Comparison of Spectral Reflectance-Based Smart Farming Tools and a Conventional Approach to Determine Herbage Mass and Grass Quality on Farm
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiments
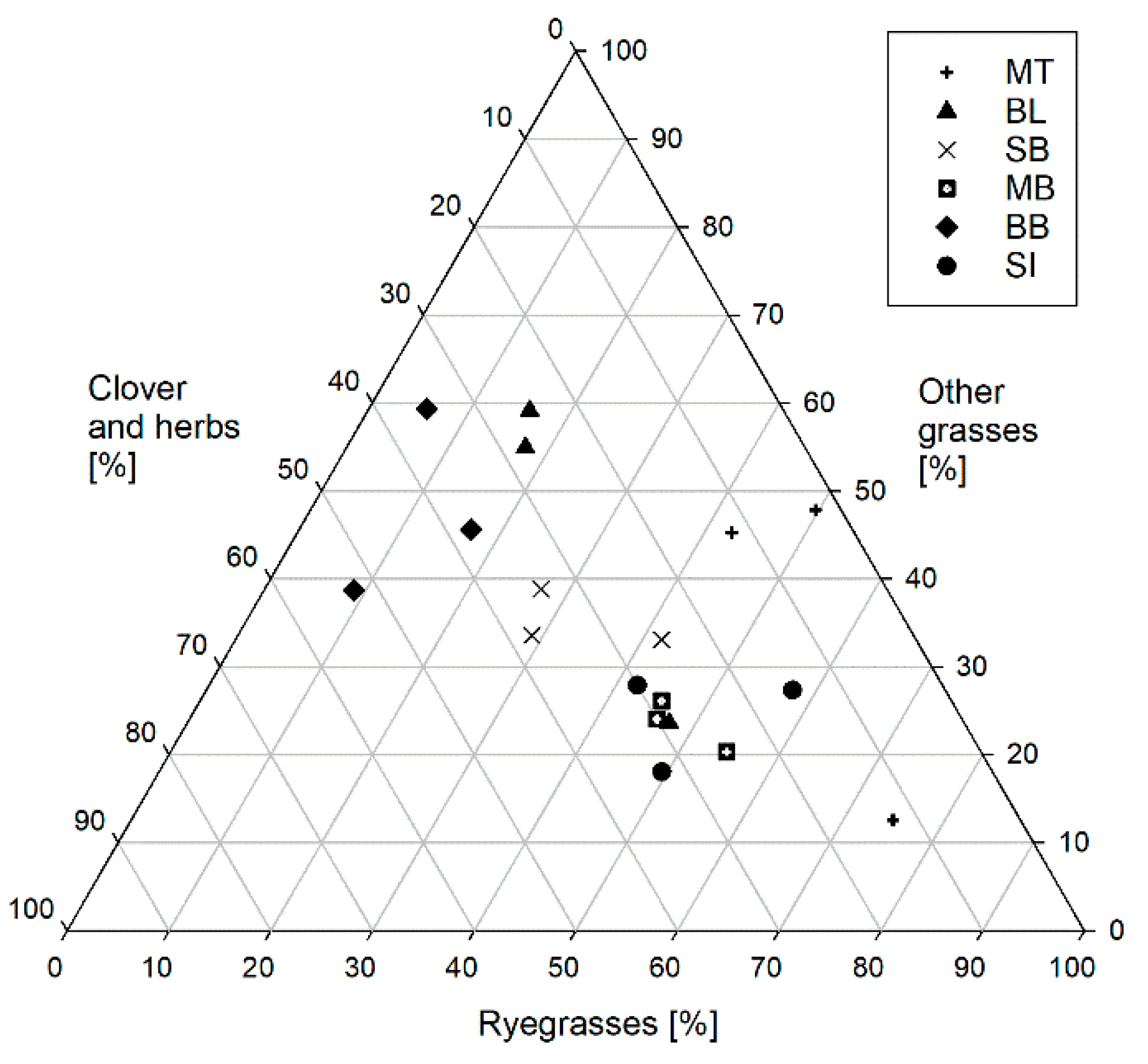
2.1.1. Grassland Study Sites
2.1.2. Experimental Design
2.1.3. Herbage Sampling
2.2. Smart Farming Tools
2.2.1. Multispectral Imagery Model
2.2.2. On-Site NIRS
2.3. Conventional Method
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Validation of the Laboratory NIRS as a Reference Method
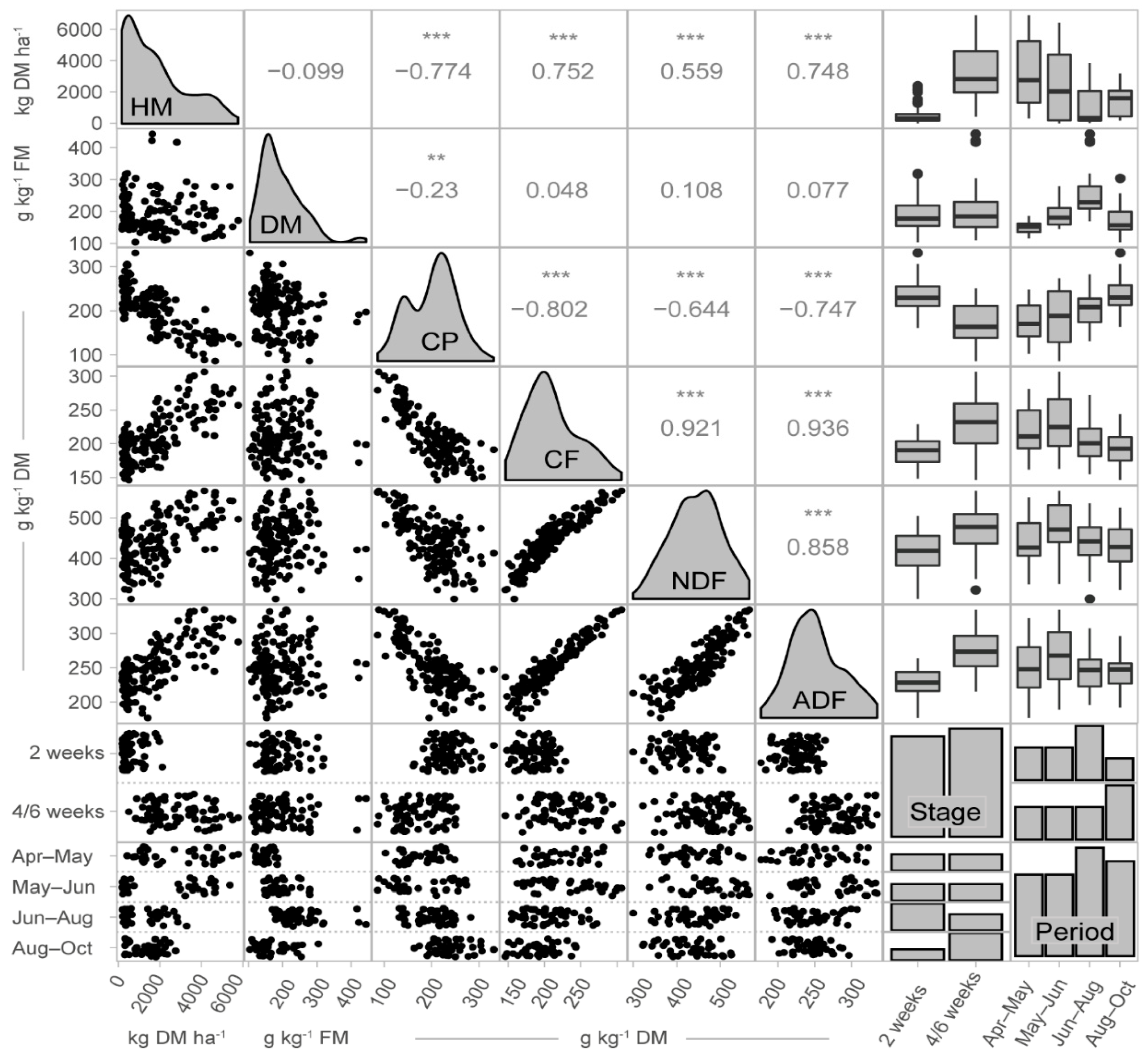
3.2. Sample Characteristics
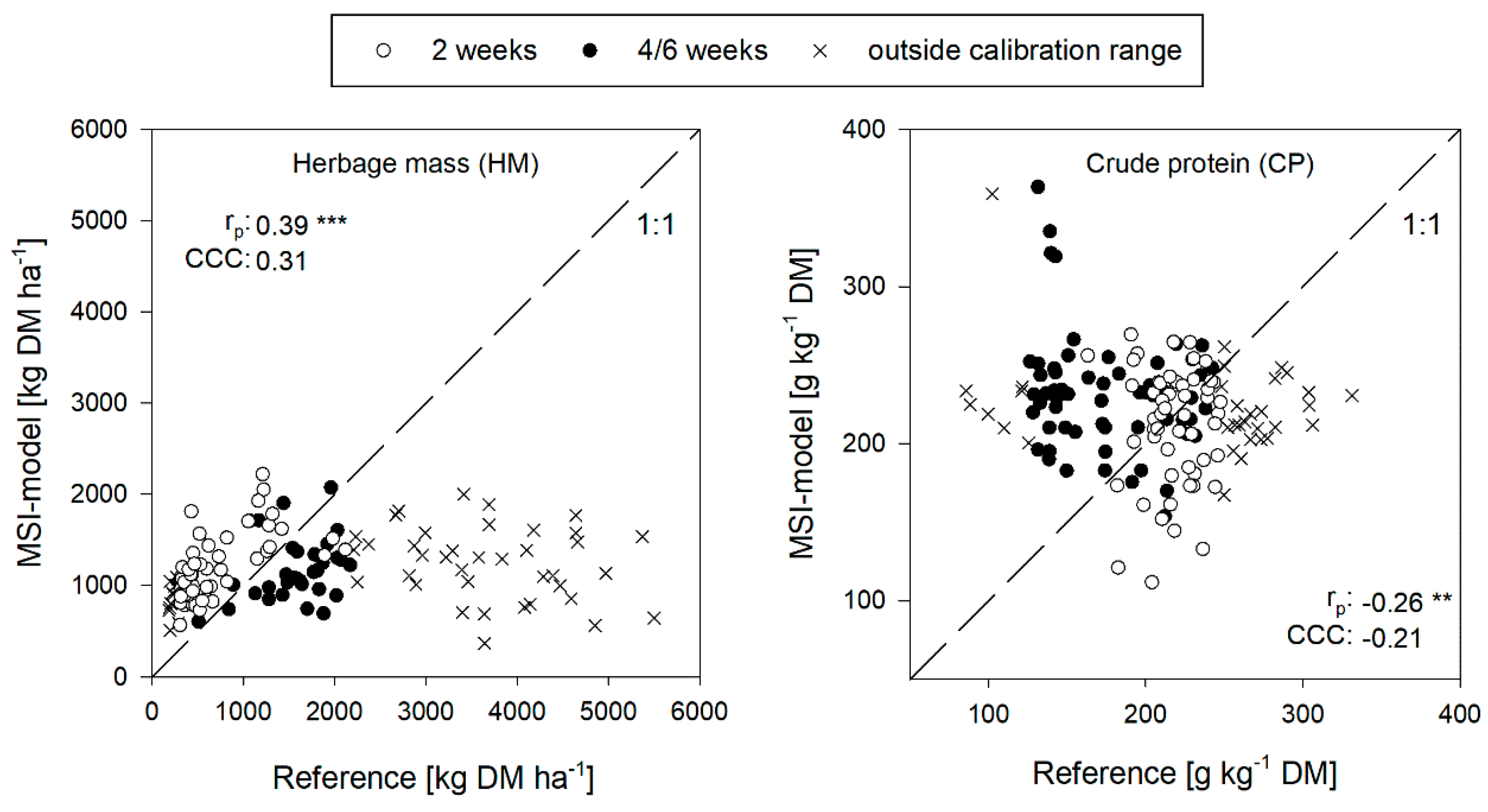
3.3. Performance of the MSI-Model
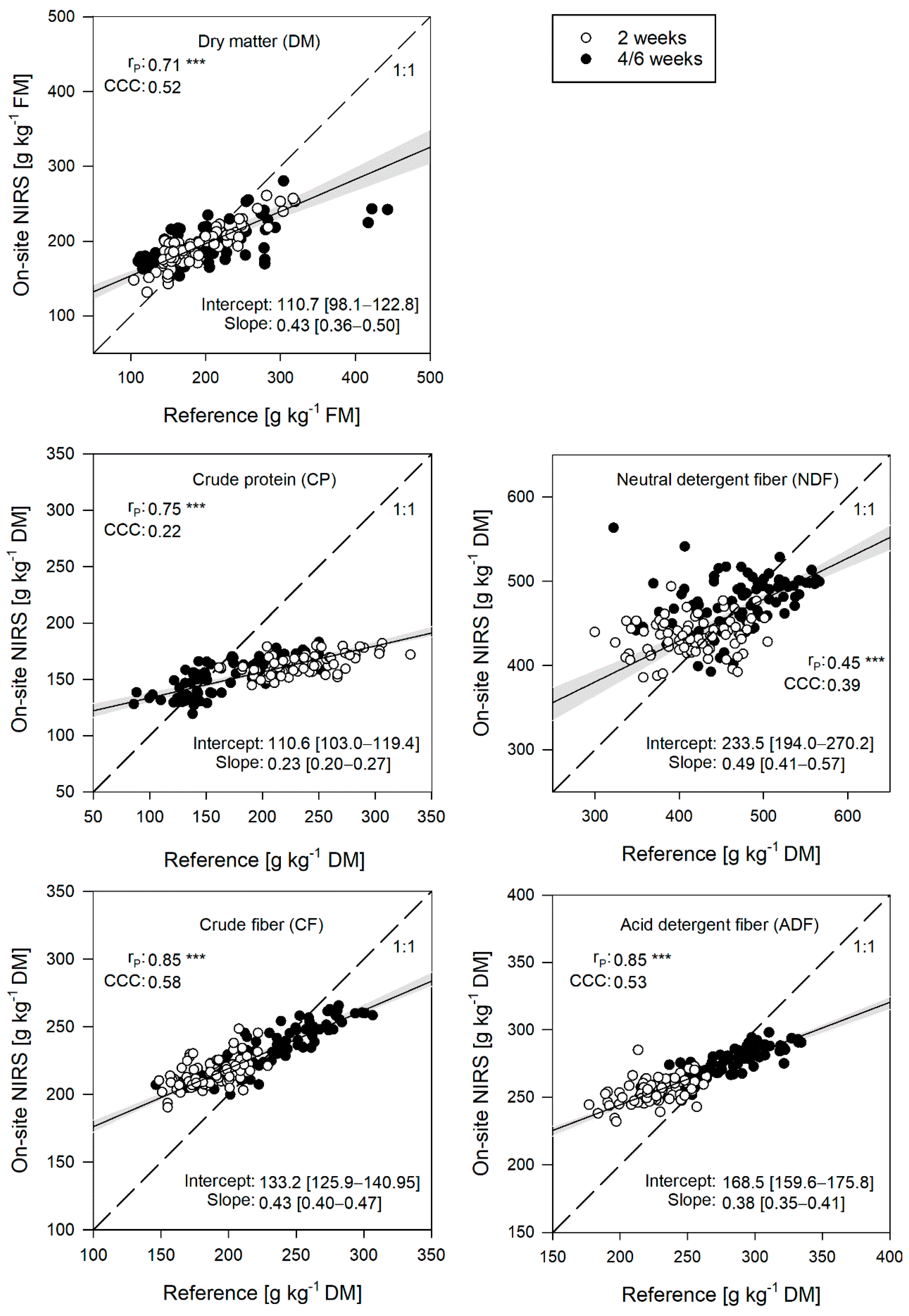
3.4. Performance of the On-Site NIRS
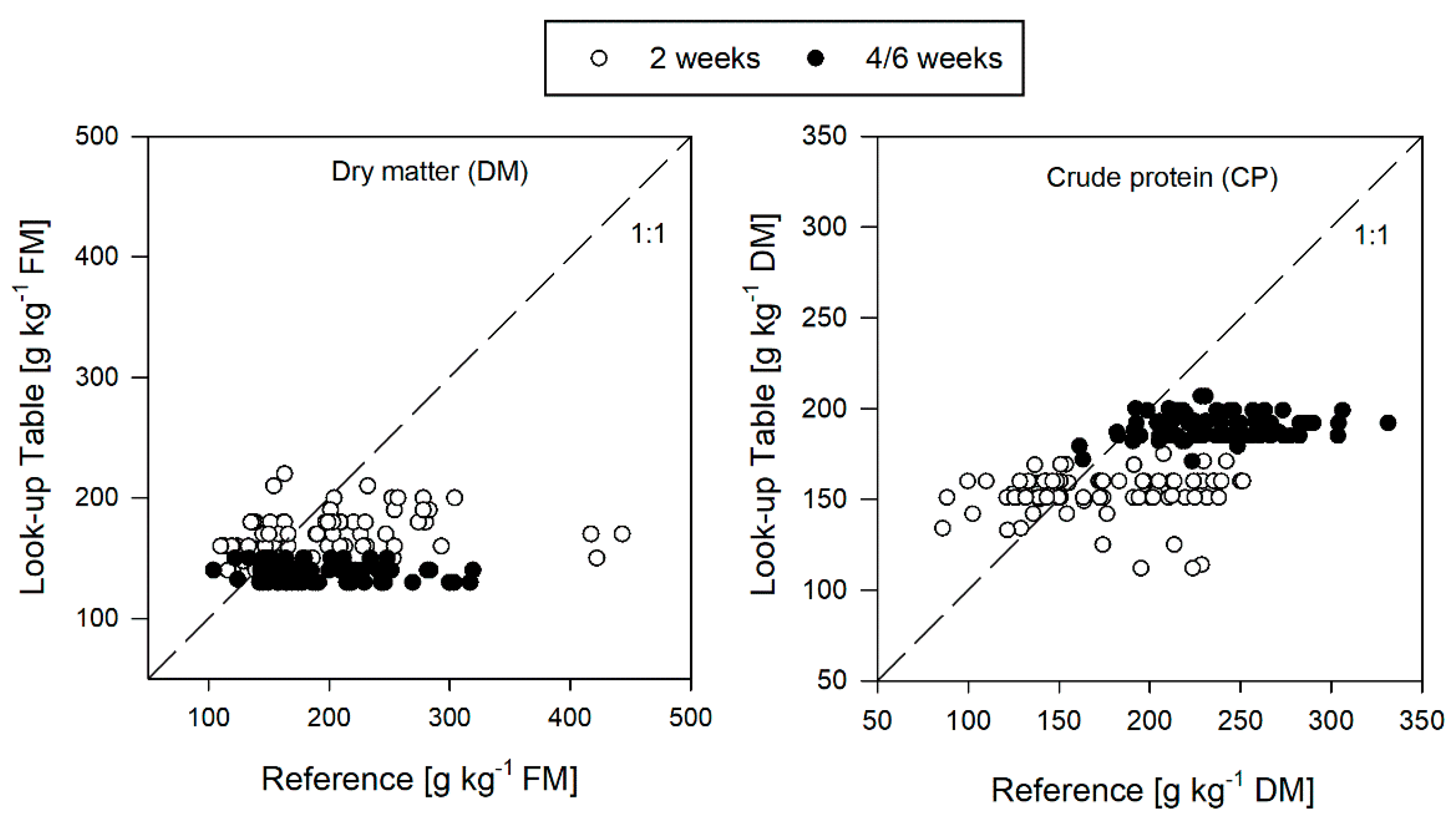
3.5. Performance of the Look-Up Tables
3.6. Comparison Between Methods
4. Discussion
4.1. Generalizability of the MSI-Model
4.2. Difficulty of Remotely Sensing High-Biomass Grasslands by MSI
4.3. Perspectives of MSI on Grasslands
4.4. Improving the Decision Support Platform for Remote Grassland Assessment
4.5. Potential for Correcting Systematic Errors of the On-Site NIRS
4.6. Benefits of Smart Farming Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaver, A.; Ritter, C.; von Keyserlingk, M.A.G. The Dairy Cattle Housing Dilemma: Natural Behavior Versus Animal Care. Vet. Clin. N. Am. Food A 2019, 35, 11–27. [Google Scholar]
- Van den Pol-van Dasselaar, A.; de Vliegher, A.; Hennessy, D.; Peyraud, J.L.; Pinxterhuis, J.B. Research Methodology of Grazing; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2011. [Google Scholar]
- Rutter, S.M. Can precision farming technologies be applied to grazing management. In Revitalising Grasslands to Sustain Our Communities, Proceedings of 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013; Michalk, D.L., Millar, G.D., Eds.; New South Wales Department of Primary Industry: Orange, New South Wales, Australia, 2013; pp. 617–620. [Google Scholar]
- Turner, L.; Irvine, L.; Kilpatrick, S. Incorporating data into grazing management decisions: Supporting farmer learning. Anim. Prod. Sci. 2020, 60, 138–142. [Google Scholar] [CrossRef]
- Ferraro, F.P.; Nave, R.L.G.; Sulc, R.M.; Barker, D.J. Seasonal Variation in the Rising Plate Meter Calibration for Forage Mass. Agron. J. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Bell, M.J.; Mereu, L.; Davis, J. The Use of Mobile Near-Infrared Spectroscopy for Real-Time Pasture Management. Front. Sustain. Food Syst. 2018, 2, 76. [Google Scholar] [CrossRef]
- Calanca, P.; Deléglise, C.; Martin, R.; Carrère, P.; Mosimann, E. Testing the ability of a simple grassland model to simulate the seasonal effects of drought on herbage growth. Field Crop. Res. 2016, 187, 12–23. [Google Scholar] [CrossRef]
- Le Clec’h, S.; Finger, R.; Buchmann, N.; Gosal, A.S.; Hörtnagl, L.; Huguenin-Elie, O.; Jeanneret, P.; Lüscher, A.; Schneider, M.K.; Huber, R. Assessment of spatial variability of multiple ecosystem services in grasslands of different intensities. J. Environ. Manag. 2019, 251, 109372. [Google Scholar] [CrossRef] [PubMed]
- Lile, J.A.; Blackwell, M.B.; Thomson, N.A.; Penno, J.W.; Macdonald, K.A.; Nicholas, P.K.; Lancaster, J.A.S.; Coulter, M. Practical Use of the Rising Plate Meter (RPM) on New Zealand Dairy Farms. In Proceedings of the NZ Grassland Association, Hamilton, New Zealand, 6–8 November 2001; NZ Grassland Association Inc.: Dunedin, New Zealand, 2001; Volume 63, pp. 159–164. [Google Scholar]
- Daccord, R.; Wyss, U.; Kessler, J.; Arrigo, Y.; Rouel, M.; Lehmann, J.; Jeangros, B.; Meisser, M. Nutritional value of roughage. In Feed Recommendations for Ruminants (Green Book); Daccord, R., Wyss, U., Eds.; Agroscope: Posieux, Switzerland, 2017. [Google Scholar]
- Deléglise, C.; Meisser, M.; Mosimann, E.; Spiegelberger, T.; Signarbieux, C.; Jeangros, B.; Buttler, A. Drought-induced shifts in plants traits, yields and nutritive value under realistic grazing and mowing managements in a mountain grassland. Agric. Ecosyst. Environ. 2015, 213, 94–104. [Google Scholar] [CrossRef]
- Michaud, A.; Plantureux, S.; Pottier, E.; Baumont, R. Links between functional composition, biomass production and forage quality in permanent grasslands over a broad gradient of conditions. J. Agric. Sci. 2015, 153, 891–906. [Google Scholar] [CrossRef]
- Peratoner, G.; Kasal, A.; Florian, C.; Pramsohler, M. Accuracy of visual assessment of the plant stand type for the estimation of forage quality. Grassl. Sci. Eur. 2018, 23, 950–952. [Google Scholar]
- Pullanagari, R.R.; Yule, I.; King, W.; Dalley, D.; Dynes, R. The use of optical sensors to estimate pasture quality. Int. J. Smart Sens. Intell. Syst. 2011, 4, 125–137. [Google Scholar] [CrossRef]
- Lugassi, R.; Chudnovsky, A.; Zaady, E.; Dvash, L.; Goldshleger, N. Spectral Slope as an Indicator of Pasture Quality. Remote Sens. 2015, 7, 256–274. [Google Scholar] [CrossRef]
- Norris, K.H.; Barnes, R.F.; Moore, J.E.; Shenk, J.S. Predicting Forage Quality by Infrared Replectance Spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar] [CrossRef]
- Mika, V.; Pozdisek, J.; Tillmann, P.; Nerusil, P.; Buchgraber, K.; Gruber, L. Development of NIR calibration valid for two different grass sample collections. Czech J. Anim. Sci. 2003, 48, 419–424. [Google Scholar]
- Alomar, D.; Fuchslocher, R.; Cuevas, J.; Mardones, R.; Cuevas, E. Prediction of the composition of fresh pastures by near infrared reflectance or interactance-reflectance spectroscopy. Chil. J. Agric. Res. 2009, 69, 198–206. [Google Scholar] [CrossRef]
- Soldado, A.; Fearn, T.; Martinez-Fernandez, A.; de la Roza-Delgado, B. The transfer of NIR calibrations for undried grass silage from the laboratory to on-site instruments: Comparison of two approaches. Talanta 2013, 105, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Nelson, S.; Carslake, H.B.; Argo, C.M.; Wolf, R.; Fabri, F.B.; Brolsma, K.M.; van Oostrum, M.J.; Ellis, A.D. Comparison of NIRS and Wet Chemistry Methods for the Nutritional Analysis of Haylages for Horses. J. Equine Vet. Sci. 2018, 71, 13–20. [Google Scholar] [CrossRef]
- Ampuero Kragten, S.; Wyss, U. Feedstuff in near infrared light (NIRS). Agrar. Schweiz 2014, 5, 204–211. [Google Scholar]
- Long, E.A.; Ketterings, Q.M.; Russell, D.; Vermeylen, F.; DeGloria, S. Assessment of yield monitoring equipment for dry matter and yield of corn silage and alfalfa/grass. Precis. Agric. 2016, 17, 546–563. [Google Scholar] [CrossRef]
- Patton, L.; McConnell, D.; Archer, J.; O’Donovan, M.; Gilliland, T.J. Portable NIRS: A novel technology for the prediction of forage nutritive quality. Grassl. Sci. Eur. 2018, 23, 892–894. [Google Scholar]
- Berzaghi, P.; Marchesini, G. Rapid Methods of Analysis of Silages to Improve Feeding Management in Dairy Farms. In Proceedings of the 16th International Symposium Forage Conservation, Brno, Czech Republic, 3–6 June 2014; Jambor, V., Malá, S., Eds.; Mendel University: Brno, Czech Republic, 2014; pp. 65–70. [Google Scholar]
- Rueda-Ayala, V.P.; Peña, J.M.; Höglind, M.; Bengochea-Guevara, J.M.; Andújar, D. Comparing UAV-Based Technologies and RGB-D Reconstruction Methods for Plant Height and Biomass Monitoring on Grass Ley. Sensors 2019, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Wachendorf, M.; Fricke, T.; Möckel, T. Remote sensing as a tool to assess botanical composition, structure, quantity and quality of temperate grasslands. Grass Forage Sci. 2018, 73, 1–14. [Google Scholar] [CrossRef]
- Iftikhar, A.; Cawkwell, F.; Dwyer, E.; Barrett, B.; Green, S. Satellite remote sensing of grasslands: From observation to management. J. Plant. Ecol. 2016, 9, 649–671. [Google Scholar] [CrossRef]
- Bareth, G.; Schellberg, J. Replacing Manual Rising Plate Meter Measurements with Low-cost UAV-Derived Sward Height Data in Grasslands for Spatial Monitoring. PFG J. Photogramm. Rem. 2018, 86, 157–168. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative Remote Sensing at Ultra-High Resolution with UAV Spectroscopy: A Review of Sensor Technology, Measurement Procedures, and Data Correction Workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Murphy, D.; O‘Brien, B.; Askari, M.S.; McCarthy, T.; Magee, A.; Burke, R.; Murphy, M. GrassQ—A Holistic Precision Grass Measurement and Analysis System to Optimize Pasture Based Livestock Production. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; ASABE: St. Joseph, MA, USA, 2019; p. 1900769. [Google Scholar]
- Askari, M.S.; McCarthy, T.; Magee, A.; Murphy, D.J. Evaluation of Grass Quality under Different Soil Management Scenarios Using Remote Sensing Techniques. Remote Sens. 2019, 11, 1835. [Google Scholar] [CrossRef]
- VDLUFA. The chemical analysis of feedstuffs. In VDLUFA Method Book III, 8th ed.; VDLUFA-Verlag: Darmstadt, Germany, 1976; ISBN 978-3-941273-14-6. [Google Scholar]
- Lin, L.I. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 48, 255–268. [Google Scholar] [CrossRef]
- Lindemann, R.H.; Merenda, P.F.; Gold, R.Z. Introduction to Bivariate and Multivariate Analysis; Scott Foresman and Company: Glenview, IL, USA, 1980. [Google Scholar]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures from method comparison studies in clinical chemistry, Part I. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M. epiR: Tools for the Analysis of Epidemiological Data. Available online: https://CRAN.R-project.org/package=epiR (accessed on 24 February 2020).
- Manuilova, E.; Schuetzenmeister, A.; Model, F. mcr: Method Comparison Regression. Available online: https://CRAN.R-project.org/package=mcr (accessed on 3 March 2020).
- Grömping, U. Relative Importance for Linear Regression in R: The Package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- O‘Neill, F.H.; Martin, J.R.; Devaney, F.M.; Perrin, P.M. The Irish semi-natural grasslands survey 2007–2012. In Irish Wildlife Manual No. 78; National Parks and Wildlife Service, Department of Arts, Heritage and Gaeltacht: Dublin, Ireland, 2013. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; de Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Svinurai, W.; Hassen, A.; Tesfamariam, E.; Ramoelo, A. Performance of ratio-based, soil-adjusted and atmospherically corrected multispectral vegetation indices in predicting herbaceous aboveground biomass in a Colophospermum mopane tree–shrub savanna. Grass Forage Sci. 2018, 73, 727–739. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Todd, S.W.; Hoffer, R.M.; Milchunas, D.G. Biomass estimation on grazed and ungrazed rangelands using spectral indices. Int. J. Remote Sens. 1998, 19, 427–438. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, C. Using remote sensing to estimate forage biomass and nutrient contents at different growth stages. Biomass Bioenergy 2018, 115, 74–81. [Google Scholar] [CrossRef]
- Grüner, E.; Astor, T.; Wachendorf, M. Biomass Prediction of Heterogeneous Temperate Grasslands Using an SfM Approach Based on UAV Imaging. Agronomy 2019, 9, 54. [Google Scholar] [CrossRef]
- Forsmoo, J.; Anderson, K.; Macleod, C.J.; Wilkinson, M.E.; Brazier, R. Drone-based structure-from-motion photogrammetry captures grassland sward height variability. J. Appl. Ecol. 2018, 55, 2587–2599. [Google Scholar]
- Näsi, R.; Viljanen, N.; Kaivosoja, J.; Alhonoja, K.; Hakala, T.; Markelin, L.; Honkavaara, E. Estimating Biomass and Nitrogen Amount of Barley and Grass Using UAV and Aircraft Based Spectral and Photogrammetric 3D Features. Remote Sens. 2018, 10, 1082. [Google Scholar] [CrossRef]
- Forsmoo, J.; Anderson, K.; Macleod, C.J.A.; Wilkinson, M.E.; DeBell, L.; Brazier, R.E. Structure from motion photogrammetry in ecology: Does the choice of software matter? Ecol. Evol. 2019, 9, 12964–12979. [Google Scholar] [CrossRef] [PubMed]
- Fearn, T. Standardisation and Calibration Transfer for near Infrared Instruments: A Review. J. Near Infrared Spec. 2001, 9, 229–244. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Li, L.-M.; Yu, R.-Q.; Littlejohn, D.; Nordon, A.; Morris, J.; Dann, A.S.; Jeffkins, P.A.; Richardson, M.D.; Stimpson, S.L. Systematic prediction error correction: A novel strategy for maintaining the predictive abilities of multivariate calibration models. Analyst 2011, 136, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Andueza, D.; Picard, F.; Jestin, M.; Andrieu, J.; Baumont, R. NIRS prediction of the feed value of temperate forages: Efficacy of four calibration strategies. Animal 2011, 5, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, M.; van Waes, C.; Dardenne, P.; Baert, J.; Reheul, D. Comparison of near infrared reflectance spectroscopy calibration strategies for the botanical composition of grass-clover mixtures. Grass Forage Sci. 2013, 69, 167–175. [Google Scholar] [CrossRef]

| Measurement Tools (Number of Samples) | Sensing Method | Measured Herbage Parameters [Unit] | Reference Methods |
|---|---|---|---|
| MSI-model (n = 147) | MSI of the bands green, red, red-edge, and NIR | HM [kg DM ha−1] | Cutting defined area + oven-drying |
| CP [g kg−1 DM] | Laboratory NIRS | ||
| On-site NIRS (n = 162) | NIRS with diode array | DM [g kg−1 FM] | Oven-drying |
| CP [g kg−1 DM] | Laboratory NIRS | ||
| CF [g kg−1 DM] | Laboratory NIRS | ||
| NDF [g kg−1 DM] | Laboratory NIRS | ||
| ADF [g kg−1 DM] | Laboratory NIRS |
| Measurement System (Number of Samples) | Sensing Method | Measured Herbage Parameters [Unit] | Validation Methods |
|---|---|---|---|
| Laboratory NIRS (n = 54) | Fourier-transform NIRS | CP [g kg−1 DM] | Dumas method |
| CF [g kg−1 DM] | Gravimetric analysis | ||
| NDF [g kg−1 DM] | Gravimetric analysis | ||
| ADF [g kg−1 DM] | Gravimetric analysis |
| Response Variables | Explanatory Variables | R2 | Significance When Added Last § | Relative Importance [% of R2] (Mathematical Sign of Regression Coefficient °) |
|---|---|---|---|---|
| HMerror | HMref | 0.29 | 0.093 (*) | 7.3 (+) |
| growth period | 0.581 | 5.9 | ||
| plot | 0.101 | 83.6 | ||
| DMref | 0.222 | 3.2 (+) | ||
| CPerror | HMref | 0.59 | <0.000 *** | 63.8 (+) |
| growth period | 0.004 ** | 18.3 | ||
| plot | 0.163 | 15.7 | ||
| DMref | 0.117 | 2.3 (+) |
| Herbage Parameter | Look-Up Tables ° | On-Site NIRS | On-Site NIRS Corrected * | On-Site NIRS Corrected with LOFO † | MSI-Model ‡ |
|---|---|---|---|---|---|
| Absolute error§: | |||||
| HM (kg DM ha−1) | - | - | - | - | 1043.1 ± 1003.1 |
| DM (g kg−1 FM) | 51.0 ± 49.5 | 32.1 ± 31.1 | 14.7 ± 13.0 | 15.0 ± 13.1 | - |
| CP (g kg−1 DM) | 41.4 ± 30.1 | 49.1 ± 33.7 | 7.5 ± 5.4 | 7.7 ± 5.4 | 54.4 ± 45.2 |
| CF (g kg−1 DM) | 29.5 ± 23.3 | 23.0 ± 15.3 | 7.1 ± 5.4 | 7.5 ± 5.6 | - |
| NDF (g kg−1 DM) | 53.9 ± 41.2 | 41.0 ± 33.7 | 24.8 ± 22.1 | 26.6 ± 22.9 | - |
| ADF (g kg−1 DM) | 27.4 ± 22.4 | 21.8 ± 15.8 | 5.8 ± 4.8 | 6.0 ± 5.0 | - |
| Absolute percentage error#: | |||||
| HM (%) | - | - | - | - | 99.7 ± 84.9 |
| DM (%) | 23.2 ± 15.2 | 16.9 ± 13.6 | 7.7 ± 6.1 | 7.9 ± 6.1 | - |
| CP (%) | 19.6 ± 12.7 | 22.2 ± 12.2 | 4.1 ± 3.4 | 4.2 ± 3.4 | 33.2 ± 39.1 |
| CF (%) | 14.4 ± 11.7 | 12.0 ± 9.5 | 3.5 ± 2.8 | 3.7 ± 2.9 | - |
| NDF (%) | 11.7 ± 8.5 | 9.9 ± 9.8 | 5.9 ± 6.0 | 6.3 ± 6.2 | - |
| ADF (%) | 10.8 ± 8.5 | 9.3 ± 7.8 | 2.4 ± 2.1 | 2.5 ± 2.2 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, L.; Huguenin-Elie, O.; Latsch, R.; Simmler, M.; Dubois, S.; Umstatter, C. Comparison of Spectral Reflectance-Based Smart Farming Tools and a Conventional Approach to Determine Herbage Mass and Grass Quality on Farm. Remote Sens. 2020, 12, 3256. https://doi.org/10.3390/rs12193256
Hart L, Huguenin-Elie O, Latsch R, Simmler M, Dubois S, Umstatter C. Comparison of Spectral Reflectance-Based Smart Farming Tools and a Conventional Approach to Determine Herbage Mass and Grass Quality on Farm. Remote Sensing. 2020; 12(19):3256. https://doi.org/10.3390/rs12193256
Chicago/Turabian StyleHart, Leonie, Olivier Huguenin-Elie, Roy Latsch, Michael Simmler, Sébastien Dubois, and Christina Umstatter. 2020. "Comparison of Spectral Reflectance-Based Smart Farming Tools and a Conventional Approach to Determine Herbage Mass and Grass Quality on Farm" Remote Sensing 12, no. 19: 3256. https://doi.org/10.3390/rs12193256
APA StyleHart, L., Huguenin-Elie, O., Latsch, R., Simmler, M., Dubois, S., & Umstatter, C. (2020). Comparison of Spectral Reflectance-Based Smart Farming Tools and a Conventional Approach to Determine Herbage Mass and Grass Quality on Farm. Remote Sensing, 12(19), 3256. https://doi.org/10.3390/rs12193256







