) | 300 |
| Depth of cut (mm) | 6 |
| Hole diameter (mm) | 3.3 |
| Tapping tool | TTT_M4C |
| Workpiece material | Ti6Al4V pre-drilled |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedicto, E.; Rubio, E.M.; Carou, D.; Santacruz, C. The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys. Metals 2020, 10, 1388. https://doi.org/10.3390/met10101388
Benedicto E, Rubio EM, Carou D, Santacruz C. The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys. Metals. 2020; 10(10):1388. https://doi.org/10.3390/met10101388
Chicago/Turabian StyleBenedicto, Elisabet, Eva María Rubio, Diego Carou, and Coral Santacruz. 2020. "The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys" Metals 10, no. 10: 1388. https://doi.org/10.3390/met10101388
APA StyleBenedicto, E., Rubio, E. M., Carou, D., & Santacruz, C. (2020). The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys. Metals, 10(10), 1388. https://doi.org/10.3390/met10101388






