Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Characterization of GCP Powder
2.2.1. Sample Preparation
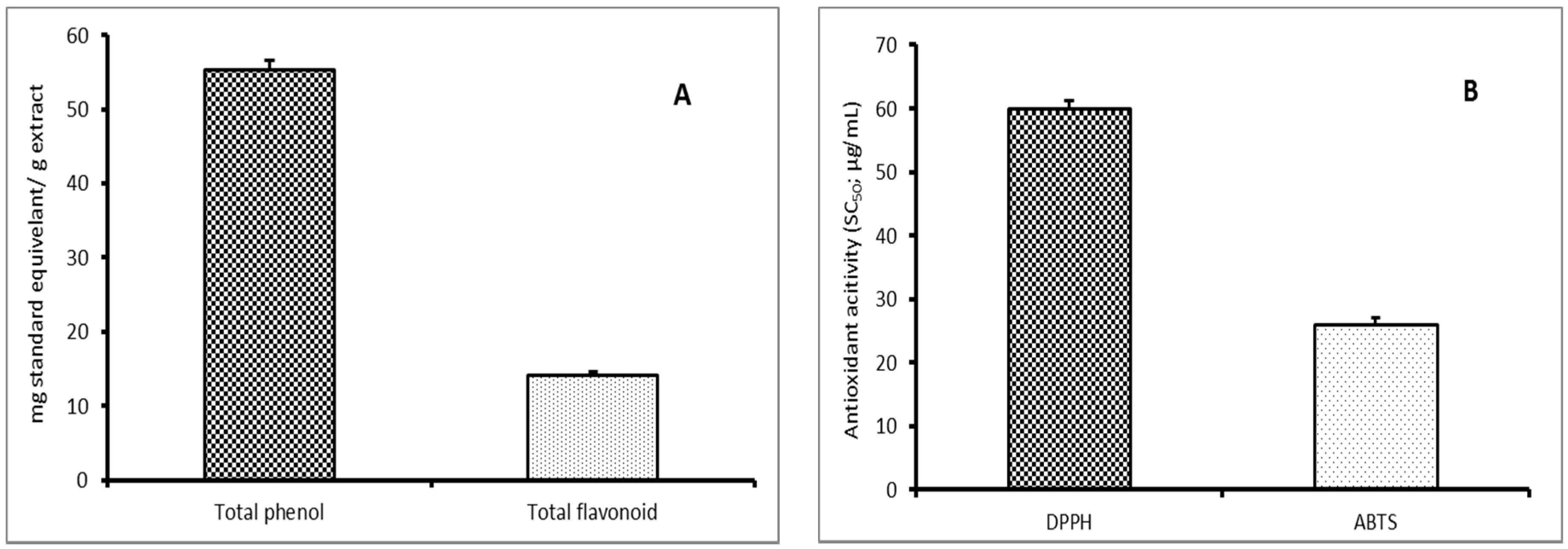
2.2.2. Total Phenolic Compounds Estimation
2.2.3. Total Flavonoids Estimation
2.2.4. Scavenging Activity Estimation
2.3. Chicks, Diets, and Experimental Design
2.4. Measured Traits
2.5. Breast Meat Quality and Sensory Evaluation
2.6. Antimicrobial Activity
2.6.1. Preparation of Coffee Bean Seed Extract (CBSE)
2.6.2. Antibacterial Activity of Coffee Bean Seed Extract against Six Bacterial Strains
2.6.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Coffee Bean Seed Extract
2.6.4. Microbial Count
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization
3.2. Growth Performance
3.3. Carcass Characteristics
3.4. Blood Biochemical Parameters
3.5. Meat Quality Parameters
3.5.1. pH
3.5.2. Thiobarbituric Acid Reactive Species
3.5.3. Total Volatile Base Nitrogen (TVBN)
3.5.4. Meat Color
3.5.5. Sensory Evaluation
3.6. Antibacterial Activity
3.7. Determination of MIC and MBC
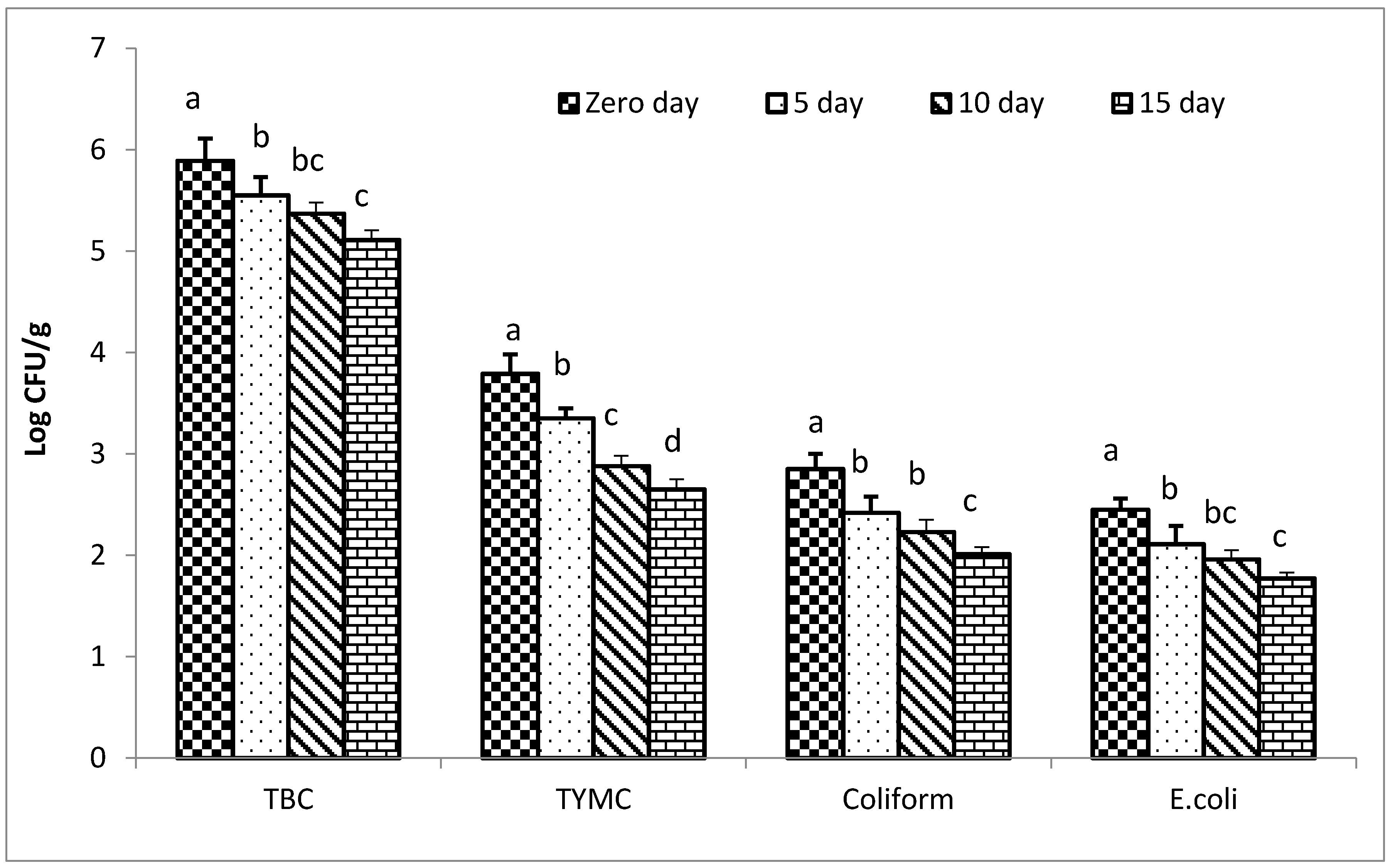
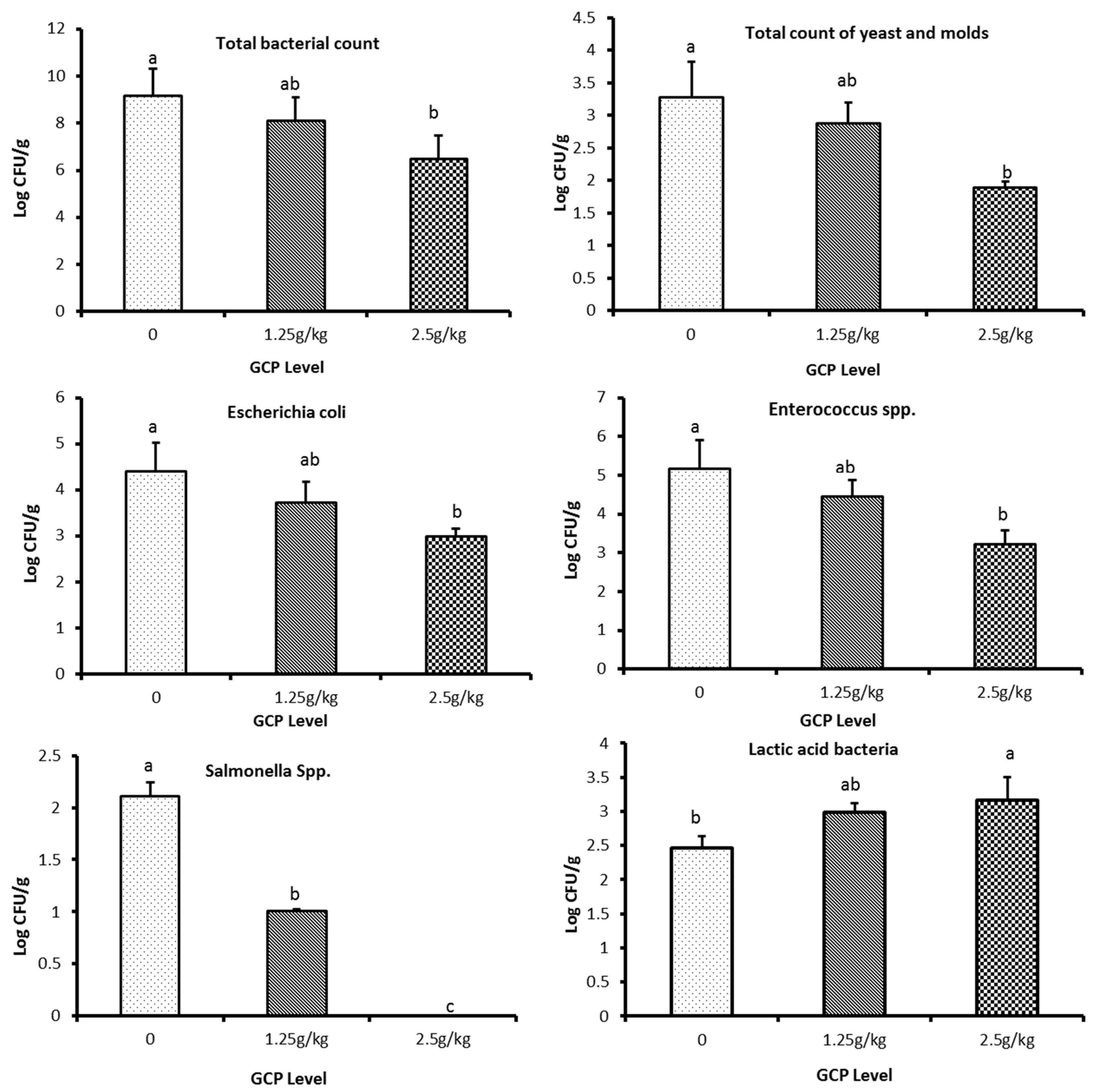
3.8. Impacts of the Coffee Extract on Microbial Count
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.; Bhatt, P.; Marappan, G.; et al. Use of licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: Current knowledge and prospects. Animal 2019, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animal 2019, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Ayasan, T.; Abdel-Daim, M.M. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2019, 703, 134399. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, E.M.; Mahrose, K.M.; Attia, F.A.M.; Swelum, A.A.; Taha, A.E.; Shewita, R.S.; Hussein, E.O.S.; Alowaimer, A.N. Laying performance, physical, and internal egg quality criteria of hens fed distillers dried grains with solubles and exogenous enzyme mixture. Animals 2019, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, E.M.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotic 2020, 9, 210. [Google Scholar] [CrossRef]
- Gado, A.R.; Ellakany, H.F.; Elbestawy, A.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Mahgoub, S.A. Herbal medicine additives as powerful agents to control and prevent avian influenza virus in poultry—A review. Ann. Anim. Sci. 2019, 19, 905–935. [Google Scholar] [CrossRef]
- Arif, M.; Hayat, Z.; Abd El-Hack, M.E.; Saeed, M.; Imran, H.M.; Alowaimer, A.N.; Saadeldin, I.M.; Taha, A.E.; Swelum, A.A. Impacts of supplementing broiler diets with a powder mixture of black cumin, Moringa and chicory seeds. S. Afr. J. Anim. Sci. 2019, 49, 564–572. [Google Scholar] [CrossRef]
- Mahgoub, S.A.M.; Abd El-Hack, M.E.; Saadeldin, I.M.; Hussein, M.A.; Swelum, A.A.; Alagawany, M. Impact of Rosmarinus officinalis cold-pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail. Poult. Sci. 2019, 98, 2139–2149. [Google Scholar] [CrossRef]
- Ocak, N.; Erener, G.; Burak, A.K.F.; Sungu, M.; Altop, A.; Ozmen, A. Performance of broilers fed diets supplemented with dry peppermint (Mentha piperita L.) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech J. Anim. Sci. 2008, 53, 69–175. [Google Scholar] [CrossRef]
- Fotina, A.A.; Fisinin, V.I.; Surai, P.F. Recent developments in usage of natural antioxidants to improve chicken meat production and quality. Bulg. J. Agric. Sci. 2013, 19, 889–896. [Google Scholar]
- Taha, A.E.; Hassan, S.S.; Shewita, R.S.; El-seidy, A.A.; Abd El-Hack, M.E.; Hussein, E.S.O.; El-Edel, M.A. Effects of supplementing broiler diets with coriander seed powder on growth performance, blood haematology, ileum microflora and economic efficiency. J. Anim. Aphysiol. Anim. Nutr. 2019, 103, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Ashour, E.A.; Bin-Jumah, M.; Abou Sayed-Ahmed, E.T.; Osman, A.O.; Taha, A.E.; Momenah, M.A.; Abd El-Hack, M.E. Effects of dried okra fruit (Abelmoschus esculentus L.) powder on growth, carcass characteristics, blood indices, and meat quality of stored broiler meat. Poult. Sci. 2020, 99, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Bin-Jumah, M.; Abd El-Hack, M.E.; Elaraby, G.; Swelum, A.A.; Taha, A.E.; Ashour, E.A. Dietary supplementation of soybean glycinin can alter the growth, carcass traits, blood biochemical indices, and meat quality of broilers. Poult. Sci. 2020, 99, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Tosh, J.; Ruch, N.; Fay, M. Growing coffee: On the bases of plastid and nuclear DNA sequences; implication for the size, morphology, distribution and evolutionary history of coffee. Bot. J. Linn. Soc. 2011, 167, 357–377. [Google Scholar] [CrossRef]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Acheson, K.J.; Zahorska-Markiewicz, B.; Pittet, P.; Anantharaman, K.; Jéquier, E. Caffeine and coffee: Their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial activity and chemical constitution of the crude, phenolic-rich extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.; Sitohy, M. Hindering milk quality storage deterioration by mild thermization combined with methylated chickpea protein. Int. Food Res. J. 2014, 21, 693–701. [Google Scholar]
- Sitohy, M.; Osman, A.; Gharib, A.; Chobert, J.-M.; Haertlé, T. Preliminary assessment of potential toxicity of methylated soybean protein and methylated β-lactoglobulin in male Wistar rats. Food Chem. Toxic. 2013, 59, 618–625. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Microstructure and thermal characteristics of Thai indigenous and broiler chicken muscles. Poult. Sci. 2005, 84, 328–336. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Fermandez-Lopez, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatball. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef]
- Sow, T.M.A.; Grongnet, J.F. Sensory characteristics and consumer reference for chicken meat in Guinea. Poult. Sci. 2010, 89, 2281–2292. [Google Scholar] [CrossRef]
- Zhuang, H.; Savage, E.M. Effect of postmortem deboning time on sensory descriptive flavor and texture profiles of cooked boneless skinless broiler thighs. Food Sci. Technol. 2011, 44, 2087–2090. [Google Scholar] [CrossRef]
- Antonio, A.G.; Iorio, N.L.P.; Pierro, V.S.S.; Candreva, M.S.; Farah, A.; Dos Santos, K.R.N.; Maia, L.C. Inhibitory properties of Coffee canephora extract against oral bacteria and its effect on demineralization of deciduous teeth. Arch. Oral Biol. 2011, 56, 556–564. [Google Scholar] [CrossRef] [PubMed]
- ELSayed, M.N. Determination of antibacterial activity of Green Coffee Arabica bean extract on multidrug resistance Pseudomonas aeruginosa (ATCC 27853). IOSR J. Pharm. 2018, 6, 33–38. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clinic. Path. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Akl, B.A.; Nader, M.M.; El-Saadony, M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp. sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020, 11, 1–8. [Google Scholar]
- El-Saadony, M.T.; Elsadek, M.F.; Alaa, S.; Mohamed Taha, A.E.; Ahmed, B.M.; Saad, A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods 2020, 9, 639. [Google Scholar] [CrossRef]
- Tien, L.; Divyaraj, Y.M.; Deepak, R.D. Comparative evaluation of antimicrobial efficacy of coffee extract and 0.2% chlorhexidine mouthwash on the periodontal pathogens Porphyromonas Gingivalis, Prevotella Intermedia, Fusobacterium Nucleatum and Aggregatibacter Actinomycetemcomitans an In Vitro Study. Adv. Hum. Biol. 2016, 6, 99–103. [Google Scholar]
- Dash, S.K.; Chakraborty, S.P.; Mandal, D.; Roy, S. Isolation and characterization of multidrug resistant uropathogenic Escherichia coli from urine sample of urinary tract infected patients. Int. J. Life Sci. Pharm. Res. 2012, 2, 25–39. [Google Scholar]
- Harrigen, W.F.; Mccance-Margart, E. Laboratory Methods in Food and Dairy Microbiology; Academic Press: London, UK, 1976; pp. 1–115. [Google Scholar]
- Sheiha, A.M.; Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Metwally, K.A.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; El-Saadony, M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals 2020, 10, 430. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef]
- Richard, A.; Szabo, E.; Todd, C.D.; Jean, R.E. Method to isolate Escherichia coli 0157.H7 from food. J. Food Prot. 1986, 49, 768–772. [Google Scholar]
- Edwards, E.A.; Hilderbrand, R.L. Method for identifying Salmonella and Shigella directly from the primary isolation plate by coagglutination of protein A-containing staphylococci sensitized with specific antibody. J. Clin. Microbiol. 1976, 3, 339–343. [Google Scholar] [PubMed]
- Kurtzman, C.; Fell, J.W. The Yeasts, a Taxonomic Study; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Argyri, A.A.; Zoumpopoulou, G.; Karatizas, K.A.; Tsakalidou, E.; Nychas, G.J.E.; Paganou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by In Vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Franco, C.M.; Va´zquez, B.I.; Fente, C.A.; Barros-Vela´zquez, J.; Cepeda, A. Evaluation of Chromocult enterococci agar for the isolation and selective enumeration of Enterococcus spp. in broilers. Lett. Appl. Microbiol. 2005, 41, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. EurAsian J. BioSci. 2019, 13, 105–112. [Google Scholar]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffee species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical profile and antioxidant capacity of coffee plant organs compared to green and roasted coffee beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef]
- Perić, L.; Žikić, D.; Lukić, M. Application of alternative growth promoters in broiler production. Biotechnol. Anim. Husb. 2009, 25, 387–397. [Google Scholar] [CrossRef]
- Cardoso, V.S.; De-Lima, C.A.R.; De-Lima, M.E.F.; Dorneles, L.E.G.; Danelli, M.G.M. Piperine as a phytogenic additive in broiler diets. Pesqui. Agropecu. Bras. 2012, 47, 489–496. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, H.J.; Kim, I.H. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest. Sci. 2014, 160, 82–88. [Google Scholar] [CrossRef]
- Scheuermann, G.N.; Cunha Junior, A.; Cypriano, L.; Gabbi, A.M. Phytogenic additive as an alternative to growth promoters in broiler chickens. Ciênc. Rural. 2009, 39, 522–527. [Google Scholar] [CrossRef]
- Kohlert, C.; van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Méd. 2000, 66, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002, 9, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Yang, I.Y.; Oh, D.H.; Bae, I.H.; Cho, S.G.; Kong, I.G.; Uuganbayar, D.; Nou, I.S.; Choi, K.S. Effect of green tea by-product on performance and body composition in broiler chicks. Asian-Australas. J. Anim. Sci. 2003, 16, 867–872. [Google Scholar] [CrossRef]
- Guray, E.; Ocak, N.; Altop, A.; Cankaya, S.; Aksoy, H.M.; Ozturk, E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian-Australas. J. Anim. Sci. 2011, 24, 1128. [Google Scholar]
- Marzoni, M.; Chiarini, R.; Castillo, A.; Romboli, I.; De Marco, M.; Schiavone, A. Effects of dietary natural antioxidant supplementation on broiler chicken and Muscovy duck meat quality. ASPR 2014, 32, 359–368. [Google Scholar]
- Swain, B.K.; Johri, T.S. Effects of supplementation of combinations of different levels of selenium and vitamin E on relative weight of some organs and serum enzymes level in broilers. Ind. J. Poult. Sci. 2000, 35, 66–69. [Google Scholar]
- Biswas, A.; Ahmed, M.; Bharti, V.K.; Singh, S.B. Effect of antioxidants on physio-biochemical and hematological parameters in broiler chicken at high altitude. Asian-Australas. J. Anim. Sci. 2011, 24, 246–249. [Google Scholar] [CrossRef]
- Nasir, A.; Aasima, R.; Faneshwar, K.; Vijay, S.; Vivek, S. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Pearson, A.M.; Gillette, T.A. Processed Meats, 3rd ed.; Aspen: Albany, NY, USA; Bonn, Germany; Boston, MA, USA; London, UK, 1996. [Google Scholar]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; Cheang, W.S.; Xiao, J. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef]
- Yi, S.; Li, J.; Zhu, J.; Lin, Y.; Fu, L.; Chen, W.; Li, X. Effect of tea polyphenols on microbiological and biochemical quality of collichthys fish ball. J. Sci. Food Agric. 2011, 91, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Balamatsia, C.C.; Patsias, A.; Kontominas, M.G.; Savvaidis, I. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chem. 2007, 104, 1622–1628. [Google Scholar] [CrossRef]
- Adeyemi, K.D.; Sazili, A.Q. Efficacy of carcass electrical stimulation in meat quality enhancement: A review. Asian-Australas. J. Anim. Sci. 2014, 27, 447–456. [Google Scholar] [CrossRef]
- Xiong, Y.L. Protein oxidation and implications for muscle food quality. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley and Sons: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Gok, V.; Obuz, E.; Akkaya, L. Effect of packaging method and storage time on the chemical, microbiological, and sensory properties of Turkish pastirma—A dry cured beef product. Meat Sci. 2008, 80, 335–344. [Google Scholar] [CrossRef]
- Beach- Larsen, T.; Scholderer, J. Functional foods in Europe: Consumer research, market experiences and regulatory aspects. Trends Food Sci. Technol. 2007, 18, 231–234. [Google Scholar] [CrossRef]
- Banon, S.P.; Daz, M.; Rodrguez, M.D.; Garrido, A.P. Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Sci. 2007, 77, 626–633. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, S.E.; Huh, J.; Han, Y.H.; Lee, M.J. Antibacterial and antioxidative activity of roasted coffee and red ginseng mixture extracts. J Korean Soc. Food Sci. Nutr. 2012, 41, 320–326. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.S. Influence of roasting time on antibacterial and antioxidative effects of coffee extract. Korean J. Food Cook. Sci. 2009, 25, 496–505. [Google Scholar]
- Stauder, M.; Papetti, A.; Mascherpa, D.; Schito, A.M.; Gazzani, G.; Pruzzo, C.; Daglia, M. Antiadhesion and antibiofilm activities of high molecular weight coffee components against Streptococcus mutans. J. Agric. Food Chem. 2010, 58, 11662–11666. [Google Scholar] [CrossRef]
- Deressa, T.; Tamiru, T.; Biadgo, B.; Belete, D.; Zewdu, S. Antimicrobial Potentials of Apis Multiflora honey in combination with coffee and cinnamon extracts against common human pathogenic bacteria. Med. Aromat. Plants 2015, 4, 2167-0412. [Google Scholar]
- Sura, I. The antibacterial activity of ethanolic extract of some Plant containing polyphenols component against Streptococcus mutans isolated from teeth caries International. J. Med. Pharm. 2016, 2, 82–86. [Google Scholar]
- Akhlaghi, N.; Sadeghi, M.; Fazeli, F.; Akhlaghi, S.; Mehnati, M.; Sadeghi, M. The antibacterial effects of coffee extract, chlorhexidine, and fluoride against Streptococcus mutans and Lactobacillus plantarum: An in vitro study. Dent. Res. J. 2019, 16, 346–353. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against Enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G.; da Costa, T.H. Is coffee a functional food? Br. J. Nutr. 2005, 93, 773–782. [Google Scholar] [CrossRef]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef]
- Vinod, D. Rangari, Pharmacognosy and Phytochemistry, 1st ed.; Carrier Publication: Nashik, Maharashtra, India, 2004; Volume II, pp. 311–312. [Google Scholar]
| Items | Basal Diets | |
|---|---|---|
| Starter (1–3 Weeks) | Finisher (4–5 Weeks) | |
| Ingredients (%) | ||
| Soybean meal (44% CP) | 31.65 | 27.15 |
| Gluten meal | 6.50 | 6.1 |
| Yellow corn | 57.13 | 60.53 |
| Vitamin and mineral premix * | 0.30 | 0.3 |
| Ca2HPO4 | 1.70 | 1.5 |
| Salt | 0.30 | 0.3 |
| Limestone | 1.24 | 1.15 |
| Vegetable oil | 1.00 | 2.85 |
| L-Lysine hydrochloride | 0.13 | 0.1 |
| DL Methionine | 0.05 | 0.02 |
| Nutrients (%) | ||
| Metabolizable Energy (MJ/kg diet) | 12.35 | 12.97 |
| Crude protein | 23.00 | 21.00 |
| Available phosphorous | 0.45 | 0.40 |
| Calcium | 1.00 | 0.90 |
| Methionine + cysteine | 0.83 | 0.74 |
| Lysine | 1.20 | 1.05 |
| Items | Green Coffee Powder (g/kg Diet) | SEM n = 6 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| Live body weight (g) | |||||
| 1 week | 129.7 | 130.3 | 130.3 | 0.20 | 0.332 |
| 3 week | 822.5 | 753.2 | 803.4 | 9.05 | 0.049 |
| 5 week | 1824.3 b | 1772.7 c | 1840.3 a | 11.43 | 0.036 |
| Body weight gain (g/day) | |||||
| 1–3 week | 49.5 | 44.5 | 48.1 | 1.15 | 0.195 |
| 3–5 week | 71.6 | 72.9 | 74.1 | 1.32 | 0.790 |
| 1–5 week | 60.5 | 58.7 | 61.1 | 0.94 | 0.615 |
| Daily feed intake (g/day) | |||||
| 1–3 week | 63.7 | 57.8 | 61.9 | 1.37 | 0.215 |
| 3–5 week | 112.8 | 112.4 | 111.3 | 2.10 | 0.967 |
| 1–5 week | 88.2 | 85.1 | 86.6 | 1.13 | 0.598 |
| Feed conversion ratio (g feed/g gain) | |||||
| 1–3 week | 1.29 | 1.31 | 1.29 | 0.03 | 0.969 |
| 3–5 week | 1.58 a | 1.55 b | 1.50 c | 0.04 | 0.027 |
| 1–5 week | 1.46 a | 1.46 a | 1.42 b | 0.03 | 0.043 |
| Traits (% of LBW) | Green Coffee Powder (g/kg Diet) | SEM n = 12 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| Carcass | 75.40 | 74.14 | 75.16 | 0.46 | 0.557 |
| Dressing | 79.14 | 78.27 | 79.19 | 0.35 | 0.543 |
| Liver | 1.95 c | 2.21 b | 2.36 a | 0.13 | 0.045 |
| Gizzard | 1.25 | 1.47 | 1.20 | 0.07 | 0.222 |
| Heart | 0.54 | 0.45 | 0.47 | 0.03 | 0.358 |
| Abdominal fat | 1.07 b | 1.40 a | 0.90 c | 0.10 | 0.015 |
| Intestine | 4.52 | 4.87 | 4.96 | 0.27 | 0.829 |
| Intestine length (cm) | 88.33 a | 81.67 c | 85.00 b | 2.04 | 0.041 |
| Lymphoid organs | |||||
| Spleen | 0.08 b | 0.14 a | 0.13 a | 0.01 | 0.013 |
| Bursa | 0.09 b | 0.12 a | 0.08 b | 0.01 | 0.031 |
| Traits | Green Coffee Powder (g/kg Diet) | SEM n = 12 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| Total protein (mg/dL) | 6.40 | 6.47 | 6.30 | 0.04 | 0.664 |
| Albumin (mg/dL) | 3.23 | 3.54 | 3.31 | 0.11 | 0.584 |
| Globulin (mg/dL) | 3.17 | 2.93 | 2.99 | 0.10 | 0.982 |
| A/G ratio | 1.03 | 1.27 | 1.10 | 0.08 | 0.867 |
| ALT (IU/L) | 11.50 | 9.87 | 11.08 | 0.30 | 0.475 |
| AST (IU/L) | 122.67 | 124.00 | 119.00 | 0.98 | 0.188 |
| Urea (mg/dL) | 43.67 | 38.33 | 35.33 | 1.23 | 0.123 |
| Creatinine (mg/dL) | 0.53 | 0.54 | 0.64 | 0.02 | 0.167 |
| Traits | Green Coffee Powder (g/kg Diet) | SEM n = 12 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| Glutathione peroxidase (U/mg protein) | 168.00 | 172.00 | 174.50 | 1.06 | 0.796 |
| Glutathione-S-transferase (U/mg protein) | 5.65 | 6.15 | 6.35 | 0.11 | 0.981 |
| Glutathione reduced (U/mg protein) | 74.85 | 80.50 | 81.50 | 1.05 | 0.980 |
| MDA (nmol/mL) | 3.85 | 3.80 | 3.16 | 0.13 | 0.733 |
| Traits | Green Coffee Powder (g/kg Diet) | SEM n = 12 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| pH | 5.37 c | 5.67 b | 6.33 a | 0.15 | <0.001 |
| TBA (mg/kg) | 0.50 a | 0.41 b | 0.40 b | 0.02 | 0.005 |
| TVBN (mg/100 g) | 6.23 a | 6.02 a | 5.47 b | 0.12 | 0.004 |
| Meat color | |||||
| L* | 48.14 c | 52.09 b | 54.99 a | 0.99 | <0.001 |
| a* | 3.17 c | 3.46 b | 3.96 a | 0.11 | <0.001 |
| b* | 11.61 a | 10.94 b | 10.87 b | 0.12 | <0.001 |
| Traits | Green Coffee Powder (g/kg Diet) | SEM n = 12 Replicates | p Value | ||
|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | |||
| Tenderness | 4.60 | 3.62 | 4.00 | 0.19 | 0.086 |
| Juiciness | 4.53 a | 3.87 ab | 3.20 b | 0.22 | 0.014 |
| Aroma | 4.47 | 3.57 | 3.53 | 0.21 | 0.111 |
| Taste | 4.33 | 3.83 | 3.53 | 0.24 | 0.462 |
| Pathogenic Bacteria | Green Coffee Extract (mg/mL) | ||||
|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |
| Zone of Inhibition (IDZ) in mm | |||||
| Bacillus subtilis ATCC 23857 | 17 ± 0.9 e | 21 ± 0.6 d | 23 ± 0.5 c | 26 ± 0.3 b | 29 ± 0.2 a |
| Bacillus cereus ATCC 14579 | 15 ± 0.4 e | 17 ± 0.5 d | 20 ± 0.6 c | 23 ± 0.5 b | 27 ± 0.3 a |
| Staphylococcus aureus ATCC 25923 | 16 ± 0.5 e | 18 ± 0.6 d | 22 ± 0.6 c | 25 ± 0.3 b | 28 ± 0.4 a |
| Escherichia coli ATCC 43886 | 12 ± 0.6 e | 14 ± 0.5 d | 17 ± 0.5 c | 19 ± 0.2 b | 24 ± 0.1 a |
| Klebsiella pneumoniae ATCC BAA-1705 | 11 ± 0. e | 13 ± 0.4 d | 16 ± 0.3 c | 18 ± 0.1 b | 21 ± 0.5 a |
| Pseudomonas aeruginosa ATCC 27853 | 13 ± 0.2 e | 15 ± 0.1 d | 18 ± 0.3 c | 22 ± 0.2 b | 25 ± 0.2 a |
| Pathogenic Bacteria | Green Coffee Extract (mg/mL) | |
|---|---|---|
| MIC µg/mL | MBC µg/mL | |
| Bacillus subtilis ATCC 23857 | 50 d | 80 d |
| Bacillus cereus ATCC 14579 | 60 c | 100 c |
| Staphylococcus aureus ATCC 25923 | 60 c | 100 c |
| Escherichia coli ATCC 43886 | 80 b | 120 b |
| Klebsiella pneumoniae ATCC BAA-1705 | 80 b | 120 b |
| Pseudomonas aeruginosa ATCC 27853 | 100 a | 150 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, E.A.; El-Hack, M.E.A.; Shafi, M.E.; Alghamdi, W.Y.; Taha, A.E.; Swelum, A.A.; Tufarelli, V.; Mulla, Z.S.; El-Ghareeb, W.R.; El-Saadony, M.T. Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers. Agriculture 2020, 10, 457. https://doi.org/10.3390/agriculture10100457
Ashour EA, El-Hack MEA, Shafi ME, Alghamdi WY, Taha AE, Swelum AA, Tufarelli V, Mulla ZS, El-Ghareeb WR, El-Saadony MT. Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers. Agriculture. 2020; 10(10):457. https://doi.org/10.3390/agriculture10100457
Chicago/Turabian StyleAshour, Elwy A., Mohamed E. Abd El-Hack, Manal E. Shafi, Wed Y. Alghamdi, Ayman E. Taha, Ayman A. Swelum, Vincenzo Tufarelli, Zohair S. Mulla, Waleed R. El-Ghareeb, and Mohamed T. El-Saadony. 2020. "Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers" Agriculture 10, no. 10: 457. https://doi.org/10.3390/agriculture10100457
APA StyleAshour, E. A., El-Hack, M. E. A., Shafi, M. E., Alghamdi, W. Y., Taha, A. E., Swelum, A. A., Tufarelli, V., Mulla, Z. S., El-Ghareeb, W. R., & El-Saadony, M. T. (2020). Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers. Agriculture, 10(10), 457. https://doi.org/10.3390/agriculture10100457











