Assessment of Intuitiveness and Comfort of Wearable Haptic Feedback Strategies for Assisting Level and Stair Walking
Abstract
1. Introduction
2. Materials and Methods
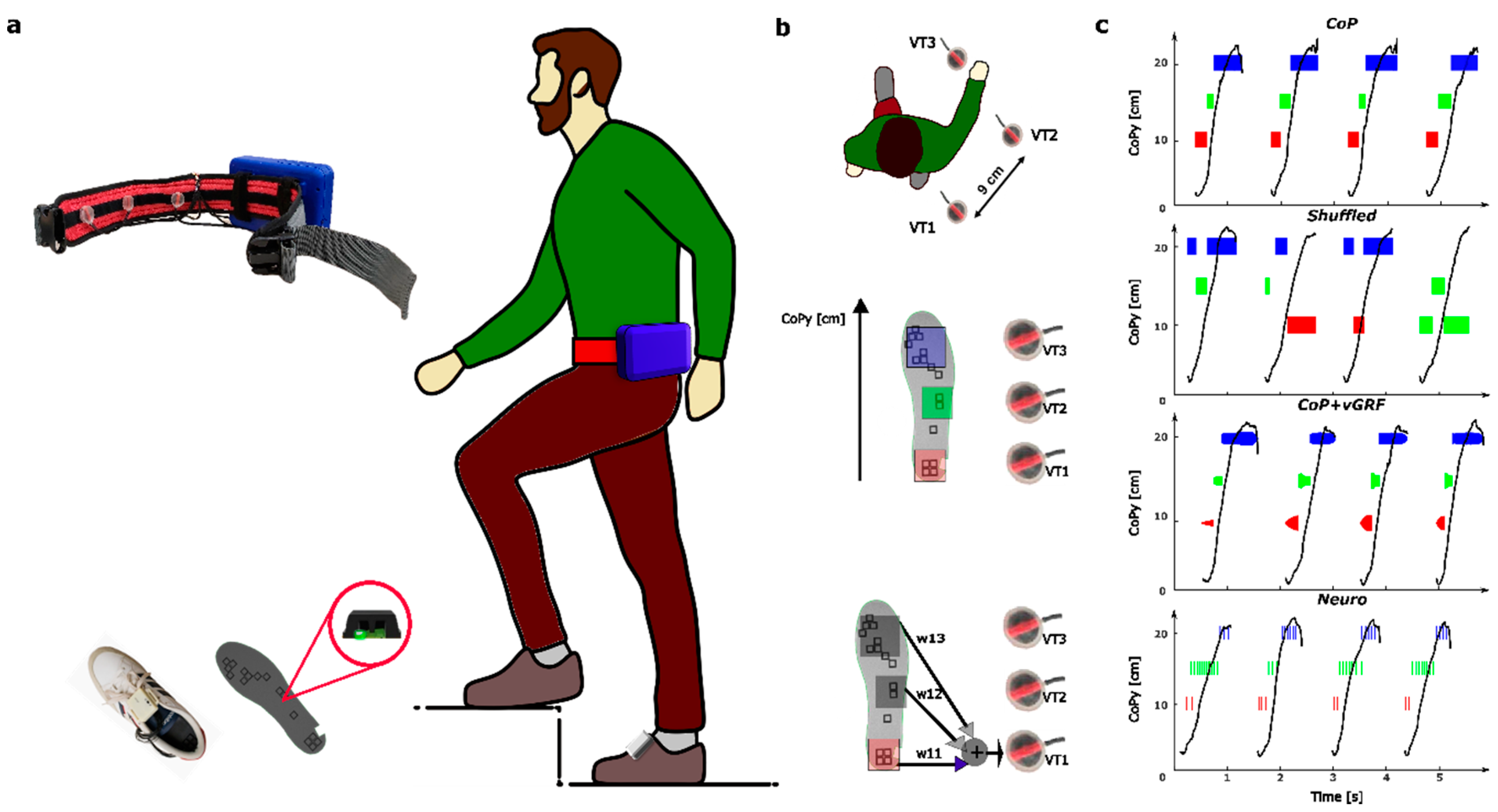
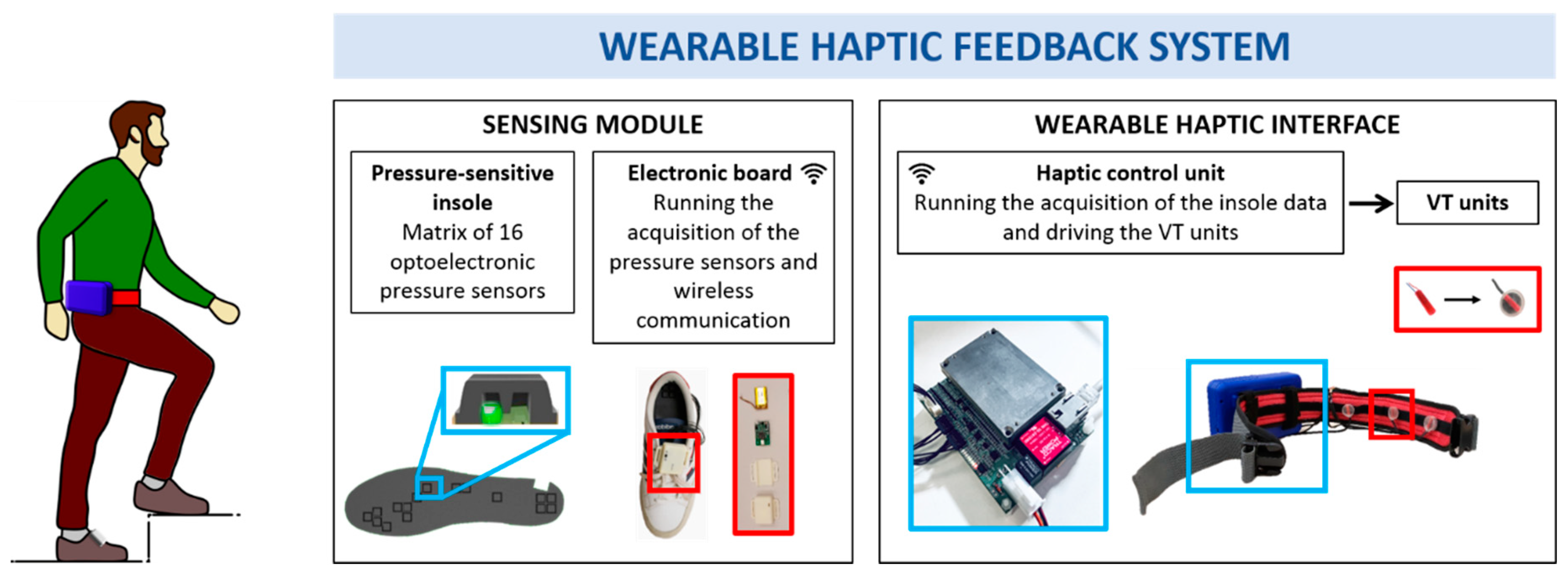
2.1. Wearable Haptic Feedback System
2.2. Stimulation Strategies
2.3. Experimental Protocol
2.4. Elo-Based Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, R.E.; Culjat, M.O.; King, C.-H.; Franco, M.L.; Boryk, R.; Bisley, J.W.; Dutson, E.; Grundfest, W.S. A Haptic Feedback System for Lower-Limb Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Sie, A.; Boe, D.; Rombokas, E. Design and Evaluation of a Wearable Haptic Feedback System for Lower Limb Prostheses During Stair Descent. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 219–224. [Google Scholar]
- Foumani, M.; Smith-Miles, K.; Gunawan, I. Scheduling of two-machine robotic rework cells: In-process, post-process and in-line inspection scenarios. Rob. Auton. Syst. 2017. [Google Scholar] [CrossRef]
- Aboseria, M.; Clemente, F.; Engels, L.F.; Cipriani, C. Discrete Vibro-Tactile Feedback Prevents Object Slippage in Hand Prostheses More Intuitively Than Other Modalities. IEEE Trans. Neural Syst. Rehabil. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grazi, L.; Crea, S.; Parri, A.; Molino Lova, R.; Micera, S.; Vitiello, N. Gastrocnemius Myoelectric Control of a Robotic Hip Exoskeleton Can Reduce the User’s Lower-Limb Muscle Activities at Push Off. Front. Neurosci. 2018, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Saracino, A.; Deguet, A.; Staderini, F.; Boushaki, M.N.; Cianchi, F.; Menciassi, A.; Sinibaldi, E. Haptic feedback in the da Vinci Research Kit (dVRK): A user study based on grasping, palpation and incision tasks. Int. J. Med. Robot. Comput. Assist. Surg. 2019, e1999. [Google Scholar] [CrossRef]
- Shull, P.B.; Damian, D.D. Haptic wearables as sensory replacement, sensory augmentation and trainer—A review. J. Neuroeng. Rehabil. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rusaw, D.; Hagberg, K.; Nolan, L.; Ramstrand, N. Can vibratory feedback be used to improve postural stability in persons with transtibial limb loss? J. Rehabil. Res. Dev. 2012, 49, 1239. [Google Scholar] [CrossRef]
- Plauche, A.; Villarreal, D.; Gregg, R.D. A Haptic Feedback System for Phase-Based Sensory Restoration in Above-Knee Prosthetic Leg Users. IEEE Trans. Haptics 2016, 9, 421–426. [Google Scholar] [CrossRef]
- Pagel, A.; Arieta, A.H.; Riener, R.; Vallery, H. Effects of sensory augmentation on postural control and gait symmetry of transfemoral amputees: A case description. Med. Biol. Eng. Comput. 2016, 54, 1579–1589. [Google Scholar] [CrossRef]
- Peterka, R.J.; Wall, C., III; Kentala, E. Determining the effectiveness of a vibrotactile balance prosthesis. J. Vestib. Res. Equilib. Orientat. 2006, 16, 45–56. [Google Scholar]
- Duclos, C.; Roll, R.; Kavounoudias, A.; Roll, J.-P.; Forget, R. Vibration-induced post-effects: A means to improve postural asymmetry in lower leg amputees? Gait Posture 2007, 26, 595–602. [Google Scholar] [CrossRef]
- Zambarbieri, D.; Schmid, M.; Verni, G. Sensory feedback for lower limb prostheses. In Proceedings of the Intelligent Systems and Technologies in Rehabilitation Engineering; CRC Press, Inc.: Boca Raton, FL, USA, 2001; pp. 129–151. [Google Scholar]
- Lauretti, C.; Pinzari, G.; Ciancio, A.L.; Davalli, A.; Sacchetti, R.; Sterzi, S.; Guglielmelli, E.; Zollo, L. A vibrotactile stimulation system for improving postural control and knee joint proprioception in lower-limb amputees. In Proceedings of the RO-MAN 2017—26th IEEE International Symposium on Robot and Human Interactive Communication, Lisbon, Portugal, 28 August–1 September 2017; pp. 88–93. [Google Scholar]
- Sienko, K.H.; Balkwill, M.; Oddsson, L.I.E.; Wall, C. The effect of vibrotactile feedback on postural sway during locomotor activities. J. Neuroeng. Rehabil. 2013, 10, 93. [Google Scholar] [CrossRef]
- Sabolich, J.A.; Ortega, G.M. Sense of Feel for Lower-Limb Amputees: A Phase-One Study. JPO J. Prosthet. Orthot. 1994, 6, 36–41. [Google Scholar] [CrossRef]
- Yang, L.; Dyer, P.S.; Carson, R.J.; Webster, J.B.; Bo Foreman, K.; Bamberg, S.J.M. Utilization of a lower extremity ambulatory feedback system to reduce gait asymmetry in transtibial amputation gait. Gait Posture 2012, 36, 631–634. [Google Scholar] [CrossRef]
- Crea, S.; Edin, B.B.; Knaepen, K.; Meeusen, R.; Vitiello, N. Time-Discrete Vibrotactile Feedback Contributes to Improved Gait Symmetry in Patients With Lower Limb Amputations: Case Series. Phys. Ther. 2017, 97, 198–207. [Google Scholar] [CrossRef]
- Jones, L.A.; Sarter, N.B. Tactile displays: Guidance for their design and application. Hum. Factors J. Hum. Factors Ergon. Soc. 2008, 50, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.R.; Olivier, J.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; Del Millán, J.R.; Riener, R.; Vallery, H.; Gassert, R. Control strategies for active lower extremity prosthetics and orthotics: A review. J. Neuroeng. Rehabil. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Canino, J.M.; Fite, K.B. Haptic feedback in lower-limb prosthesis: Combined haptic feedback and EMG control of a powered prosthesis. In Proceedings of the 2016 IEEE EMBS International Student Conference (ISC), Ottawa, ON, Canada, 29–31 May 2016; pp. 1–4. [Google Scholar]
- Husman, M.A.B.; Maqbool, H.F.; Awad, M.I.; Dehghani-Sanij, A.A. Portable haptic device for lower limb amputee gait feedback: Assessing static and dynamic perceptibility. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–21 July 2017; pp. 1562–1566. [Google Scholar]
- Cesini, I.; Spigler, G.; Prasanna, S.; Taxis, D.; Dell’Agnello, F.; Martini, E.; Crea, S.; Vitiello, N.; Mazzoni, A.; Oddo, C.M. A wearable haptic feedback system for assisting lower-limb amputees in multiple locomotion tasks. In Biosystems and Biorobotics; Springer: Cham, Switzerland, 2019; Volume 22, pp. 115–119. [Google Scholar]
- Leong, J.; Parzer, P.; Perteneder, F.; Babic, T.; Rendl, C.; Vogl, A.; Egger, H.; Olwal, A.; Haller, M. proCover: Sensory Augmentation of Prosthetic Limbs Using Smart Textile Covers. In Proceedings of the 29th Annual Symposium on User Interface Software and Technology—UIST ’16, Tokyo, Japan, 16–19 October 2016; ACM Press: New York, NY, USA, 2016; pp. 335–346. [Google Scholar]
- Filosa, M.; Cesini, I.; Martini, E.; Spigler, G.; Vitiello, N.; Oddo, C.; Crea, S. A new sensory feedback system for lower-limb amputees: Assessment of discrete vibrotactile stimuli perception during walking. In Biosystems and Biorobotics; Springer: Cham, Switzerland, 2019; Volume 22, pp. 105–109. [Google Scholar]
- Sie, A.; Realmuto, J.; Rombokas, E. A Lower Limb Prosthesias Haptic Feedback System for Stir Descent. In Proceedings of the 2017 Design of Medical Devices Conference, Minneapolis, MN, USA, 10–13 April 2017; ASME: New York, NY, USA; p. V001T05A004. [Google Scholar]
- Crea, S.; Cipriani, C.; Donati, M.; Carrozza, M.C.; Vitiello, N. Providing Time-Discrete Gait Information by Wearable Feedback Apparatus for Lower-Limb Amputees: Usability and Functional Validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.E.; Wottawa, C.; Mulgaonkar, A.; Boryk, R.J.; Sander, T.C.; Wyatt, M.P.; Dutson, E.; Grundfest, W.S.; Culjat, M.O. Pilot testing of a haptic feedback rehabilitation system on a lower-limb amputee. In Proceedings of the 2009 ICME International Conference on Complex Medical Engineering, Tempe, AZ, USA, 9–11 April 2009; pp. 1–4. [Google Scholar]
- Husman, M.A.B. A Haptic Feedback System for Lower Limb Amputees Based on Gait Event Detection. Ph.D. Thesis, University of Leeds, Leeds, UK, 2017. [Google Scholar]
- Rongala, U.B.; Mazzoni, A.; Oddo, C.M. Neuromorphic Artificial Touch for Categorization of Naturalistic Textures. IEEE Trans. Neural Netw. Learn. Syst. 2015, 1–11. [Google Scholar] [CrossRef]
- Joucla, S.; Ambroise, M.; Levi, T.; Lafon, T.; Chauvet, P.; Saïghi, S.; Bornat, Y.; Lewis, N.; Renaud, S.; Yvert, B. Generation of Locomotor-Like Activity in the Isolated Rat Spinal Cord Using Intraspinal Electrical Microstimulation Driven by a Digital Neuromorphic CPG. Front. Neurosci. 2016, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Oddo, C.M.; Raspopovic, S.; Artoni, F.; Mazzoni, A.; Spigler, G.; Petrini, F.; Giambattistelli, F.; Vecchio, F.; Miraglia, F.; Zollo, L.; et al. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 2016, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Spigler, G.; Oddo, C.M.; Carrozza, M.C. Soft-neuromorphic artificial touch for applications in neuro-robotics. In Proceedings of the IEEE RAS EMBS International conference on biomedical robotics and biomechatronics (BioRob), Rome, Italy, 24 June 2012; pp. 1913–1918. [Google Scholar] [CrossRef]
- Oddo, C.M.; Mazzoni, A.; Spanne, A.; Enander, J.M.D.; Mogensen, H.; Bengtsson, F.; Camboni, D.; Micera, S.; Jörntell, H. Artificial spatiotemporal touch inputs reveal complementary decoding in neocortical neurons. Sci. Rep. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Oh, M.K.; Lee, C.H.; Park, Y.S.; Yoon, J. A Portable Gait Asymmetry Rehabilitation System for Individuals with Stroke Using a Vibrotactile Feedback. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Rokhmanova, N.; Rombokas, E. Vibrotactile Feedback Improves Foot Placement Perception on Stairs for Lower-Limb Prosthesis Users. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR) 2019, Toronto, ON, Canada, 24 June 2019; pp. 1215–1220. [Google Scholar]
- Tsang, C.S.C.; Ngan, H.Y.T.; Pang, G.K.H. Fabric inspection based on the Elo rating method. Pattern Recognit. 2016. [Google Scholar] [CrossRef]
- Elo, A.E. The Rating of Chess Players, Past & Present; Arco Pub.: New York, NY, USA, 1978; ISBN 9780923891275. [Google Scholar]
- Teh, K.C.; Aziz, A.R. Heart rate, oxygen uptake, and energy cost of ascending and descending the stairs. Med. Sci. Sports Exerc. 2002. [Google Scholar] [CrossRef]
- Riener, R.; Rabuffetti, M.; Frigo, C. Stair ascent and descent at different inclinations. Gait Posture 2002. [Google Scholar] [CrossRef]
- Al Kandari, J.R.; Mohammad, S.; Al-Hashem, R.; Telahoun, G.; Barac-Nieto, M. Practical Use of Stairs to Assess Fitness, Prescribe and Perform Physical Activity Training. Health 2016. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Bassett, D.R.; Vachon, J.A.; Kirkland, A.O.; Howley, E.T.; Duncan, G.E.; Johnson, K.R. Energy cost of stair climbing and descending on the college alumnus questionnaire. Med. Sci. Sports Exerc. 1997, 29, 1250–1254. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Tan, C.W.; Mukherjee, M.; Davidson, A.J.; Stergiou, N. Biomechanical analyses of stair-climbing while dual-tasking. J. Biomech. 2015. [Google Scholar] [CrossRef]
- Cesini, I.; Martini, E.; Filosa, M.; Spigler, G.; Sabatini, A.M.; Vitiello, N.; Oddo, C.M.; Crea, S. Perception of time-discrete haptic feedback on the waist is invariant with gait events. IEEE Trans. Neural Syst. Rehabil. Eng. 2020. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Fiumalbi, T.; Dell’agnello, F.; Ivanić, Z.; Munih, M.; Vitiello, N.; Crea, S. Pressure-sensitive insoles for real-time gait-related applications. Sensors 2020, 20, 1448. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Simple model of spiking neurons. IEEE Trans. Neural Networks 2003, 14, 1569–1572. [Google Scholar] [CrossRef]
- Prasanna, S.; Massari, L.; Sinibaldi, E.; Detry, R.J.; Bowkett, J.; Carpenter, K.C.; Oddo, C.M. Neuromorphic tactile sensor array based on fiber Bragg gratings to encode object qualities. Opt. Photonics Inf. Process. XIII 2019, 6. [Google Scholar] [CrossRef]
- Gunasekaran, H.; Spigler, G.; Mazzoni, A.; Cataldo, E.; Oddo, C.M. Convergence of regular spiking and intrinsically bursting Izhikevich neuron models as a function of discretization time with Euler method. Neurocomputing 2019. [Google Scholar] [CrossRef]
- Sorgini, F.; Massari, L.; D’Abbraccio, J.; Palermo, E.; Menciassi, A.; Petrovic, P.; Mazzoni, A.; Carrozza, M.; Newell, F.; Oddo, C.; et al. Neuromorphic Vibrotactile Stimulation of Fingertips for Encoding Object Stiffness in Telepresence Sensory Substitution and Augmentation Applications. Sensors 2018, 18, 261. [Google Scholar] [CrossRef]
- D’Abbraccio, J.; Massari, L.; Prasanna, S.; Baldini, L.; Sorgini, F.; Airò Farulla, G.; Bulletti, A.; Mazzoni, M.; Capineri, L.; Menciassi, A.; et al. Haptic Glove and Platform with Gestural Control For Neuromorphic Tactile Sensory Feedback In Medical Telepresence †. Sensors 2019, 19, 641. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |






| Feedback | Intuitiveness M [Q1, Q3] | Comfort M [Q1, Q3] |
|---|---|---|
| CoP | 68.23 [38.98, 140.52] | −56.24 [−81.39, 14.23] |
| CoP + vGRF | 72.9 [10.76, 149.16] | 56.08 [−16.49, 110.4] |
| Shuffled | −51.91 [−173.8, 62.79] | −71.74 [−137.01, −30.14] |
| Neuro | −111.82 [−168.34, 22.27] | 52.48 [−33.43, 179.86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesini, I.; Spigler, G.; Prasanna, S.; D’Abbraccio, J.; De Luca, D.; Dell’Agnello, F.; Crea, S.; Vitiello, N.; Mazzoni, A.; Oddo, C.M. Assessment of Intuitiveness and Comfort of Wearable Haptic Feedback Strategies for Assisting Level and Stair Walking. Electronics 2020, 9, 1676. https://doi.org/10.3390/electronics9101676
Cesini I, Spigler G, Prasanna S, D’Abbraccio J, De Luca D, Dell’Agnello F, Crea S, Vitiello N, Mazzoni A, Oddo CM. Assessment of Intuitiveness and Comfort of Wearable Haptic Feedback Strategies for Assisting Level and Stair Walking. Electronics. 2020; 9(10):1676. https://doi.org/10.3390/electronics9101676
Chicago/Turabian StyleCesini, Ilaria, Giacomo Spigler, Sahana Prasanna, Jessica D’Abbraccio, Daniela De Luca, Filippo Dell’Agnello, Simona Crea, Nicola Vitiello, Alberto Mazzoni, and Calogero Maria Oddo. 2020. "Assessment of Intuitiveness and Comfort of Wearable Haptic Feedback Strategies for Assisting Level and Stair Walking" Electronics 9, no. 10: 1676. https://doi.org/10.3390/electronics9101676
APA StyleCesini, I., Spigler, G., Prasanna, S., D’Abbraccio, J., De Luca, D., Dell’Agnello, F., Crea, S., Vitiello, N., Mazzoni, A., & Oddo, C. M. (2020). Assessment of Intuitiveness and Comfort of Wearable Haptic Feedback Strategies for Assisting Level and Stair Walking. Electronics, 9(10), 1676. https://doi.org/10.3390/electronics9101676






