Trichoderma Contributes to the Germination and Seedling Development of Açaí Palm
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination of açaí Seeds
2.2. Initial Development of açaí Seedlings
- (1)
- application of Trichoderma isolates in seeds;
- (2)
- application of Trichoderma isolates on the preplanting substrate;
- (3)
- biweekly applications of suspensions of Trichoderma isolates on the postplanting substrate;
- (4)
- application of Trichoderma isolates in seeds + application of Trichoderma isolates in the preplanting substrate;
- (5)
- application of Trichoderma isolates in seeds + biweekly applications of suspensions of Trichoderma isolates on the postplanting substrate;
- (6)
- application of the Trichoderma isolates to the preplanting substrate + biweekly applications of suspensions of the Trichoderma isolates on the postplanting substrate
- (7)
- application of Trichoderma isolates in seeds + application of Trichoderma isolates in the preplanting substrate + biweekly applications of suspensions of Trichoderma isolates in the postplanting substrate and,
- (8)
- Treatment control (without any application of Trichoderma).
2.3. Data Analysis
3. Results
3.1. Germination of açaí Seeds
3.2. Initial Development of açaí Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzi, H.; Souza, H.M.; Costa, J.T.M.; Cerqueira, L.S.C.; van Behr, N. Palmeiras No Brasil: Nativas e Exóticas, 1st ed.; Plantarum: Nova Odessa, Brasil, 1996. [Google Scholar]
- Coelho, J.J.V. Forest Economies: A Remedy to Amazonian Deforestation? Indiana Univ. J. Res. 2016, 2, 63–71. [Google Scholar]
- Martinot, J.F.; Pereira, H.D.S.; Silva, S.C.P. Coletar ou Cultivar: As escolhas dos produtores de açaí-da-mata (Euterpe precatoria) do Amazonas. Rev. Econ. Sociol. Rural. 2017, 55, 751–766. [Google Scholar] [CrossRef]
- Conab-Companhia Nacional de Abastecimento. Conjuntura Mensal: Açaí. Setembro 2016; Conab: Brasília, Brazil, 2016. [Google Scholar]
- Tagore, M.P.B.; Canto, O.; Sobrinho, M.V. Políticas públicas e riscos ambientais em áreas de várzea na Amazônia: o caso do PRONAF para produção do açaí. Desenvolv. Meio Ambient. 2018, 45, 194–214. [Google Scholar] [CrossRef]
- Brandão, B.B.; Costa, S.J.; Numes, D.P.; Marinho, G.A.; Erasmo, E.A.L. Seletividade de herbicidas no crescimento inicial da cultura do açaí (Euterpe oleraceae Mart.). J. Biotechnol. Biodivers. 2014, 5, 95–100. [Google Scholar] [CrossRef]
- Oliveira, M.S.P.; Carvalho, J.E.U.; Nascimento, W.M.O. Açaí (Euterpe oleracea Mart.); Funep: Jaboticabal, Brazil, 2000. [Google Scholar]
- Nascimento, W.M.O.; Silva, W.R. Comportamento fisiológico de sementes de açaí (Euterpe oleracea Mart.) submetidas à desidratação. Rev. Bras. Frutic. 2005, 27, 349–351. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Silva, C.F.B.; Oliveira, L.L.B.; Sousa, A.B.O.; Rebouças, J.R.L. Uso do Trichoderma na cultura do melão (479–492). In Trichoderma – Uso na Agricultura; Meyer, M.C., Mazaro, S.M., Silva, J.C., Eds.; Embrapa: Brasília, Brazil, 2019; 538p. [Google Scholar]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions between Trichoderma harzianum Strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 146–153. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed Treatment with Trichoderma harzianum Alleviates Biotic, Abiotic, and Physiological Stresses in Germinating Seeds and Seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- Machado, D.F.M.; Parzianello, F.R.; Silva, A.C.F.; Antoniolli, Z.I. Trichoderma no Brasil: o fungo e o bioagente. Rev. Ciências Agrárias 2012, 35, 274–288. [Google Scholar]
- Santos, G.R.; Ferreira, W.X.; Oliveira, T.M.; Machado, R.C.L.; Silva, G.B. Indução de crescimento em mudas de açaizeiro com o fungo Trichoderma asperrellum. In Ciência, Tecnologia e Desenvolvimento Rural: Compartilhando conhecimentos inovadores e experiências. Intl. J. Edu. Teach 2018. [Google Scholar] [CrossRef]
- Monte, E.; Bettiol, W.; Hermosa, R. Trichoderma e seus mecanismos de ação para o controle de doenças de plantas. In Trichoderma – Uso na Agricultura; Meyer, M.C., Mazaro, S.M., Silva, J.C., Eds.; Embrapa: Brasília, Brazil, 2019; 538p. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Martins, C.C.; Nakagawa, J.; Bovi, M.L.A. Desiccation tolerance of four seedlots of Euterpe edulis Mart. Seed Sci. Tech. 2000, 28, 1–13. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Alfenas, C.A.; Mafia, R.G. Métodos em Fitopatologia; UFV: Viçosa, Brazil, 2007. [Google Scholar]
- Silva, F.A.S. Assistat: Versão 7.7 Beta; DEAG-CTRN-UFCG: João Pessoa, 2014; Available online: http://www.assistat.com (accessed on 20 May 2014).
- Nascimento, W.M.O. Açaí (Euterpe Oleracea Mart.); Rede de Sementes da Amazônia: Manaus, Brazil, 2008. [Google Scholar]
- Cadore, L.S.; Silva, N.G.; Vey, R.T.; Silva, A.C.F. Inoculação de sementes com Trichoderma harzianum e Azospirillum brasiliense no desenvolvimento inicial de arroz. Enciclopédia Biosfera 2016, 13, 1725–1731. [Google Scholar] [CrossRef]
- Silva, F.F.; Castro, E.M.; Moreira, S.I.; Ferreira, T.C.; Lima, A.E.; Alves, E. Emergência e análise ultraestrutural de plântulas de soja inoculadas com Sclerotinia sclerotiorum sob efeito da aplicação de Trichoderma harzianum. Summa Phytopathol. 2017, 43, 41–45. [Google Scholar] [CrossRef][Green Version]
- Bernardes, V.P.; Andrade, D.S.; Lustosa, D.C.; Vieira, T.A.; Rayol, B.P.; Silva, G.B. Avaliação de isolados de Trichoderma na germinação de sementes de jacarandá-do-pará (Dalbergia spruceana). In 44° Congresso Brasileiro de Fitopatologia; Annals of XLIV Congresso Brasileiro de Fitopatologia: Bento Gonçalves, Brazil, 2011. [Google Scholar]
- Machado, D.F.M.; Tavares, A.P.; Lopes, S.J.; Silva, A.C.F. Trichoderma spp. na emergência e crescimento de mudas de cambará (Gochnatia polymorpha (Less.) Cabrera). Revista Árvore 2015, 39, 167–176. [Google Scholar] [CrossRef]
- Popinigs, F. Fisiologia de Sementes, 2nd ed.; AGIPLAN: Brasília, Brazil, 1985. [Google Scholar]
- Santos, M.F.; Costa, D.L.; Matos, J.C.N.; Silva, G.B.; Vieira, T.A.; Lustosa, D.C. Tratamento biológico de sementes de cupuaçu para o controle de fitopatógenos e promoção da germinação. Cadernos de Agroecologia 2018, 13, 1. [Google Scholar]
- Resende, M.L.; Oliveira, J.A.; Guimarães, R.M.; Von Pinho, R.G.; Vieira, A.R. Inoculação de sementes de milho utilizando o Trichoderma harzianum como promotor de crescimento. Ciência e Agrotecnologia 2004, 28, 793–798. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2013. [Google Scholar]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control. 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Gomes, J.M.; Paiva, H.N. Viveiros Florestais-Propagação Sexuada, 3rd ed.; Editora UFV: Viçosa, Brazil, 2004. [Google Scholar]
- Rudek, A.; Garcia, F.A.O.; Bandeira, F.S.P. Avalição da qualidade de mudas de Eucalipto pela mensuração da área foliar com o uso de imagens digitais. Enciclopédia Biosfera 2013, 9, 3776. [Google Scholar]
- Gomes, J.M.; Couto, L.; Leite, H.G.; Xavier, A.; Garcia, S.L.R. Parâmetros morfológicos na avaliação de qualidade de mudas de Eucalyptus grandis. Revista Árvore 2002, 26, 655–664. [Google Scholar] [CrossRef]
- Lima, J.D.; Silva, B.M.S.; Moraes, W.S.; Dantas, V.A.V.; Almeida, C.C. Efeitos da luminosidade no crescimento de mudas de Caesalpinia ferrea Mart. Ex Tul. (Leguminosae, Caesalpinoideae). Acta Amaz. 2008, 38, 5–10. [Google Scholar] [CrossRef]
- França, D.V.C. Interação de Isolados de Trichoderma spp. e Preparados Homeopáticos: Estudo In Vitro e No Desenvolvimento Inicial do Tomateiro-Cereja. Master’s Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2016. [Google Scholar]
- Gava, C.; Menezes, M.E.L. Eficiência de isolados de Trichoderma spp no controle de patógenos de solo em meloeiro amarelo. Revista Ciência Agrônomica 2012, 43, 633–640. [Google Scholar] [CrossRef][Green Version]
- Patekoski, K.D.S.; Pires-Zottarelli, C.L.A. Efeito de Trichoderma spp no controle de podridão de raiz causada por Pythium aphanidermatum e na promoção de crescimento de alface hidropônica. Acta Sci. Biol. Res. 2016, 1, 59–74. [Google Scholar] [CrossRef]
- Jesus, E.P.; Souza, C.H.E.; Pomella, A.W.V.; Costa, R.L.C.; Seixas, L.; Silva, R.B. Avaliação do potencial de Trichoderma asperellum como condicionador de substrato para a produção de mudas de café. Cerrado Agrociências 2011, 2, 7–19. [Google Scholar]
- Carvalho Filho, M.R.; Mello, S.C.M.; Santos, R.P.; Menêzes, J.E. Avaliação de Isolados de Trichoderma na Promoção de Crescimento, Produção de ácido Indolacético In Vitro e Colonização Endofítica de Mudas de Eucalipto; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2008. [Google Scholar]
- Milanesi, P.M.; Blume, E.; Antonioli, Z.I.; Muniz, M.F.B.; Santos, R.F.; Finger, G.; Durigon, M.R. Biocontrole de Fusarium spp. com Trichoderma spp. e promoção de crescimento em plântulas de soja. Rev. Ciências Agrárias 2013, 36, 347–356. [Google Scholar]
- Azevedo, G.B.; Novaes, Q.S.; Azevedo, G.T.O.S.; Silva, H.F.; Sobrinho, G.G.R.; Novaes, A.B. Efeito de Trichoderma spp. no crescimento de mudas clonais de Eucalyptus camaldulensis. Sci. For. 2017, 45, 343–352. [Google Scholar] [CrossRef]
- Junges, E.; Manzoni, C.G.; Milanesi, P.; Brand, S.C.; Durigon, M.R.; Blume, E.; Muniz, M.F.B. Germinação e vigor de sementes de arroz semeadas em substrato tratado com o bioprotetor Trichoderma spp em formulação líquida ou pó. Cadernos de Agroecologia 2007, 2, 2. [Google Scholar]
- Silva, V.N.; Guzzo, S.D.; Lucon, C.M.M.; Harakava, R. Promoção de crescimento e indução de resistência à antracnose por Trichoderma spp. em pepineiro. Pesquisa Agropecuária Brasileira 2011, 46, 1609–1618. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 149–260. [Google Scholar]
- Junges, E.; Muniz, M.F.; Mezzomo, R.; Bastos, B.; Machado, R.T. Trichoderma spp. na produção de mudas de espécies florestais. Floresta Ambient. 2016, 23, 237–244. [Google Scholar] [CrossRef]
- Silva, B.D.S. Ação Antimicrobiana de Enzimas Hidrolíticas Produzidas por Trichoderma asperellum e Imobilizadas em Blendas de Polímeros Biodegradáveis. Master’s Thesis, Universidade Federal de Goiás, São Carlos, Brazil, 2011. [Google Scholar]
- Souza, E.P.; Amaral, H.F.; Santos Neto, J.; Nunes, M.P. Alta dosagem de Trichoderma harzianum em tomateiro influencia negativamente a produção de mudas e produção. Revista Terra Cultura 2018, 34, 20–36. [Google Scholar]
- De França, S.K.S.; Cardoso, A.F.; Lustosa, D.C.; Ramos, E.M.L.S.; De Filippi, M.C.C.; Silva, G.B. Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agro. Sustain. Dev. 2014, 35, 317–324. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Wiethan, M.M.S.; Vey, R.T.; Oliveira, J.C.P.; Köpp, M.M.; Silva, A.C.F. Trichoderma na promoção do desenvolvimento de plantas de Paspalum regnellii Mez. Rev. Ciências Agrárias 2019, 42, 135–145. [Google Scholar] [CrossRef]
- Carvalho Filho, M.R.; Martins, I.; Peixoto, G.H.S.; Muniz, P.H.P.; Carvalho, D.D.C.; Mello, S.C. Biological control of leaf spot and growth promotion of Eucalyptus plants by Trichoderma spp. J. Agric. Sci. 2018, 10, 459–467. [Google Scholar] [CrossRef][Green Version]
- Soldan, A.; Watzlawick, L.F.; Botelho, R.V.; Faria, C.M.D.R.; Maia, A.J. Development of forestry species inoculated with Trichoderma spp. fertilized with rock phosphate. Floresta Ambient. 2018, 25, e20160643. [Google Scholar] [CrossRef]
- Amaral, P.P.; Steffen, G.P.K.; Maldaner, J.; Missio, E.L.; Saldanha, C.W. Promotores de crescimento na propagação de caroba. Pesq. Flor. Bras. 2017, 37, 149–157. [Google Scholar] [CrossRef]
- Santos, M.F.; Costa, D.L.; Vieira, T.A.; Lustosa, D.C. Effect of Trichoderma spp. fungus for production of seedlings in Enterolobium Schomburgkii (Benth.) Benth. Aust. J. Crop. Sci. 2019, 13, 1706–1711. [Google Scholar] [CrossRef]
- Lustosa, D.C.; Araújo, A.J.C.; Campo, B.F.; Vieira, T.A. Trichoderma spp. and its effects on the physiological quality of seeds and development of seedlings of African mahogany. Rev. Bras. Cienc. Agrar. 2020, 15. [Google Scholar] [CrossRef]
- Rosmana, A.; Taufik, M.; Asman, A.; Jayanti, N.J.; Hakkar, A.A. Dynamic of Vascular Streak Dieback Disease Incidence on Susceptible Cacao Treated with Composted Plant Residues and Trichoderma asperellum in Field. Agronomy 2019, 9, 650. [Google Scholar] [CrossRef]
- Santos, M.F.; Santos, L.E.; Costa, D.L.; Vieira, T.A.; Lustosa, D.C. Trichoderma spp. on treatment of Handroanthus serratifolius seeds: Effect on seedling germination and development. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Brewer, M.T. Effects of Crop Rotation and Biocontrol Amendments on Rhizoctonia Disease of Potato and Soil Microbial Communities. Agriculture 2020, 10, 128. [Google Scholar] [CrossRef]

| Treatment | Germination (%) | SG | Hypocotyl (mm) |
|---|---|---|---|
| Control | 95.0 ± 4.7 b | 28.4 ± 2.9 ab | 18.4 ± 6.9 ab |
| TAM01 | 98.5 ± 2.2 ab | 28.4 ± 1.1ab | 16.7 ± 5.4 ab |
| TAM02 | 97.0 ± 2.7 ab | 28.9 ± 1.1 a | 18.2 ± 6.3 ab |
| TAM03 | 100.0 ± 0.0 a | 26.4 ± 1.83 b | 16.5 ± 6.4 b |
| Tc | 97.0 ± 2.1 ab | 26.9 ± 2.25 ab | 19.1 ± 9.4 a |
| Tce | 96.0 ± 1.4 b | 28.4 ± 1.33 ab | 17.6 ± 5.9 ab |
| CV (%) | 6.6 | 3.6 | 6.4 |
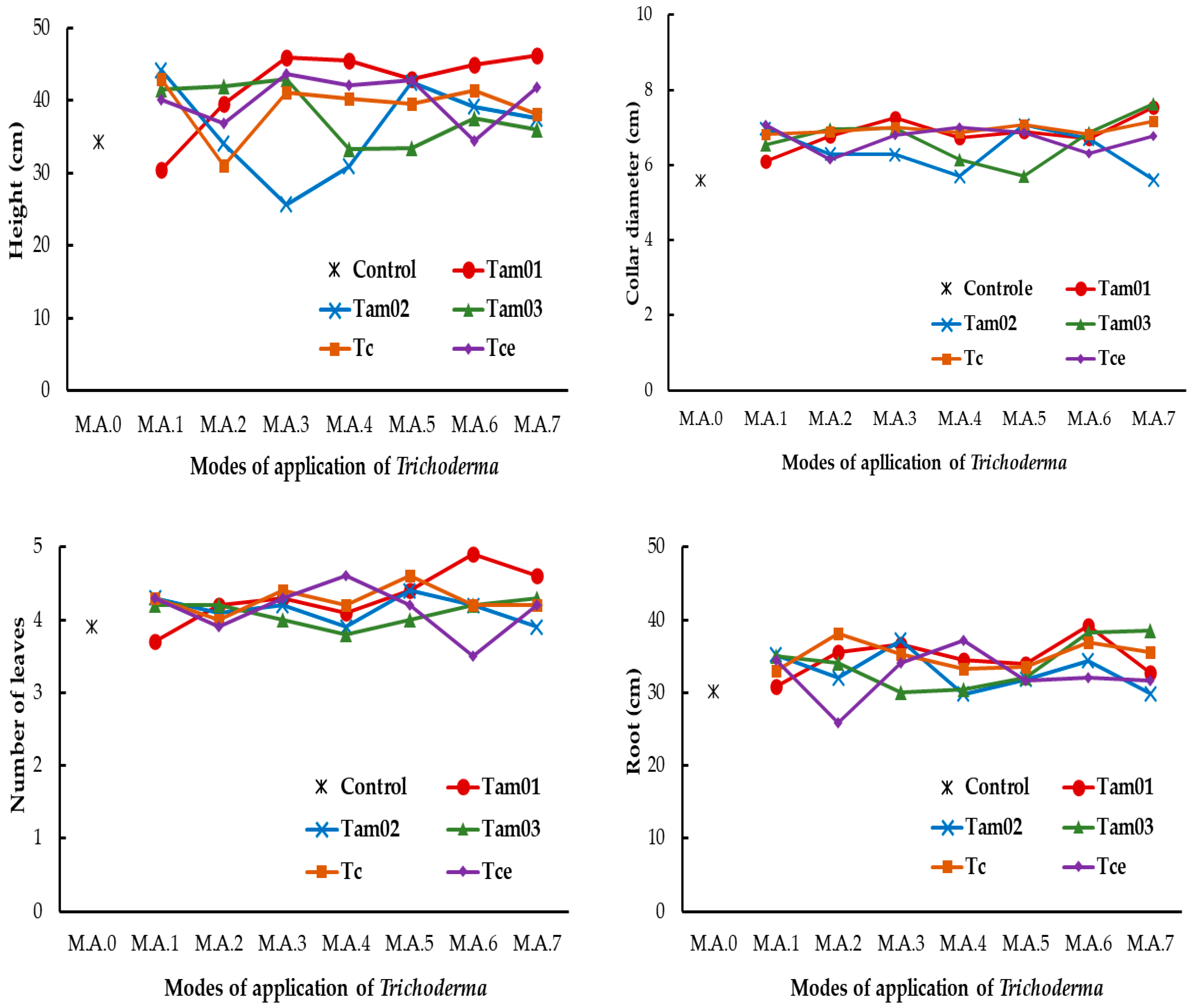
| Source of Variation | Height (cm) | Collar Diameter (cm) | Number of Leaves | Leaf Area (cm) | Root (cm) | Root DM (g) | Aerial Part DM (g) |
|---|---|---|---|---|---|---|---|
| Trichoderma | F = 7.4, p < 0.001 | F = 12.6, p < 0.001 | F = 1.4, p = 0.214 | F = 1.9, p < 0.103 | F = 16.4, p < 0.001 | F = 2.0, p = 0.084 | F = 3.8, p = 0.005 |
| Modes of application | F = 5.2, p < 0.001 | F = 37.9, p < 0.001 | F = 2.3, p < 0.028 | F = 3.4, p = 0.001 | F = 7.3, p < 0.001 | F = 3.4, p = 0.001 | F = 3.4, p = 0.001 |
| Trichoderma × Modes | F = 5.9, p < 0.001 | F = 9.3, p < 0.001 | F = 2.0, p < 0.002 | F = 1.8, p = 0.007 | F = 3.2, p < 0.001 | F = 2.5, p < 0.001 | F = 2.3, p < 0.001 |
| Treatments | Height (cm) | Collar Diameter (cm) | Number of Leaves | Root (cm) | Leaf Area (cm²) | Root DM (g) | Aerial Part DM (g) |
|---|---|---|---|---|---|---|---|
| TAM01 applied to seeds | 30.4 ± 3.8 ns | 6.1 ± 0.6 ns | 3.7 ± 0.6 ns | 30.9 ± 6.3 ns | 107.9 ± 28.5 ns | 1.1 ± 0.3 ns | 2.5 ± 0.7 ns |
| TAM01 applied to preplanting substrate | 39.6 ± 10.3 ns | 6.7 ± 1.0 ** | 4.2 ± 0.6 ns | 35.6 ± 6.4 ns | 105.8 ± 59.3 ns | 1.5 ± 0.5 ns | 3.6 ± 1.5 ns |
| TAM01 applied biweekly to postplanting substrate | 46.0 ± 7.9 * | 7.2 ± 0.9 ** | 4.3 ± 0.6 ns | 36.6 ± 7.4 ns | 128.2 ± 46.8 ** | 1.8 ± 0.4 ns | 4.1 ± 1.0 ** |
| TAM01 applied to seeds + preplanting substrate | 45.5 ± 6.3 * | 6.7 ± 0.9 ** | 4.1 ± 0.5 ns | 34.5 ± 4.5 ns | 104.5 ± 40.1 ns | 1.8 ± 0.6 ns | 3.8 ± 1.0 ns |
| TAM01 applied to seeds + biweekly on postplanting substrate | 42.9 ± 8.8 * | 6.9 ± 1.2 ** | 4.4 ± 0.5 ns | 34.0 ± 7.6 ns | 123.8 ± 45.1 ** | 1.7 ± 0.7 ns | 3.5 ± 1.2 ns |
| TAM01 applied to preplanting substrate + biweekly to substrate | 44.9 ± 7.6 * | 6.7 ± 1.4 ** | 4.9 ± 0.7 ** | 39.2 ± 8.7 ** | 112.6 ± 37.8 ns | 2.3 ± 1.0 ** | 4.5 ± 1.4 ** |
| TAM01 applied to seeds + substrate + biweekly to substrate | 46.2 ± 9.5 * | 7.5 ± 1.4 ** | 4.6 ± 0.5 ns | 32.7 ± 10.0 ns | 96.2 ± 24.1 ns | 1.7 ± 1.0 ns | 3.3 ± 1.6 ns |
| TAM02 applied to seeds | 44.2 ± 7.7 * | 7.0 ± 1.1 ** | 4.3 ± 0.7 ns | 35.2 ± 8.4 ns | 83.2 ± 22.7 ns | 2.0 ± 0.8 ns | 3.6 ± 1.5 ns |
| TAM02 applied to preplanting substrate | 34.0 ± 7.3 ns | 6.3 ± 1.2 ** | 4.1 ± 0.8 ns | 32.0 ± 5.1 ns | 73.7 ± 39.2 ns | 1.3 ± 0.5 ns | 2.6 ± 1.0 ns |
| TAM02 applied biweekly to postplanting substrate | 25.7 ± 6.1 * | 6.3 ± 1.1 ** | 4.2 ± 1.0 ns | 37.2 ± 9.8 ns | 58.6 ± 17.8 ** | 1.9 ± 0.9 ns | 3.1 ± 1.1 ns |
| TAM02 applied to seeds + preplanting substrate | 30.9 ± 4.5 ns | 5.7 ± 0.5 ns | 3.9 ± 0.9 ns | 29.8 ± 1.8 ns | 95.7 ± 19.0 ns | 1.4 ± 0.3 ns | 2.6 ± 0.6 ns |
| TAM02 applied to seeds + biweekly on postplanting substrate | 42.5 ± 6.2 * | 7.1 ± 0.7 ** | 4.4 ± 0.6 ns | 31.8 ± 6.7 ns | 96.9 ± 25.5 ns | 1.7 ± 0.7 ns | 3.8 ± 0.9 ns |
| TAM02 applied to preplanting substrate + biweekly to substrate | 39.1 ± 5.6 ns | 6.7 ± 0.9 ** | 4.2 ± 0.5 ns | 34.4 ± 5.1 ns | 114.6 ± 39.5 ns | 1.5 ± 0.6 ns | 3.1 ± 1.0 ns |
| TAM02 applied to seeds + substrate + biweekly to substrate | 37.5 ± 6.8 ns | 5.6 ± 1.1 ns | 3.8 ± 0.5 ns | 29.9 ± 6.3 ns | 105.2 ± 27.9 ns | 1.0 ± 0.4 ns | 2.3 ± 1.0 ns |
| TAM03 applied to seeds | 41.6 ± 6.4 ns | 6.5 ± 1.2 ** | 4.2 ± 0.6 ns | 35.0 ± 6.1 ns | 125.4 ± 43.7 ** | 1.3 ± 0.3 ns | 3.2 ± 0.8 ns |
| TAM03 applied to preplanting substrate | 41.9 ± 5.5 ns | 6.9 ± 1.0 ** | 4.2 ± 0.5 ns | 34.1 ± 7.3 ns | 105.2 ± 33.0 ns | 1.4 ± 0.6 ns | 3.2 ± 1.1 ns |
| TAM03 applied biweekly to postplanting substrate | 43.0 ± 8.4 * | 6.9 ± 1.1 ** | 4.0 ± 0.6 ns | 30.0 ± 4.1 ns | 103.2 ± 44.7 ns | 1.3 ± 0.3 ns | 3.1 ± 0.8 ns |
| TAM03 applied to seeds + preplanting substrate | 33.3 ± 4.9 ns | 6.1 ± 0.9 ns | 3.8 ± 0.8 ns | 30.5 ± 5.3 ns | 99.2 ± 38.2 ns | 1.2 ± 0.5 ns | 2.5 ± 0.8 ns |
| TAM03 applied to seeds + biweekly on postplanting substrate | 33.4 ± 6.9 ns | 5.7 ± 0.9 ns | 4.0 ± 0.7 ns | 32.1 ± 6.4 ns | 99.0 ± 28.3 ns | 1.2 ± 0.7 ns | 2.5 ± 1.1 ns |
| TAM03 applied to preplanting substrate + biweekly to substrate | 37.5 ± 7.0 ns | 6.9 ± 1.2 ** | 4.2 ± 0.6 ns | 38.3 ± 9.6 ns | 117.8 ± 41.3 ns | 1.2 ± 0.6 ns | 2.7 ± 1.2 ns |
| TAM03 applied to seeds + substrate + biweekly to substrate | 36.0 ± 6.1 ns | 7.6 ± 1.1 ** | 4.3 ± 0.8 ns | 38.5 ± 6.4 ns | 94.1 ± 34.1 ns | 2.3 ± 1.1 ** | 3.6 ± 1.2 ns |
| TC applied to seeds | 42.9 ± 8.0 * | 6.8 ± 1.2 ** | 4.3 ± 0.4 ns | 33.0 ± 4.9 ns | 124.4 ± 52.4 ** | 1.8 ± 0.7 ns | 3.5 ± 1.1 ns |
| TC applied to preplanting substrate | 31.0 ± 7.1 ns | 6.9 ± 1.3 ** | 4.0 ± 0.7 ns | 38.1 ± 7.6 ns | 96.0 ± 47.7 ns | 1.6 ± 0.5 ns | 3.0 ± 0.8 ns |
| TC applied biweekly to postplanting substrate | 41.1 ± 7.3 ns | 7.0 ± 1.2 ** | 4.4 ± 0.5 ns | 35.3 ± 6.1 ns | 91.5 ± 38.8 ns | 1.7 ± 0.7 ns | 3.9 ± 1.4 ns |
| TC applied to seeds + preplanting substrate | 40.2 ± 8.2 ns | 6.9 ± 1.1 ** | 4.2 ± 0.6 ns | 33.3 ± 6.4 ns | 118.1 ± 32.7 ns | 1.4 ± 0.7 ns | 3.4 ± 1.3 ns |
| TC applied to seeds + biweekly on postplanting substrate | 39.5 ± 10.5 ns | 7.1 ± 1.0 ** | 4.6 ± 0.5 ns | 33.6 ± 8.9 ns | 114.1 ± 28.3 ns | 1.6 ± 0.7 ns | 3.5 ± 1.2 ns |
| TC applied to preplanting substrate + biweekly to substrate | 41.4 ± 6.5 ns | 6.8 ± 1.1 ** | 4.2 ± 0.5 ns | 36.9 ± 7.7 ns | 113.1 ± 39.4 ns | 1.6 ± 0.6 ns | 3.4 ± 1.2 ns |
| TC applied to seeds + substrate + biweekly to substrate | 38.1 ± 6.2 ns | 7.2 ± 1.1 ** | 4.2 ± 0.6 ns | 35.6 ± 5.1 ns | 86.0 ± 31.8 ns | 1.9 ± 1.0 ns | 3.1 ± 1.0 ns |
| TCE applied to seeds | 40.1 ± 6.2 ns | 7.1 ± 0.8 ** | 4.3 ± 0.5 ns | 34.5 ± 7.2 ns | 117.5 ± 41.7 ns | 1.6 ± 0.6 ns | 3.4 ± 0.9 ns |
| TCE applied to preplanting substrate | 36.9 ± 7.9 ns | 6.1 ± 1.1 ns | 3.9 ± 0.6 ns | 25.9 ± 4.5 ns | 121.2 ±4 0.0 ** | 1.2 ± 0.6 ns | 2.7 ± 1.2 ns |
| TCE applied biweekly to postplanting substrate | 43.6 ± 6.7 * | 6.8 ± 0.8 ** | 4.3 ± 0.5 ns | 34.1 ± 6.7 ns | 139.9 ± 50.1 ** | 1.4 ± 0.3 ns | 3.4 ± 0.6 ns |
| TCE applied to seeds + preplanting substrate | 42.1 ± 7.6 ns | 7.0 ± 0.9 ** | 4.6 ± 0.5 ns | 37.2 ± 7.6 ns | 114.6 ± 36.6 ns | 1.8 ± 0.6 ns | 3.9 ± 1.0 ns |
| TCE applied to seeds + biweekly on postplanting substrate | 42.8 ± 9.5 * | 6.9 ± 1.4 ** | 4.2 ± 0.6 ns | 31.7 ± 8.8 ns | 122.0 ± 30.7 ** | 1.6 ± 0.7 ns | 3.8 ± 1.3 ns |
| TCE applied to preplanting substrate + biweekly to substrate | 34.4 ± 6.5 ns | 6.3 ± 1.1 ** | 3.5 ± 0.8 ns | 32.0 ± 5.0 ns | 128.7 ± 41.2 * | 1.0 ± 0.5 ns | 2.2 ± 1.1 ns |
| TCE applied to seeds + substrate + biweekly to substrate | 41.8 ± 7.1 ns | 6.8 ± 0.9 ** | 4.2 ± 0.4 ns | 31.6 ± 5.2 ns | 112.4 ± 33.4 ns | 1.6 ± 0.6 ns | 3.4 ± 1.1 ns |
| Control | 34.3 ± 7.9 | 5.6 ± 0.9 | 3.9 ± 0.5 | 30.2 ± 6.2 | 89.8 ± 30.5 | 1.1 ± 0.4 | 2.6 ± 0.9 |
| Trichoderma Isolates | Average Height (cm) | ||||||
|---|---|---|---|---|---|---|---|
| Mode of Application | |||||||
| TS | AS | AQ | TS + AS | TS + AQ | AS + AQ | S + AS + AQ | |
| TAM01 | 30.4 bB | 39.6 abA | 46.0 aA | 45.5 aA | 42.9 aA | 44.9 aA | 46.2 aA |
| TAM02 | 44.2 aA | 34.1 bcBC | 25.7 bD | 30.9 cCD | 42.5 aA | 39.1 abAB | 37.5 bBC |
| TAM03 | 41.6 aA | 41.9 aA | 43.0 aA | 33.3 bcB | 33.4 bB | 37.5 abB | 36.0 bB |
| Tc | 42.9 aA | 31.1 cB | 41.1 aA | 40.2 abA | 39.5 abA | 41.4 abA | 38.1 bAB |
| Tce | 40.1 aAB | 36.9 bcB | 43.6 aA | 42.1 aAB | 42.8 aA | 34.4 bB | 41.8 abAB |
| CV(%) 15.8 | |||||||
| Trichoderma Isolates | Average Collar Diameter (cm) | ||||||
|---|---|---|---|---|---|---|---|
| Modes of Application | |||||||
| TS | AS | AQ | TS + AS | TS + AQ | AS + AQ | TS + AS + AQ | |
| TAM01 | 6.1 bC | 6.8 aB | 7.2 aAB | 6.7 abBC | 6.9 aAB | 6.7 aBC | 7.5 aA |
| TAM02 | 7.0 aA | 6.3 bcBC | 6.3 bBC | 5.7 cCD | 7.1 aA | 6.7 aAB | 5.6 cD |
| TAM03 | 6.5 aBC | 7.0 aB | 7.0 aB | 6.1 bcCD | 5.7 bD | 6.9 aB | 7.6 aA |
| Tc | 6.8 aA | 6.9 aA | 7.0 aA | 6.9 aA | 7.1 aA | 6.8 aA | 7.2 abA |
| Tce | 7.1 aA | 6.1 cC | 6.8 abAB | 7.0 aA | 6.9 aAB | 6.3 aBC | 6.8 abAB |
| CV(%) 7.3 | |||||||
| Trichoderma isolates | Average Leaf Area (cm2) | ||||||
|---|---|---|---|---|---|---|---|
| Modes of Application | |||||||
| TS | AS | AQ | TS + AS | TS + AQ | AS + AQ | TS + AS + AQ | |
| TAM01 | 107.9 abAB | 105.8 aAB | 128.2 abA | 104.5 aAB | 123.8 aAB | 112.6 aAB | 96.2 aB |
| TAM02 | 83.2 bBCD | 73.7 bCD | 58.6 dD | 95.7 aBC | 96.9 aBC | 114.6 aA | 105.2 aAB |
| TAM03 | 125.4 aA | 105.2 aAB | 103.2 bcAB | 99.2 aAB | 99.0 aAB | 117.8 aAB | 94.1 aB |
| Tc | 124.4 aA | 96.0 bBC | 91.5 cBC | 118.1 aAB | 114.1 aAB | 113.1 aAB | 86.0 aC |
| Tce | 117.5 aA | 121.2 aA | 139.9 aA | 114.6 aA | 122.0 aA | 128.7 aA | 112.4 aA |
| CV (%) 21.7 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, B.F.; Araújo, A.J.C.; Felsemburgh, C.A.; Vieira, T.A.; Lustosa, D.C. Trichoderma Contributes to the Germination and Seedling Development of Açaí Palm. Agriculture 2020, 10, 456. https://doi.org/10.3390/agriculture10100456
Campos BF, Araújo AJC, Felsemburgh CA, Vieira TA, Lustosa DC. Trichoderma Contributes to the Germination and Seedling Development of Açaí Palm. Agriculture. 2020; 10(10):456. https://doi.org/10.3390/agriculture10100456
Chicago/Turabian StyleCampos, Bruno Fróes, Anselmo Junior Corrêa Araújo, Cristina Aledi Felsemburgh, Thiago Almeida Vieira, and Denise Castro Lustosa. 2020. "Trichoderma Contributes to the Germination and Seedling Development of Açaí Palm" Agriculture 10, no. 10: 456. https://doi.org/10.3390/agriculture10100456
APA StyleCampos, B. F., Araújo, A. J. C., Felsemburgh, C. A., Vieira, T. A., & Lustosa, D. C. (2020). Trichoderma Contributes to the Germination and Seedling Development of Açaí Palm. Agriculture, 10(10), 456. https://doi.org/10.3390/agriculture10100456






