Current Developments in Lignocellulosic Biomass Conversion into Biofuels Using Nanobiotechology Approach
Abstract
:1. Introduction
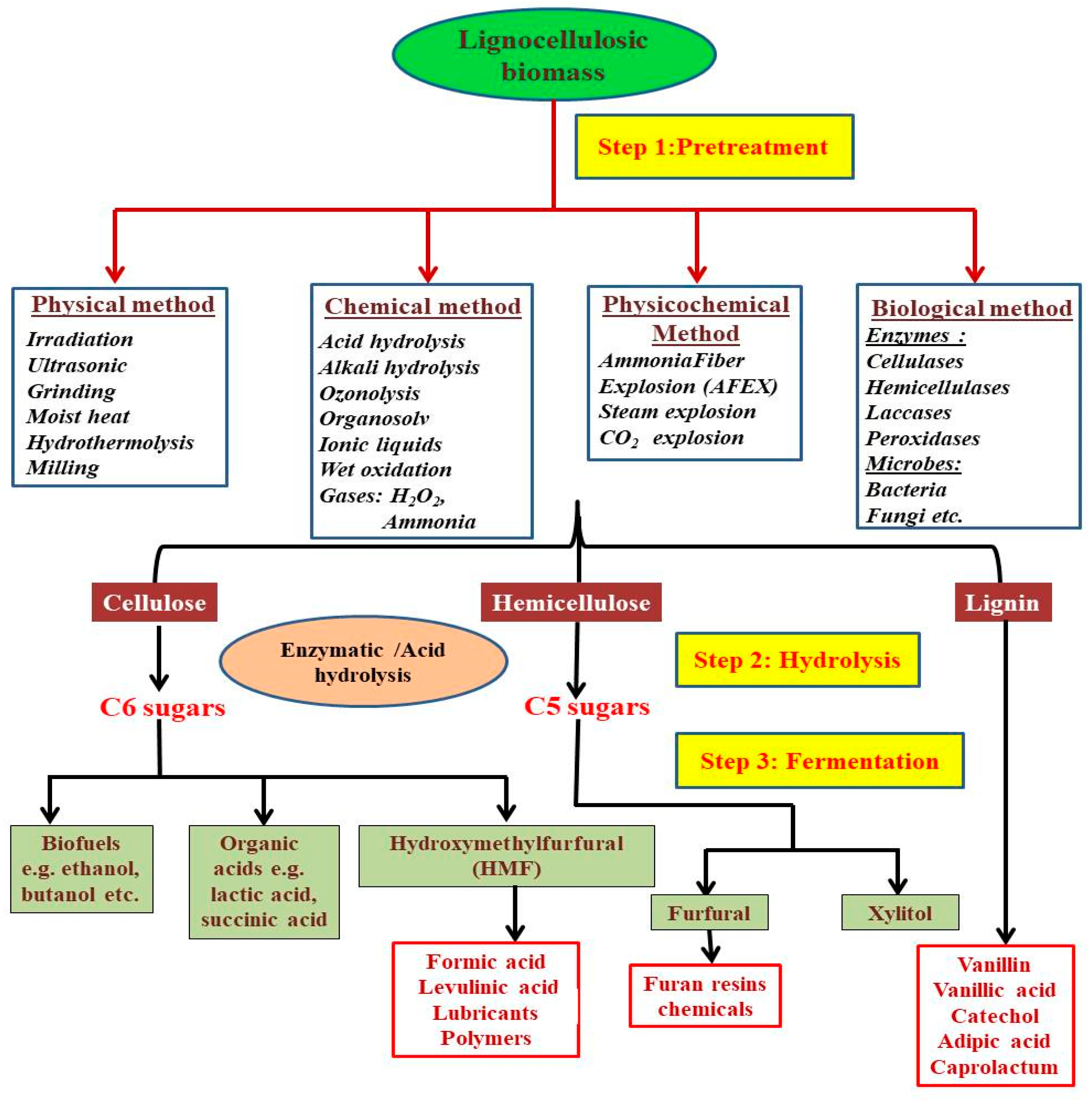
2. Lignocellulosic Biomass Pre-Treatment
Pre-Treatment of Lignocellulosic Biomass Using Nanobiotechology Approach
3. Lignocellulosic Biomass Hydrolysis
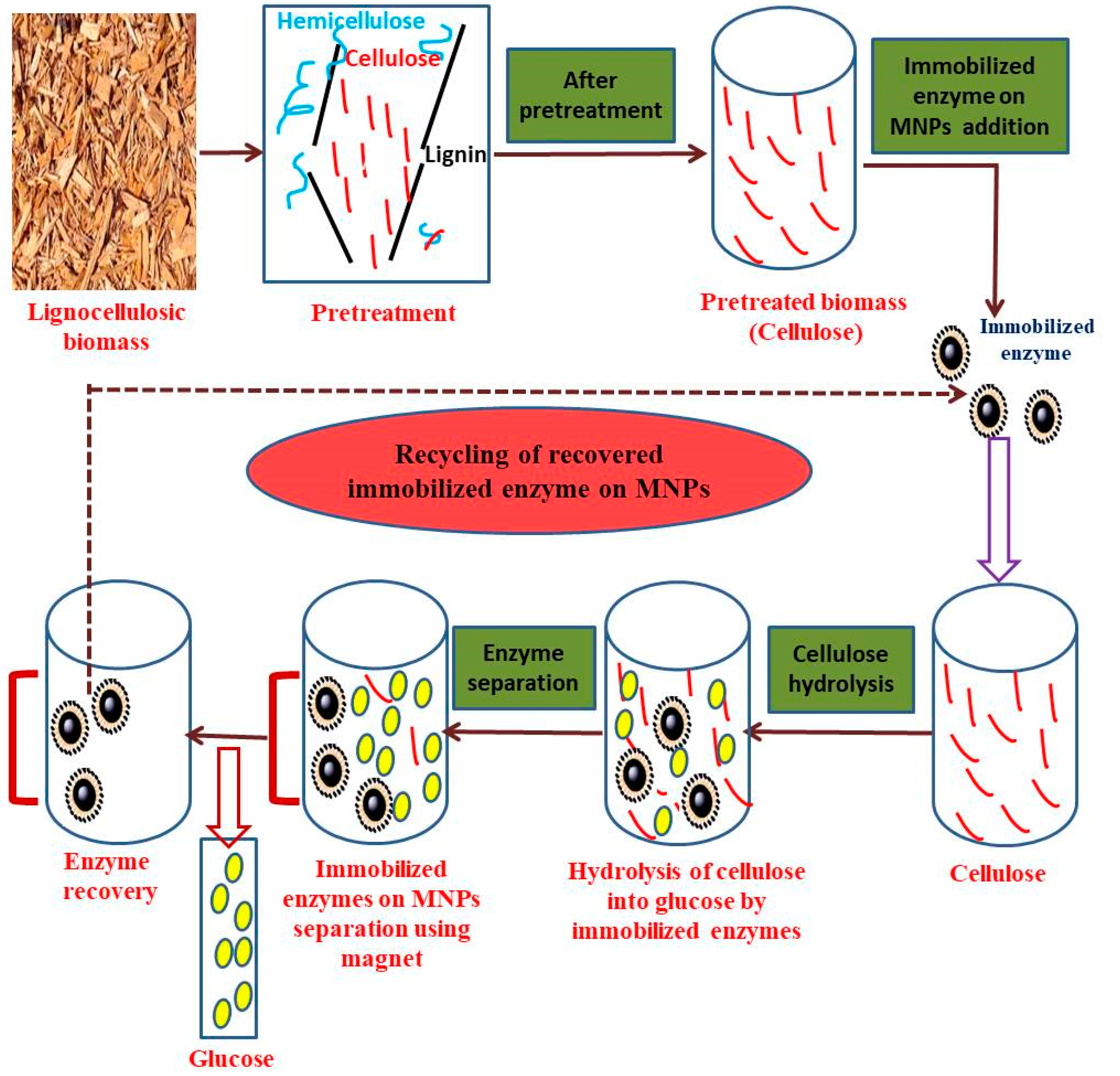
Lignocellulosic Biomass Hydrolysis Using Nanobiotechnology Approach
4. Effect of Nanomaterials on Enzyme Properties
5. Enzyme Immobilization
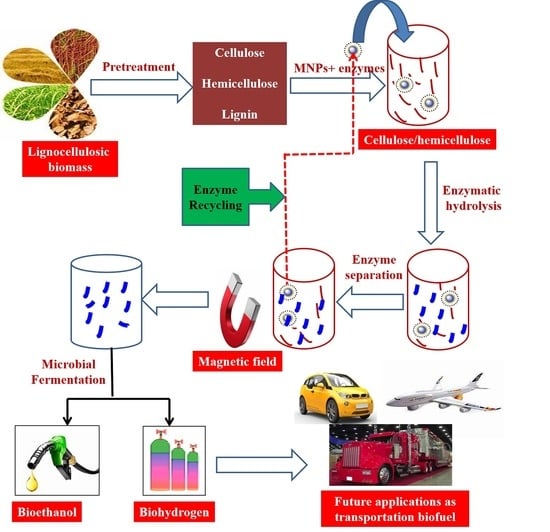
6. Microbial Fermentation for Biofuel Production Using Nanobiocatalyst
6.1. Bioethanol Production Using Various Nanomaterials
6.2. Biohydrogen Production Using Nanomaterials
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LB | Lignocellulosic biomass |
| NPs | Nanoparticles |
| MNPs | Magnetic nanoparticles |
| NCs | Nanocomposites |
| Fe3O4 | Iron (III) oxide |
| Fe2O3 | Ferric oxide |
| FeO | Ferrous oxide |
| FeNPs | Iron nanoparticles |
| SiNPs | Silica nanoparticles |
| NiNPs | Nickel nanoparticles |
| MgNPs | Magnesium nanoparticles |
| ZnO | Zinc oxide |
| NiCo2O4 | Nickel cobaltite |
| TiO2 | Titanium dioxide |
| LiP | Lignin peroxidase |
| MnP | Manganese peroxidase |
| SSAF | Simultaneous saccharification and fermentation |
References
- Rittmann, B.E. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.; Anbalagan, K.; Rajaguru, P.; Pugalenthi, V. Effects of phytogenic copper nanoparticles on fermentative hydrogen production by Enterobacter cloacae and clostridium acetobutylicum. Int. J. Hydrogen Energy 2016, 41, 10639–10645. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Ali, D.M.; Kathiresan, K.; Thajuddin, N.; Chen, J. Biogenic metallic nanoparticles as catalyst for bioelectricity production: A novel approach in microbial fuel cells. Mater. Sci. Eng. B 2016, 203, 27–34. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M. Value-Added Bioproducts Development from Lignocellulosic Biomass; Auburn University: Auburn, AL, USA, 2014. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Clark, D.P.; Pazdernik, N.J. Basics of Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–31. [Google Scholar]
- Li, C.; Aston, J.E.; Lacey, J.A.; Thompson, V.S.; Thompson, D.N. Impact of feedstock quality and variation on biochemical and thermochemical conversion. Renew. Sustain. Energy Rev. 2016, 65, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Rubin, E.M. Genomics of cellulosic biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [PubMed]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pre-treatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pre-treatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; da Silva, S.S. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal. Rev. 2019, 61, 1–26. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gaikwad, S.; Dussán, K.J.; da Silva, S.S. Role of nanoparticles in enzymatic hydrolysis of lignocellulose in ethanol. In Nanotechnology for Bioenergy and Biofuel Production, 2nd ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2017; pp. 153–171. [Google Scholar]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Boyer, A.; Subramanian, S. Bioconversion of lignocellulosic biomass to fermentable sugars by immobilized magnetic cellulolytic enzyme cocktails. Langmuir 2018, 34, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, J.; Feng, H.; Zang, L.; Sakai, E. Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J. Magn. Magn. Mater. 2015, 375, 117–123. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 2017, 22, 269. [Google Scholar] [CrossRef]
- Verma, M.L. Nanobiotechnology advances in enzymatic biosensors for the agri-food industry. Environ. Chem. Lett. 2017, 15, 555–560. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Chandel, A.K.; Teran-Hilares, R.; Ingle, A.P.; Rai, M.; dos Santos Milessi, T.S.; da Silva, S.S.; dos Santos, J.C. Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: Emerging role of nano, biotechnological and promising approaches. 3 Biotech 2019, 9, 230. [Google Scholar] [CrossRef]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. J. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Joshi, M.; Nigam, S.; Shree, S.; Avasthi, D.K.; Adelung, R.; Srivastava, S.K.; Mishra, Y. ZnO tetrapods and activated carbon based hybrid composite: Adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution. Chem. Eng. J. 2019, 358, 540–551. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Manikanta, A.; Singh, P.; Ramteke, P.W.; Mishra, P.K. Nanomaterials for biofuel production using lignocellulosic waste. Environ. Chem. Lett. 2017, 15, 179–184. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pre-treatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Martín, C.; da Silva, V.F.N.; Gómez, E.O.; Gonçalves, A.R. Mass balance of pilot-scale pre-treatment of sugarcane bagasse by steam explosion followed by alkaline delignification. Bioresour. Technol. 2012, 111, 447–452. [Google Scholar] [CrossRef]
- Furlan, F.F.; Filho, R.T.; Pinto, F.H.P.B.; Costa, C.B.B.; Cruz, A.J.G.; Giordano, R.L.C.; Giordano, R.C. Bioelectricity versus bioethanol from sugarcane bagasse: Is it worth being flexible? Biotechnol. Biofuels 2013, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Leidy, P.; Ikenberry, M.; Hohn, K.L.; Wang, D. Acid-functionalized nanoparticles for pre-treatment of wheat straw. J. Biomater. Nanobiotechnol. 2012, 3, 342–352. [Google Scholar]
- Khalid, M.J. Effect of Alkaline Pretreatment and Iron Oxide Nanoparticles on Biogas Production from Rice Straw; National University of Sciences and Technology (NUST): Islamabad, Pakistan, 2018. [Google Scholar]
- Kumar, A.; Purohit, B.; Maurya, P.K.; Pandey, L.M.; Chandra, P. Engineered nanomaterial assisted signal-amplification strategies for enhancing analytical performance of electrochemical biosensors. Electroanalysis 2019, 31, 1615–1629. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Enzyme immobilization on chitin and chitosan-based supports for biotechnological applications. In Sustainable Agriculture Reviews, 2nd ed.; Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 147–173. [Google Scholar]
- Bootsma, J.A.; Shanks, B.H. Cellobiose hydrolysis using organic–inorganic hybrid mesoporous silica catalysts. Appl. Catal. A 2007, 327, 44–51. [Google Scholar] [CrossRef]
- Zhang, M.; Cushing, B.L.; O’Connor, C.J. Synthesis and characterization of monodisperse ultra-thin silica-coated magnetic nanoparticles. Nanotechnology 2008, 19, 085601. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Dutta, N.; Saha, M.K. Nanoparticle-induced enzyme pretreatment method for increased glucose production from lignocellulosic biomass under cold conditions. J. Sci. Food Agric. 2019, 99, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Joo, H.S.; Yang, Y.H. Biowaste-to-bioenergy using biological methods—A mini-review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neil, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Mittal, A.; Black, S.K.; Vinzant, T.B.; O’Brien, M.; Tucker, M.P.; Johnson, D.K. Production of furfural from process-relevant biomass-derived pentoses in a biphasic reaction system. ACS Sustain. Chem. Eng. 2017, 5, 5694–5701. [Google Scholar] [CrossRef]
- Perna, M.D.S.C.; Bastos, R.G.; Ceccato-Antonini, S.R. Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 2018, 8, 119. [Google Scholar] [CrossRef]
- Reynaldo, P.B.; Adriano, E.; Marcelo, M.; Silvia, N. Enzymatic hydrolysis of sugarcane biomass and heat integration as enhancers of ethanol production. J. Renew. Mater. 2018, 6, 183–194. [Google Scholar]
- Chen, H.Z.; Liu, Z.H. Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, V.; Verma, P. An overview of key pre-treatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. 2013, 3, 415–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verardi, A.; De Bari, I.; Ricca, E.; Calabrò, V. Hydrolysis of lignocellulosic biomass: Current status of processes and technologies and future perspectives. In Bioethanol, 1st ed.; Lima, M.A.P., Natalense, A.P.P., Eds.; Intech Publisher: London, UK, 2012; p. 100. [Google Scholar]
- De Souza, W.R. Microbial Degradation of lignocellulosic biomass. In Sustainable Degradation of Lignocellulosic Biomass: Techniques, Applications and Commercialization, 2nd ed.; Chandel, A.K., Da Silva, S.S., Eds.; InTech Publisher: London, UK, 2013; Volume 9, pp. 1–13. [Google Scholar]
- Victoria, J.; Odaneth, A.; Lali, A. Importance of cellulase cocktails favouring hydrolysis of cellulose. Prep. Biochem. Biotechnol. 2017, 47, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lei, T.; Yang, Y.; Wu, N.; Su, P.; Yang, Y. Attachment of enzymes to hydrophilic magnetic nanoparticles through DNA-directed immobilization with enhanced stability and catalytic activity. New J. Chem. 2018, 42, 8458–8468. [Google Scholar] [CrossRef]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar] [CrossRef] [Green Version]
- Ingle, A.P.; Rathod, J.; Pandit, R.; da Silva, S.S.; Rai, M. Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulase 2017, 24, 5529–5540. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Husain, Q.; Ansari, S.A.; Alam, F.; Azam, A. immobilization of Aspergillus oryzae beta galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. Int. J. Biol. Macromol. 2011, 49, 37–43. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.; Chang, J.H.; Lee, J.H. Inorganic nanomaterial-based biocatalysts. BMB Rep. 2011, 44, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Ahmad, R.; Khare, S.K. Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour. Technol. 2017, 242, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid functionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Su, T.C.; Fang, Z.; Zhang, F.; Luo, J.; Li, X.K. Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave. Sci. Rep. 2015, 5, 17538. [Google Scholar]
- Huang, P.J.; Chang, K.L.; Hsieh, J.F.; Chen, S.T. Catalysis of rice straw hydrolysis by the combination of immobilized cellulase from Aspergillus niger on β-cyclodextrin-Fe3O4 nanoparticles and ionic liquid. Biomed. Res. Int. 2015, 1, 2015. [Google Scholar]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases immobilization on chitosan-coated magnetic nanoparticles: Application for agave atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosys. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Jordan, J.; Kumar, C.S.S.; Theegala, C. Preparation and characterization of cellulase bound magnetite nanoparticles. J. Mol. Catal. B Enzymol. 2011, 68, 139–146. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 2014, 53, 3448–3454. [Google Scholar] [CrossRef]
- Manasa, P.; Saroj, P.; Korrapati, N. Immobilization of cellulase enzyme on zinc ferrite nanoparticles in increasing enzymatic hydrolysis on ultrasound-assisted alkaline pretreated Crotalaria juncea biomass. Indian J. Sci. Technol. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.H.Y.; Jang, J.; Wu, K.C.W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem. 2011, 13, 2844–2850. [Google Scholar] [CrossRef]
- Srivastava, N.; Rawat, R.; Sharma, R.; Oberoi, H.S.; Srivastava, M.; Singh, J. Effect of Nickel–Cobaltite Nanoparticles on Production and Thermostability of Cellulases from Newly Isolated Thermotolerant Aspergillus fumigatus NS (Class: Eurotiomycetes). Appl. Biochem. Biotechnol. 2014, 174, 1092–1103. [Google Scholar] [CrossRef]
- Allen, S.G.; Schulman, D.; Lichwa, J.; Antal, M.J.; Jennings, E.; Elander, R. A comparison of aqueous and dilute-acid single-temperature pre-treatment of yellow poplar sawdust. Ind. Eng. Chem. Res. 2001, 40, 2352–2361. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 2014, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Wang, J.; Lu, C.; Xu, Y.; Sun, Z. Immobilized β-glucosidase on magnetic chitosan microspheres for hydrolysis of straw cellulose. Process Biochem. 2013, 48, 683–687. [Google Scholar] [CrossRef]
- Song, Q.; Mao, Y.; Wilkins, M.; Segato, F.; Prade, R. Cellulase immobilization on superparamagnetic nanoparticles for reuse in cellulosic biomass conversion. AIMS Bioeng. 2016, 3, 264–276. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.; Ramteke, P.W. Application of ZnO nanoparticles for improving the thermal and pH stability of crude cellulase obtained from Aspergillus fumigatus AA001. Front. Microbiol. 2016, 7, 514. [Google Scholar] [CrossRef] [Green Version]
- Cherian, E.; Dharmendirakumar, M.; Baskar, G. Immobilization of cellulase onto MnO2 nanoparticles for bioethanol production by enhanced hydrolysis of agricultural waste. Chin. J. Catal. 2015, 36, 1223–1229. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger Cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.A.; Lu, J.; Lee, I. Immobilization of cellulase on magnetoresponsive graphene nano-supports. J. Mol. Catal. B Enzym. 2013, 90, 76–86. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Cui, M.; Wei, L.; Yang, H.; Shen, J. Enhancement effect of hematite nanoparticles on fermentative hydrogen production. Bioresour. Technol. 2011, 102, 7903–7909. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The effects of FeO and NiO nanoparticles versus Fe2+ and Ni2+ ions on dark hydrogen fermentation. Int. J. Hydrogen Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Enhancement effect of hematite and nickel nanoparticles on biohydrogen production from dairy wastewater. Int. J. Hydrogen Energy 2015, 40, 4502–4511. [Google Scholar] [CrossRef]
- Singh, J.; Roychoudhury, A.; Srivastava, M.; Solanki, P.R.; Lee, D.W.; Lee, S.H.; Malhotra, B.D. A dual enzyme functionalized nanostructured thulium oxide based interface for biomedical application. Nanoscale 2014, 6, 1195–1208. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Farhadi, K.; Siahkamari, S.; Azizi, B. Catalytic wet peroxide oxidation of phenol over ZnFe2O4 nano spinel. Can. J. Chem. 2016, 95, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.; Hassan, M.A.; Soon, C.S. Effect of retention time on biohydrogen production by microbial consortia immobilised in polydimethylsiloxane. Afr. J. Biotechnol. 2011, 10, 601–609. [Google Scholar]
- Zhu, S.; Xu, X.; Rong, R.; Li, B.; Wang, X. Evaluation of zinc-doped magnetite nanoparticle toxicity in the liver and kidney of mice after sub-chronic intragastric administration. Toxicol. Res. 2016, 5, 97–106. [Google Scholar] [CrossRef]
- Xu, J.; Huo, S.; Yuan, Z.; Zhang, Y.; Xu, H.; Guo, Y.; Liang, C.; Zhuang, X. Characterization of direct cellulase immobilization with superparamagnetic nanoparticles. Biocatal. Biotransform. 2011, 29, 71–76. [Google Scholar] [CrossRef]
- Jariyaboon, R.; Sompong, O.; Kongjan, P. Bio-hydrogen and bio-methane potentials of skim latex serum in batch thermophilic two-stage anaerobic digestion. Bioresour. Technol. 2015, 198, 198–206. [Google Scholar] [CrossRef]
- Kirli, B.; Kapdan, I.K. Selection of microorganism immobilization particle for dark fermentative biohydrogen production by repeated batch operation. Renew. Energy 2016, 87, 697–702. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, J.; Ramteke, P.W.; Mishra, P.K.; Srivastava, M. Improved production of reducing sugars from rice straw using crude cellulase activated with Fe3O4/alginate nanocomposite. Bioresour. Technol. 2015, 183, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J. Chem. Eng. 2016, 33, 216–222. [Google Scholar] [CrossRef]
- Harmoko, C.; Sucipto, K.I.; Ery, R.S.; Hartono, S.B. Vinyl functionalized cubic mesoporous silica nanoparticles as supporting material to enhance cellulase enzyme stability. ARPN J. Eng. Appl. Sci. 2016, 11, 2981–2992. [Google Scholar]
- Yang, C.; Mo, H.; Zang, L.; Chen, J.; Wang, Z.; Qiu, J. Surface functionalized natural inorganic nanorod for highly efficient cellulase immobilization. RSC Adv. 2016, 6, 76855–76860. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.Y.; Jiang, X.P.; Ye, J.J.; Zhang, Y.W.; Zhang, X.Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J. Nanopart. Res. 2015, 17, 8. [Google Scholar] [CrossRef]
- Lima, J.S.; Araújo, P.H.H.; Sayer, C.; Souza, A.A.U.; Viegas, A.C.; de Oliveira, D. Cellulase immobilization on magnetic nanoparticles encapsulated in polymer nanospheres. Bioprocess Biosyst. Eng. 2017, 40, 511–518. [Google Scholar] [CrossRef]
- Han, J.; Rong, J.; Wang, Y.; Liu, Q.; Tang, X.; Li, C.; Ni, L. Immobilization of cellulase on thermo-sensitive magnetic microspheres: Improved stability and reproducibility. Bioprocess Biosyst. Eng. 2018, 41, 1051–1060. [Google Scholar] [CrossRef]
- Singh, N.; Mathur, A.S.; Barrow, C.J.; Puri, M. Enhanced cellulosic ethanol production via consolidated bioporcessing by Clostridium thermocellum ATCC 31924. Bioresour. Technol. 2018, 250, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Barrow, C.J.; Verma, M.L. Enzyme immobilization on nanomaterials for biofuel production. Trends Biotechnol. 2013, 31, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sardar, M. Enzyme immobilization: An overview on nanoparticles as immobilization matrix. Biochem. Anal. Biochem. 2015, 4, 178. [Google Scholar]
- Pundir, C.S. Immobilization of enzyme nanoparticles. In Enzyme Nanoparticles, 1st ed.; Pundir, C.S., Ed.; William Andrew Publishing: Boston, CA, USA, 2015; pp. 23–32. [Google Scholar]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.H. Enhanced hydrogen production in a UASB reactor by retaining microbial consortium onto carbon nanotubes (CNTs). Int. J. Hydrogen Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Sindhe, M.A.; Satyanarayan, N.D.; Pramod, S.N.; Nagaraja, K. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Padil, T.V.V.; Cernik, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Bhatia, S.K.; Kim, S.H.; Yoon, J.J.; Yang, Y.H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers. Manag. 2017, 148, 1142–1156. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, S.; Wei, X.; Wang, L.; Liu, Y. Immobilization of β-glucosidase onto magnetic nanoparticles and evaluation of the enzymatic properties. BioResources 2013, 8, 2605–2619. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of β-glucosidase from Thermatoga maritima on chitin-functionalized magnetic nanoparticle via a novel thermostable chitin-binding domain. Sci. Rep. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Tsai, C.T.; Meyer, A.S. Enzymatic cellulose hydrolysis: Enzyme reusability and visualization of beta-glucosidase immobilized in calcium alginate. Molecules 2014, 19, 19390–19406. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Tanaka, T.; Yoshino, T. Stoichiometrically controlled immobilization of multiple enzymes on magnetic nanoparticles by the magnetosome display system for efficient cellulose hydrolysis. Biomacromolecules 2015, 16, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Kumar, R.N.; Melvin, X.H.; Aiswarya, R.; Soumya, S. Sesbania aculeate biomass hydrolysis using magnetic nanobiocomposite of cellulase for bioethanol production. Renew. Energy 2016, 98, 23–28. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Logan, B.E. Biologically extracting energy from wastewater: Bio-hydrogen production and microbial fuel cells. Environ. Sci. Technol. 2004, 38, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Lay, C.H.; Sen, B.; Chu, C.Y.; Kumar, G.; Chen, C.C.; Chang, J.S. Fermentative hydrogen production from wastewaters: A review and prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Van Ginkel, S.W.; Oh, S.E.; Logan, B.E. Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy 2005, 30, 1535–1542. [Google Scholar] [CrossRef]
- Bunker, C.E.; Smith, M.J. Nanoparticles for hydrogen generation. J. Mater. Chem. 2005, 21, 12173–12180. [Google Scholar] [CrossRef]
- Malik, S.N.; Pugalenthi, V.; Vaidya, A.N.; Ghosh, P.C.; Mudliar, S.N. Kinetics of Nano-catalysed dark fermentative hydrogen production from distillery wastewater. Energy Procedia 2014, 54, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Thakur, S.; Mahapatra, D.M.; Wahid, Z.A.; Liu, H.; Singh, L. Impacts of nano-metal oxides on hydrogen production in anaerobic digestion of palm oil mill effluent—A novel approach. Int. J. Hydrogen Energy 2018, 43, 2666–2676. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ. Sci. Pollut. Control Ser. 2017, 24, 8790–8804. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Liu, M.; Zhou, J.; Cen, K. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacter aerogenes. Bioresour. Technol. 2016, 207, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jafari, O.; Zilouei, H. Enhanced biohydrogen and subsequent biomethane production from sugarcane bagasse using nano-titanium dioxide pretreatment. Bioresour. Technol. 2016, 2014, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Engliman, N.S.; Abdul, P.M.; Wu, S.Y.; Jahim, J.M. Influence of iron (II) oxide nanoparticle on biohydrogen production in thermophilic mixed fermentation. Int. J. Hydrogen Energy 2017, 42, 27482–27493. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, C.; Zhang, H.; Wang, Z.; Zhang, J.; Song, M. Ferric oxide/carbon nanoparticles enhanced bio-hydrogen production from glucose. Int. J. Hydrogen Energy 2018, 43, 8729–8738. [Google Scholar] [CrossRef]
- Nath, D.; Manhar, A.K.; Gupta, K.; Saikia, D.; Das, S.K.; Mandal, M. Phytosynthesized iron nanoparticles: Effects on fermentative hydrogen production by Enterobacter cloacae DH-89. Bull. Mater. Sci 2015, 38, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Mohanraj, S.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Comparative evaluation of fermentative hydrogen production using Enterobacter cloacae and mixed culture: Effect of Pd (II) ion and phytogenic palladium nanoparticles. J. Biotechnol. 2014, 192, 87–95. [Google Scholar] [CrossRef]
- Beckers, L.; Hiligsmann, S.; Lambert, S.D.; Heinrichs, B.; Thonart, P. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by clostridium butyricum. Bioresour. Technol. 2013, 133, 109–117. [Google Scholar] [CrossRef]
- Wimonsong, P.; Nitisoravut, R. Comparison of different catalyst for fermentative hydrogen production. J. Clean Energy Technol. 2015, 3, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Wimonsong, P.; Nitisoravut, R. Biohydrogen enhancement using highly porous activated carbon. Energy Fuels 2014, 28, 4554–4559. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Du, B.; Wei, D.; Wei, Q.; Zhao, Y. Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria. Bioresour. Technol. 2013, 142, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Lee, J.; Cho, M.H. Electrochemically active biofilm mediated bio-hydrogen production catalyzed by positively charged gold nanoparticles. Int. J. Hydrogen Energy 2013, 38, 5243–5250. [Google Scholar] [CrossRef]
- Bao, M.; Su, H.; Tan, T. Dark fermentative bio-hydrogen production: Effects of substrate pre-treatment and addition of metal ions or L-cysteine. Fuel 2013, 112, 38–44. [Google Scholar] [CrossRef]
- Mullai, P.; Yogeswari, M.K.; Sridevi, K. Optimisation and enhancement of biohydrogen production using nickel nanoparticles—A novel approach. Bioresour. Technol. 2013, 141, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Li, S.; Zhang, W.X. Renewable hydrogen generation by bimetallic zero valent iron nanoparticles. Chem. Eng. J. 2011, 170, 562–567. [Google Scholar] [CrossRef]
| Nanomaterials Used on Support | Enzymes Used | References |
|---|---|---|
| Silica based NPs with carbon-derived mesospores | Lipase | [36] |
| MgNPs | Xylanase | [41] |
| Sulfonated magnetic carbonaceous acid NPs | Cellulase | [61] |
| β-cyclodextrin conjugated MNPs | Cellulase (Aspergillus niger) | [62] |
| Fe2O3 MNPs | β-glucosidase | [65] |
| Fe3O4 MNPs | Cellulase | [66] |
| ZnFe2O4 | Cellulase | [68] |
| Sulfonated mesoporous silica modified with Fe3O4 NPs | Cellobiase | [72] |
| Zn MNPs | Cellulase | [73] |
| Chitosan-based magnetic microspheres | β-glucosidase | [74] |
| Superparamagnetic NPs | Cellulases (β -glucosidase A and cellobiohydrolase D) | [75] |
| ZnO functionalized NPs | Cellulase (Aspergillus fumigatus AA001) | [76] |
| MnO2 NPs | Cellulase | [77] |
| Multiwall carbon nanotubes functionalized with N-ethyl-N-(3-dimethylaminopropyl) carbodiimide hydrochloride | Cellulase (Aspergillus niger) | [78] |
| Magneto-responsive graphene nanosupports | Cellulase | [79] |
| Nanomaterials Used | Improved Physical Properties | Immobilization Methods | References |
|---|---|---|---|
| MnO2 NPs | Temperature stability at 70 °C and pH stability at 5.0 | Covalent binding through surface modification using glutaraldehyde | [77] |
| Fe3O4/ Chitosan NPs | Temperature stability at 60 °C and pH stability at 5.0 | Covalent binding through surface modification using glutaraldehyde | [67] |
| CoFe2O4 NPs | Temperature stability at 50 °C and pH stability at 5.0 | Covalent binding through 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride & N-hydroxysuccinimide chemistry | [92] |
| Fe3O4 NPs | Temperature stability at 60 °C and pH stability at 4.5 | Covalent binding through surface modification using glutaraldehyde | [88] |
| SiO2 NPs | pH stability at 4.8 | Physical adsorption through vinyl group | [93] |
| Fe3O4/Chitosan NPs | Temperature stability at 60 °C and pH stability at 5.5 | Covalent binding through surface modification using glutaraldehyde | [64] |
| Attapulgite@chitosan NCs | Temperature stability at 60 °C and pH stability at 4.0 | Covalent binding through surface modification using glutaraldehyde | [94] |
| Fe3O4@SiO2/ graphene oxide NCs | Temperature stability at 50 °C and pH stability at 4.0 | Covalent binding through surface modification using (3-aminopropyl)triethoxysilane chemistry | [95] |
| Fe3O4/polymer NCs | Temperature stability in the range of 10–70 °C and pH stability in the range of 2.0–8.0 | Covalent binding through surface modification using glutaraldehyde | [96] |
| Fe3O4@SiO2 NCs | Temperature stability at 60 °C and pH stability at 4.5 | Covalent binding through surface functionalization using glycidyl methacrylate | [97] |
| Microbial Strains Used | Substrates Used | NPs/ Nanomaterials Used | % Increase in H2 Yield | References |
|---|---|---|---|---|
| Bacillus anthracis | Palm oil mill effluent | NiO | 151 | [117] |
| CoO | 167 | |||
| Enterobacter sp. and Clostridium sp. | Grass | FeO | 73.1 | [118] |
| Anaerobic sludge | Sugarcane bagasse | Fe3O4 | 69 | [119] |
| Mesophilic culture | Starch | FeNPs | 200 | [82] |
| Enterobacter aerogenes | Cassava starch | Fe2O3 | 92 | [120] |
| Anaerobic sludge containing H2 producing bacteria | Molasses waste | NiO | 24 | [83] |
| Fe2O3 | 43 | |||
| Anaerobic sludge | Sugarcane bagasse | TiO2 | 127 | [121] |
| Thermophillic anaerobic mixed culture | Glucose | Fe2O3 | 53.6 | [122] |
| Anaerobic mixed bacteria | Glucose | Fe2O3-Fe3O4/carbon nanocomposite | 33.7 | [123] |
| Enterobacter cloacae | Glucose | FeNPs | 130 | [124] |
| Mixed culture | Glucose | Pd(II) NPs | 9 | [125] |
| Clostridium butyricum | Glucose | Pd, Ag, Cu, and Fe encapsulated SiO2 NPs | 38 | [126] |
| Clostridium butyricum | Sucrose | α-Fe2O3 | 32.64 | [81] |
| Anaerobic sludge | Glucose | Carbon nanotubes | ~50 | [98] |
| Anaerobic sludge | Sucrose | Activated carbon | 62.5 | [127] |
| Anaerobic sludge | Sucrose | Nano activated carbon | 70 | [128] |
| Clostridium butyricum | Glucose | AgNPs | 67.5 | [129] |
| Anaerobic sludge | Acetate | AuNPs | - | [130] |
| Rhodobacter sphaeroides | Malate | Fe | 19.4 | [131] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhvi, M.; Kim, B.S. Current Developments in Lignocellulosic Biomass Conversion into Biofuels Using Nanobiotechology Approach. Energies 2020, 13, 5300. https://doi.org/10.3390/en13205300
Singhvi M, Kim BS. Current Developments in Lignocellulosic Biomass Conversion into Biofuels Using Nanobiotechology Approach. Energies. 2020; 13(20):5300. https://doi.org/10.3390/en13205300
Chicago/Turabian StyleSinghvi, Mamata, and Beom Soo Kim. 2020. "Current Developments in Lignocellulosic Biomass Conversion into Biofuels Using Nanobiotechology Approach" Energies 13, no. 20: 5300. https://doi.org/10.3390/en13205300