Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species
Abstract
:1. Introduction
2. Methods
2.1. Conventional Extraction Methods
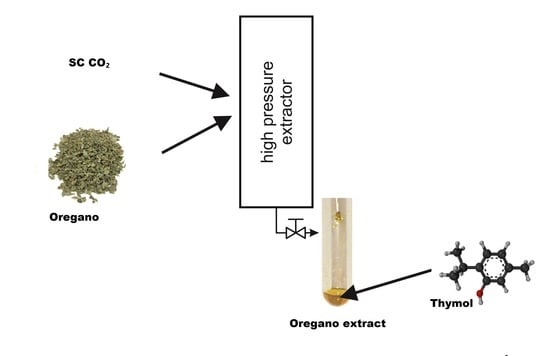
2.2. Extraction Methods Involving Sub- and Supercritical Fluids
2.3. Sub- and Supercritical Fluid Extraction by Adding Organic Modifiers
2.4. Ultra-Sound Assisted Extraction
2.5. Microwave Extraction
2.6. Clevenger Distillation
2.7. Identification and Quantitative Determination of Components
3. Results
4. Discussion
5. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related Over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Migliore, J.; Baumel, A.; Pouget, M.; Diadema, K.; Noble, V.; Leriche, A.; Medail, F. Plant biogeography in the western mediterranean basin: New insights from phylogeographical studies. Boll. Mus. Ist. Biol. Univ. Genova 2013, 75, 64–67. [Google Scholar]
- Skoula, M.; Harborne, J.B.; Harborne, J.B. The taxonomy and chemistry of Origanum. Available online: https://www.taylorfrancis.com/ (accessed on 9 May 2019).
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial Activity of Essential Oils from Plants against Selected Pathogenic and Saprophytic Microorganisms. J. Food Prot. 2001, 64, 1019–1024. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn essential oils of Greek oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Marriott, R.J. Greener chemistry preparation of traditional flavor extracts and molecules. Agro FOOD Ind. Hi Tech 2010, 21, 46–48. [Google Scholar]
- Vetter, G.; Karl, E. High-Pressure Technology. Ullmann’s Encycl. Ind. Chem. 2000, 537–575. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Li, Y.; Tixier, A.-S.F.; Chemat, F. Essential Oils: From Conventional to Green Extraction. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; pp. 9–20. [Google Scholar]
- Busatta, C.; Barbosa, J.; Cardoso, R.I.; Paroul, N.; Rodrigues, M.; De Oliveira, D.; De Oliveira, J.V.; Cansian, R.L. Chemical profiles of essential oils of marjoram (Origanum majorana) and oregano (Origanum vulgare) obtained by hydrodistillation and supercritical CO2. J. Essent. Oil Res. 2017, 29, 367–374. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Khaneghah, A.M.; Barba, F.J.; Misra, N. A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci. Technol. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Gavahian, M.; Farhoosh, R.; Javidnia, K.; Shahidi, F.; Golmakani, M.-T.; Farahnaky, A. Effects of Electrolyte Concentration and Ultrasound Pretreatment on Ohmic-Assisted Hydrodistillation of Essential Oils from Mentha piperita L. Int. J. Food Eng. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Asl, R.M.Z.; Niakousari, M.; Gahruie, H.H.; Saharkhiz, M.J.; Khaneghah, A.M. Study of two-stage ohmic hydro-extraction of essential oil from Artemisia aucheri Boiss.: Antioxidant and antimicrobial characteristics. Food Res. Int. 2018, 107, 462–469. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Barba, F.J.; Abedi, E.; Niakousari, M.; Tavakoli, J. Extraction of essential oil from Aloysia citriodora Palau leaves using continuous and pulsed ultrasound: Kinetics, antioxidant activity and antimicrobial properties. Process. Biochem. 2018, 65, 197–204. [Google Scholar] [CrossRef]
- Cervato, G.; Carabelli, M.; Gervasio, S.; Cittera, A.; Cazzola, R.; Cestaro, B. Antioxbdant Properties of Oregano (Origanum vulgare) Leaf Extracts. J. Food Biochem. 2000, 24, 453–465. [Google Scholar] [CrossRef]
- Chuang, L.-T.; Tsai, T.-H.; Lien, T.-J.; Huang, W.-C.; Liu, J.-J.; Chang, H.; Chang, M.-L.; Tsai, P.-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef] [Green Version]
- Bower, A.M.; Hernandez, L.M.R.; Berhow, M.A.; De Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S. fruticosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Hossain, M.; Camphuis, G.; Aguiló-Aguayo, I.; Gangopadhyay, N.; Rai, D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3205–3213. [Google Scholar] [CrossRef]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef]
- González, M.D.; Lanzelotti, P.L.; Luis, C.M. Chemical Fingerprinting by HPLC-DAD to Differentiate Certain Subspecies of Origanum vulgare L. Food Anal. Methods 2016, 10, 1460–1468. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2016, 54, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliora, A.C.; Kogiannou, D.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.G.; Lima, M.A.S.; Silveira, E.R. Total NMR assignments of new [C7–O–C7″]-biflavones from leaves of the limonene–carvone chemotype of Lippia alba (Mill) NE Brown. Magn. Reson. Chem. 2005, 43, 334–338. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef]
- Koldas, S.; Demirtas, I.; Özen, T.; Demirci, M.A.; Behçet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2014, 95, 786–798. [Google Scholar] [CrossRef]
- Carvalho, R.N.; Moura, L.S.; Rosa, P.D.T.V.E.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Cavero, S.; García-Risco, M.R.; Marín, F.R.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Reglero, G.; Ibáñez, E. Supercritical fluid extraction of antioxidant compounds from oregano. J. Supercrit. Fluids 2006, 38, 62–69. [Google Scholar] [CrossRef]
- Fornari, T.; Ruiz-Rodríguez, A.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Kinetic study of the supercritical CO2 extraction of different plants from Lamiaceae family. J. Supercrit. Fluids 2012, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, F.; Lu, T.; Santos, R.; Al-Duri, B. Modelling the extraction of essential oils with compressed carbon dioxide. J. Supercrit. Fluids 2003, 25, 247–260. [Google Scholar] [CrossRef]
- Simándi, B.; Oszagyán, M.; Lemberkovics, E.; Kéry, Á.; Kaszács, J.; Thyrion, F.; Mátyás, T. Supercritical carbon dioxide extraction and fractionation of oregano oleoresin. Food Res. Int. 1998, 31, 723–728. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [Google Scholar] [CrossRef] [Green Version]
- Leeke, G.A.; Gaspar, F.; Santos, R. Influence of Water on the Extraction of Essential Oils from a Model Herb Using Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2002, 41, 2033–2039. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Akbarirad, H. The Effects of Amplitudes Ultrasound-Assisted Solvent Extraction and Pretreatment Time on the Yield and Quality of Pistacia Khinjuk Hull Oil. J. Oleo Sci. 2016, 65, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Santos-Zea, L.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Lucini, L.; Benedito, J. Effect of ultrasound transducer design on the acoustically-assisted supercritical fluid extraction of antioxidants from oregano. Ultrason. Sonochemistry 2018, 47, 47–56. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Potential Application of Essential Oils as Natural Antioxidants in Meat and Meat Products: A Review. Food Rev. Int. 2013, 30, 71–90. [Google Scholar] [CrossRef]
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadoğan, T.; Sağdıç, O. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; Kishimoto, T. Development of a Method for the Quantitation of Three Thiols in Beer, Hop, and Wort Samples by Stir Bar Sorptive Extraction with in Situ Derivatization and Thermal Desorption–Gas Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6698–6706. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Harnly, J.M. A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using a Standard Analytical Approach for All Plant Materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, S.-S.; Vattem, D.A.; Lin, Y.-T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical Analysis and Evaluation of Its Antimalarial, Antioxidant, and Cytotoxic Activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, L.P.; Stanojević, J.S.; Cvetković, D.J.; Ilić, D.P. Antioxidant activity of oregano essential oil (Origanum vulgare L.). Biol. Nyssana 2018, 7, 131–139. [Google Scholar]
- Puertas-Mejía, M.; Hillebrand, S.; Stashenko, E.; Winterhalter, P. In vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L.) essential oil. Flavour Fragr. J. 2002, 17, 380–384. [Google Scholar] [CrossRef]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Farag, R.S.; Daw, Z.Y.; Abo-Raya, S.H. Influence of Some Spice Essential Oils on Aspergillus Parasiticus Growth and Production of Aflatoxins in a Synthetic Medium. J. Food Sci. 1989, 54, 74–76. [Google Scholar] [CrossRef]
- Askun, T.; Tümen, G.; Satil, F.; Kılıç, T.; Kilic, T. Effects of Some Lamiaceae Species Methanol Extracts on Potential Mycotoxin Producer Fungi. Pharm. Biol. 2008, 46, 688–694. [Google Scholar] [CrossRef] [Green Version]
- Gotsiou, P.; Naxakis, G.; Skoula, M. Diversity in the composition of monoterpenoids of Origanum microphyllum (Labiatae). Biochem. Syst. Ecol. 2002, 30, 865–879. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kürkçüoǧlu, M.; Demirci, B.; Ozek, T. The essential oil of Origanum syriacum L. var. sinaicum (Boiss.). Flavour Fragr. J. 2003, 18, 98–99. [Google Scholar] [CrossRef]
- Farhat, M.; Toth, J.; Héthelyi, B.É.; Szarka, S.; Czigle, S. Analysis of the Essential Oil Compounds of Origanum syriacum L. Acta Fac. Pharm. Univ. Comen. 2012, 59, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, A.T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Başer, K.H.; Ozek, T.; Kürkçüoğlu, M.; Tümen, G. Composition of the Essential Oil of Origanum sipyleum of Turkish Origin. J. Essent. Oil Res. 1992, 4, 139–142. [Google Scholar] [CrossRef]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus Origanum: A review. J. Pharmacogn. Phytother. 2013, 10, 170–177. [Google Scholar] [CrossRef]
- Çetin, B.; Çakamakci, S.; Cakmakci, R. The investigation of antimicrobial activity of thyme and oregano essential oils. Turk. J. Agric. For. 2011, 35, 145–154. [Google Scholar] [CrossRef]
- Bayramoglu, B.; Sahin, S.; Sumnu, G. Solvent-free microwave extraction of essential oil from oregano. J. Food Eng. 2008, 88, 535–540. [Google Scholar] [CrossRef]
- Assaf, M.H.; Ali, A.A.; Makboul, M.A.; Beck, J.P.; Anton, R. Preliminary Study of Phenolic Glycosides from Origanum majorana, Quantitative Estimation of Arbutin; Cytotoxic Activity of Hydroquinone. Planta Medica 1987, 53, 343–345. [Google Scholar] [CrossRef]
- Lemhadri, A.; Zeggwagh, N.-A.; Maghrani, M.; Jouad, H.; Eddouks, M. Anti-hyperglycaemic activity of the aqueous extract of Origanum vulgare growing wild in Tafilalet region. J. Ethnopharmacol. 2004, 92, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Goun, E.; Cunningham, G.; Solodnikov, S.; Krasnykch, O.; Miles, H. Antithrombin activity of some constituents from Origanum vulgare. Fitoter. 2002, 73, 692–694. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Puertas-Mejía, M.A.; Martínez, J.R. SPME determination of volatile aldehydes for evaluation of in-vitro antioxidant activity. Anal. Bioanal. Chem. 2002, 373, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Martucci, J.; Gende, L.; Neira, L.; Ruseckaite, R. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Flores Santurio, D.; Kunz, d.J.; Pantella, F.; Zanette, R.A.; Schlemmer, B.; Fraton, A.; Fries, M.; Leadir, L. Antimicrobial Activity of the Essential Oil of Thyme and of Thymol against Escherichia coli Strains. Acta Sci. Vet. 2014, 42, 1–4. [Google Scholar]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013, 5, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Kizil, S.; Ipek, A.; Arslan, N.; Khalid, M.K. Effect of different developing stages on some agronomical characteristics and essential oil composition of Oregano (Origanum onites). N. Z. J. Crop. Hortic. Sci. 2008, 36, 71–76. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Özer, H.; Kordali, S.; Cakmakci, R.; Dadaşoğlu, F.; Dikbas, N.; Aydin, T.; Kazaz, C. Antibacterial effects of Origanum onites against phytopathogenic bacteria: Possible use of the extracts from protection of disease caused by some phytopathogenic bacteria. Sci. Hortic. 2014, 172, 210–220. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of Action of Carvacrol on the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [Green Version]
- Alov, P.; Tsakova, I.; Pajeva, I. Computational Studies of Free Radical-Scavenging Properties of Phenolic Compounds. Available online: http://www.eurekaselect.com/126818/article (accessed on 23 December 2018).
- Fernandes, R.; Trindade, M.; Lorenzo, J.; Munekata, P.; De Melo, M. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control. 2016, 63, 65–75. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant capacity variation in the oregano (Origanum vulgare L.) collection of the German National Genebank. Ind. Crop. Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Balkan, B.; Balkan, S.; Aydoğdu, H.; Güler, N.; Ersoy, H.; Aşkın, B. Evaluation of Antioxidant Activities and Antifungal Activity of Different Plants Species Against Pink Mold Rot-Causing Trichothecium roseum. Arab. J. Sci. Eng. 2017, 42, 2279–2289. [Google Scholar] [CrossRef]
- Vichi, S.; Zitterl-Eglseer, K.; Jugl, M.; Franz, C. Determination of the presence of antioxidants deriving from sage and oregano extracts added to animal fat by means of assessment of the radical scavenging capacity by photochemiluminescence analysis. Food Nahr. 2001, 45, 101–104. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Riveros, C.G.; Nepote, V.; Zygadlo, J.A.; Grosso, N.R. Antioxidant activity of essential oil of oregano species from Argentina in relation to their chemical composition. Int. J. Food Sci. Technol. 2011, 46, 2648–2655. [Google Scholar] [CrossRef]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical Composition and Nutraceutical Potential of Indian Borage (Plectranthus amboinicus) Stem Extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, D.R.; Banu, S.; Salim, K.M. Determination of Bioactives and Antioxidant Activity in Eryngium foetidum L.: A Traditional Culinary and Medicinal Herb. Proc. Natl. Acad. Sci. India Sect. B: Boil. Sci. 2012, 83, 453–460. [Google Scholar] [CrossRef]
- Timóteo, P.; Karioti, A.; Leitão, S.G.; Vincieri, F.F.; Bilia, A.R. A validated HPLC method for the analysis of herbal teas from three chemotypes of Brazilian Lippia alba. Food Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Antioxidant and Antimutagenic Activities of Mexican Oregano (Lippia graveolens Kunth). Plant Foods Hum. Nutr. 2007, 63, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lagouri, V.; Alexandri, G. Antioxidant Properties of Greek O. Dictamnus and R. officinalis Methanol and Aqueous Extracts—HPLC Determination of Phenolic Acids. Int. J. Food Prop. 2013, 16, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Çelik, S.E.; Tufan, A.N.; Bekdeşer, B.; Özyürek, M.; Güçlü, K.; Apak, R. Identification and Determination of Phenolics in Lamiaceae Species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2016, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2014, 29, 474–479. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; De Alvarenga, J.F.R.; Martínez-Huélamo, M.; Leal, L.N.; Lamuela-Raventós, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Kogiannou, D.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef]
- Hossain, M.; Barry-Ryan, C.; Martin-Diana, A.; Brunton, N. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Heal. Dis. 2015, 26, 883. [Google Scholar] [CrossRef]
- Goze, I.; Cetin, A.; Goze, A. Investigation of effects of essential oils of Origanum minutiflorum O Schwarz PH Davis and Cyclotrichium niveum (Labiatae) plants on angiogenesis in shell-less chick embryo culture. Afr. J. Biotechnol. 2010, 9, 2156–2160. [Google Scholar]
- Ozen, F.; Ekinci, F.; Korachi, M. The inhibition of Helicobacter pylori infected cells by Origanum minutiflorum. Ind. Crop. Prod. 2014, 58, 329–334. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control. 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Bunghez, F.; Morar, M.A.; Pop, R.M.; Romanciuc, F.; Csernatoni, F.; Fetea, F.; Diaconeasa, Z.; Socaciu, C. Comparative Phenolic Fingerprint and LC-ESI+QTOF-MS Composition of Oregano and Rosemary Hydrophilic Extracts in Relation to their Antibacterial Effect. Bull. Univ. Agric. Sci. Veter. Med. Cluj Napoca. Food Sci. Technol. 2015, 72, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. hirtum. Ind. Crop. Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Pirigharnaei, M.; Zare, S.; Heidary, R.; Khara, J.; Emamali Sabzi, R.; Kheiry, F. The essential oils compositions of Iranian Oregano (Origanum vulgare L.) populations in field and provenance from Piranshahr district, West Azarbaijan province, Iran. Avicenna J. Phytomed. 2011, 1, 106–114. [Google Scholar] [CrossRef]
- Wogiatzi, E.; Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Chouliaras, N. Chemical Composition and Antimicrobial Effects of Greek Origanum Species Essential Oil. Biotechnol. Biotechnol. Equip. 2009, 23, 1322–1324. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Bousmaha-Marroki, L.; Atik-Bekkara, F.; Tomi, F.; Casanova, J. Chemical Composition and Antibacterial Activity of the Essential Oil of Thymus ciliatus (Desf.) Benth. ssp. eu-ciliatus Maire from Algeria. J. Essent. Oil Res. 2007, 19, 490–493. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Efrati, R.; Natan, M.; Pelah, A.; Haberer, A.; Banin, E.; Dotan, A.; Ophir, A. The combined effect of additives and processing on the thermal stability and controlled release of essential oils in antimicrobial films. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Bikouli, V.C.; Gardeli, C.; Mitsi, C.; Tarantilis, P.; Stamatiou, A.; Skandamis, P.N. Effect of single or combined chemical and natural antimicrobial interventions on Escherichia coli O157:H7, total microbiota and color of packaged spinach and lettuce. Int. J. Food Microbiol. 2016, 220, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E.; Henika, P.R. Addition of phytochemical-rich plant extracts mitigate the antimicrobial activity of essential oil/wine mixtures against Escherichia coli O157:H7 but not against Salmonella enterica. Food Control 2017, 73, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Skandamis, P.; Nychas, G.-J. Effect of oregano essential oil on microbiological and physico-chemical attributes of minced meat stored in air and modified atmospheres. J. Appl. Microbiol. 2001, 91, 1011–1022. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage micro-organism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef]
- Fasseas, M.; Mountzouris, K.; Tarantilis, P.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Force, M.; Sparks, W.S.; Ronzio, R.A. Inhibition of enteric parasites by emulsified oil of oregano in vivo. Phytotherapy Res. 2000, 14, 213–214. [Google Scholar] [CrossRef]
- Milhau, G.; Valentin, A.; Benoit, F.; Mallié, M.; Bastide, J.-M.; Pélissier, Y.; Bessière, J.-M. In Vitro Antimalarial Activity of Eight Essential Oils. J. Essent. Oil Res. 1997, 9, 329–333. [Google Scholar] [CrossRef]
- Ponce-Macotela, M.; Rufino-González, Y.; González-Maciel, A.; Reynoso-Robles, R.; Martínez-Gordillo, M.N. Oregano (Lippia spp.) kills Giardia intestinalis trophozoites in vitro: Antigiardiasic activity and ultrastructural damage. Parasitol. Res. 2006, 98, 557–560. [Google Scholar] [CrossRef]
- Ponce-Macotela, M.; Navarro-Alegría, I.; Martínez-Gordillo, M.N.; Alvarez-Chacón, R. In vitro effect against Giardia of 14 plant extracts. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 1994, 46, 343–347. [Google Scholar]
- Rahmatullah, M.; Hossan, S.; Khatun, A.; Seraj, S.; Jahan, R. Medicinal Plants Used by Various Tribes of Bangladesh for Treatment of Malaria. Available online: https://www.hindawi.com/journals/mrt/2012/371798/ (accessed on 22 November 2019).


| Species | Active Compound | Quantity % | Type of Isolation | References |
|---|---|---|---|---|
| O. majorana | carvacrol | 62.6 | conventional steam distillation | [51] |
| thymol | 11.5 | |||
| phenol | 8.2 | |||
| O. vulgare | camphene | 0–1 | conventional steam distillation subcritical water extraction | [52,53] |
| thymol | 3.7 | |||
| carvacrol | 75 | |||
| γ-terpinene | 2–3 | |||
| linalyl-acetate | 0–0.5 | |||
| myrcene | 0–0.05 | |||
| terpinen-4-ol | 0–2.2 | |||
| caryophyllene | 0.9 | |||
| π-cymene | 2.1–3.7 | |||
| O. microphyllum | cis-sabinene | 29.8 | conventional steam distillation | [54] |
| sabinene | 20.6 | |||
| trans-sabinene hydrate | 14.9 | |||
| γ-terpinene | 2.8 | |||
| p-cymene | 2.8 | |||
| linalool | 3.8 | |||
| O. syriacum | thymol | 24–29 | hydrodistillation steam distillation | [55,56] |
| cis-sabinene hydrate | 18–20 | |||
| γ-terpinene | 13–15 | |||
| p-cymene | 5–8 | |||
| terpinen-4-ol | 4–8 | |||
| O. onites | carvacrol | 64 | hydrodistillation steam distillation | [55,56] |
| α-pinene | 1.0 | |||
| thymol | 1.4 | |||
| myrcene | 1.6 | |||
| linalool | 13.8 | |||
| p-cymene | 7.1 | |||
| γ-terpinene | 3.5 | |||
| α-terpinene | 1.4 | |||
| O. dictamnus | carvacrol | 62 | steam distillation | [57] |
| thymol | 0.44 | |||
| p-cymene | 13.49 | |||
| γ-terpinene | 11.41 | |||
| r-terpinene | 1.88 | |||
| O. sipyleum (L.) | γ-terpinene | 10.8–26.6 | steam distillation | [58] |
| thymol | 0.23–7.3 | |||
| carvacrol | 0.82–12.2 | |||
| p-cymene | 3.7–36.6 | |||
| O. acutidens | carvacrol | 72 | hydrodistillation | [59] |
| p-cymene, | 7.5 | |||
| γ-terpinene | 5.3 | |||
| borneol | 0.9 | |||
| thymol | 0.2 | |||
| O. compactum | carvacrol | 30.53 | hydrodistillation | [59] |
| p-cymene | 7.89 | |||
| α-terpinene | 2.59 | |||
| thymol | 17.5 | |||
| γ-terpinene | 18.2 | |||
| O. floribundum | carvacrol | 63 | hydrodistillation | [59] |
| p-cymene | 6.3 | |||
| linalool | 5.6 | |||
| γ-terpinene | 16.2 | |||
| O. rotundifolium | carvacrol | 54.6 | hydrodistillation | [60] |
| p-cymene | 12.5 | |||
| borneol | 5.9 | |||
| thymol | 3.5 | |||
| linalool | 1.8 | |||
| terpinene-4-ol | 1.5 | |||
| thymohydroquinone | 1.14 | |||
| β-Caryophyllene | 1.09 | |||
| germacrene D | 1.08 | |||
| linalyl acetate | 1.07 | |||
| O. minutiflorum | carvacrol | 78.8 | hydrodistillation | [61] |
| γ-terpinene | 3.7 | |||
| p-cymene | 3.5 | |||
| thymol | 1.1 | |||
| α-pinene | 1.3 |
| Species | Biological Activity |
|---|---|
| O. majorana | antioxidant [51] antiproliferative [51] antibacterial [51] cytotoxic [62,63] |
| O. vulgare | antiproliferative activity on tumor cells of Hela [10,17] antiradical [51] antifungal [52,53] antihyperglycaemic [63] antithrombin [64] antioxidant [65,66] antimicrobal [67,68] |
| O. microphyllum | antioxidant [54] |
| O. syriacum | antibacterial [55,56] antimycotic [55,56] antioxidative [55,56] |
| O. onites | antibacterial [55,56] antimycotic [55,56] antioxidative [55,56] antispasmodic [69] antibacterial [70,71] antifungal [72] |
| O. dictammus | antibacterial [57] antifungal [57] |
| O. sipyleum (L.) | effective on respiratory and gastrointestinal disorders [58] |
| O. acutidens | antibacterial [59] antifungal [59] antioxidant [59] |
| O. compactum | antioxidant [59] antifungal [59] cytotoxic [59] |
| O. floribundum | against diarrhoea and other digestive disorders [59] |
| O. rotundifolium | antibacterial [60] |
| O. minutiflorum | antibacterial [61] |
| Oregano Species | Components | Extraction Solvent Used | Ref. |
|---|---|---|---|
| Coleus aromaticus |
| Methanol | [81] |
| Eryngium foetidum |
| Aqueous, methanol | [82] |
| Lippia alba |
| Aqueous | [83] |
| Lippia graveolens |
| Methanol | [84] |
| Methanol | [19] | |
| Origanum dictamnus |
| Sequentially with hexane, acetone and methanol; Aqueous | [85] |
| Aqueous | [25] | |
| Origanum glandulosum |
| Methanol, previously defatted with n-hexane | [85] |
| Origanum majorana |
| Methanol microwave-assisted | [86] |
| Aqueous, methanol | [87] | |
| Methanol | [19] | |
| Methanol | [21] | |
| Ethanol | [88] | |
| Origanum microphyllum |
| Aqueous | [89] |
| Origanum vulgare |
| Methanol | [77] |
| Methanol | [31] | |
| Water, methanol, ethyl acetate, hexane | [31] | |
| Methanol | [19] | |
| Origanum vulgare |
| Methanol | [90] |
| Aqueous | [76] | |
| Thymbra capitata |
| Ethyl acetate, ethanol | [17] |
| Species | Bacteria Inhibition | Ref. |
|---|---|---|
| O. minutiflorum |
| [93,94] |
| O. vulgare |
| [66,95,96,97,98,99,100,101,102] |
| O. majorana |
| [95,103,104] |
| O. compactum |
| [13,18] |
| O. syriacum |
| [101] |
| O. onites |
| [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knez Hrnčič, M.; Cör, D.; Simonovska, J.; Knez, Ž.; Kavrakovski, Z.; Rafajlovska, V. Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species. Molecules 2020, 25, 4735. https://doi.org/10.3390/molecules25204735
Knez Hrnčič M, Cör D, Simonovska J, Knez Ž, Kavrakovski Z, Rafajlovska V. Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species. Molecules. 2020; 25(20):4735. https://doi.org/10.3390/molecules25204735
Chicago/Turabian StyleKnez Hrnčič, Maša, Darija Cör, Jana Simonovska, Željko Knez, Zoran Kavrakovski, and Vesna Rafajlovska. 2020. "Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species" Molecules 25, no. 20: 4735. https://doi.org/10.3390/molecules25204735
APA StyleKnez Hrnčič, M., Cör, D., Simonovska, J., Knez, Ž., Kavrakovski, Z., & Rafajlovska, V. (2020). Extraction Techniques and Analytical Methods for Characterization of Active Compounds in Origanum Species. Molecules, 25(20), 4735. https://doi.org/10.3390/molecules25204735