Curing Behavior, Rheological, and Thermal Properties of DGEBA Modified with Synthesized BPA/PEG Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization
Abstract
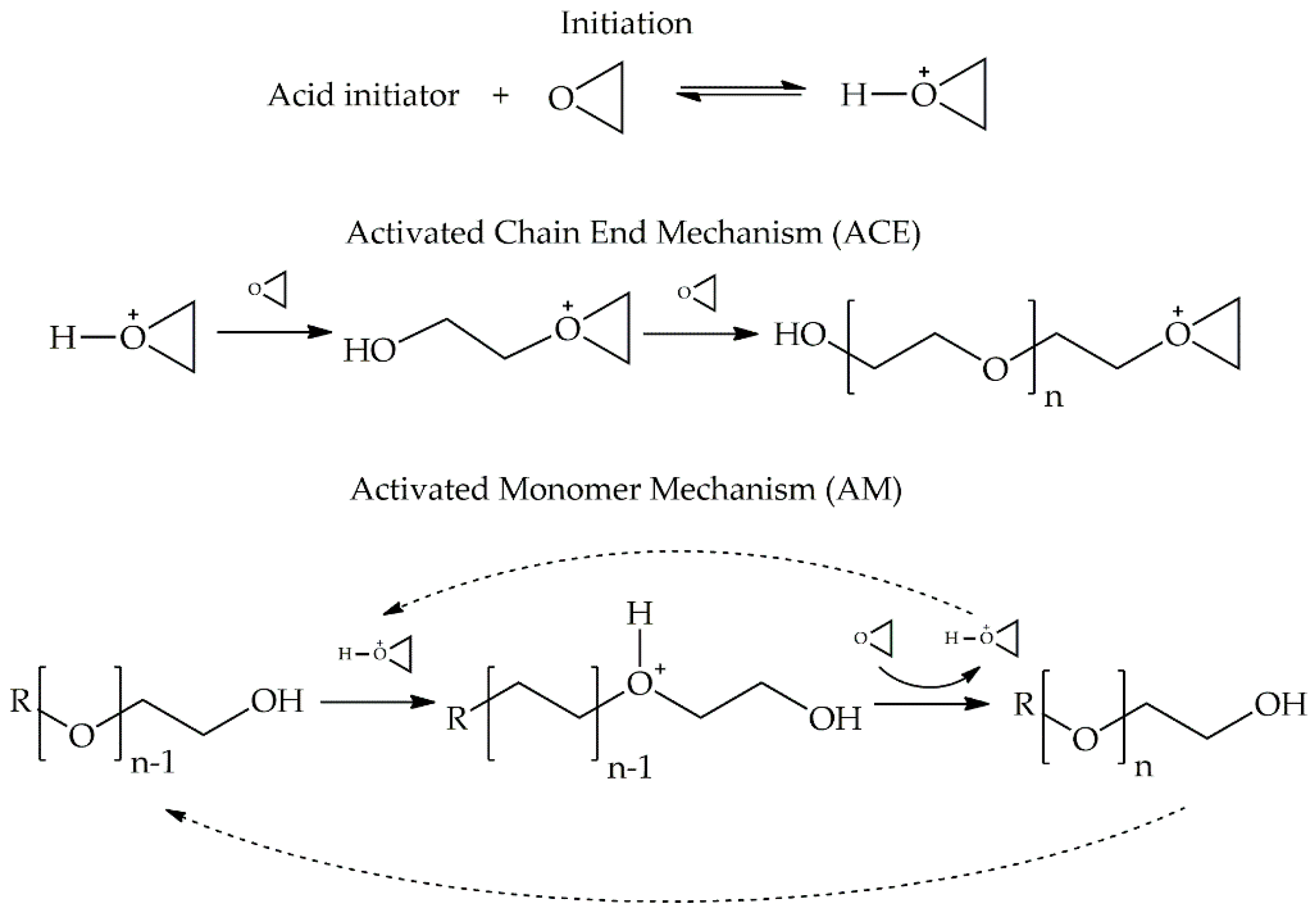
:1. Introduction
2. Materials and Methods
2.1. Materials
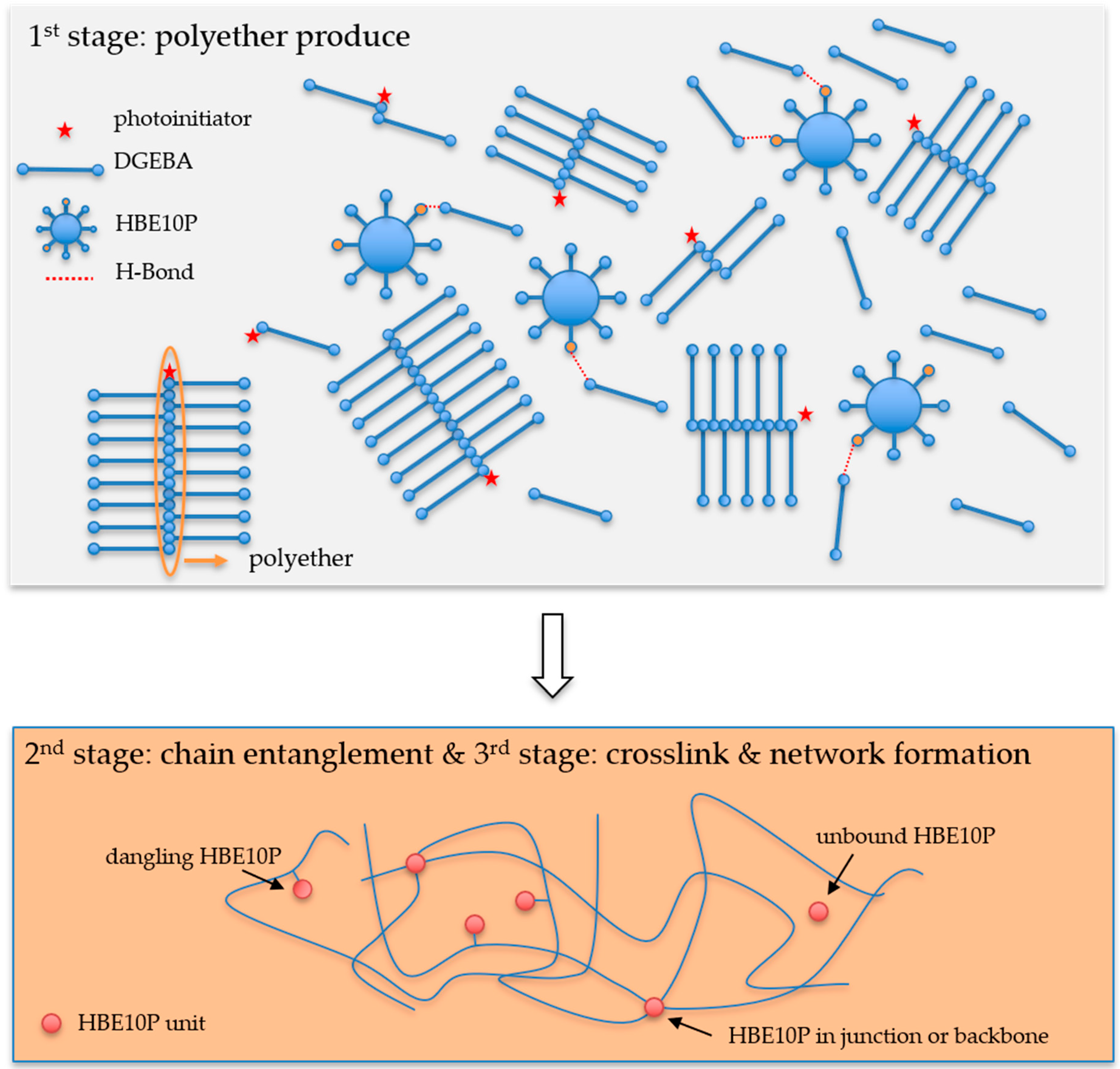
2.2. Synthesis of Hyperbranched Epoxy Resin
2.3. Preparation of Epoxy Mixtures
2.4. Curing Behavior and Thermal Property
2.5. Rheological Property
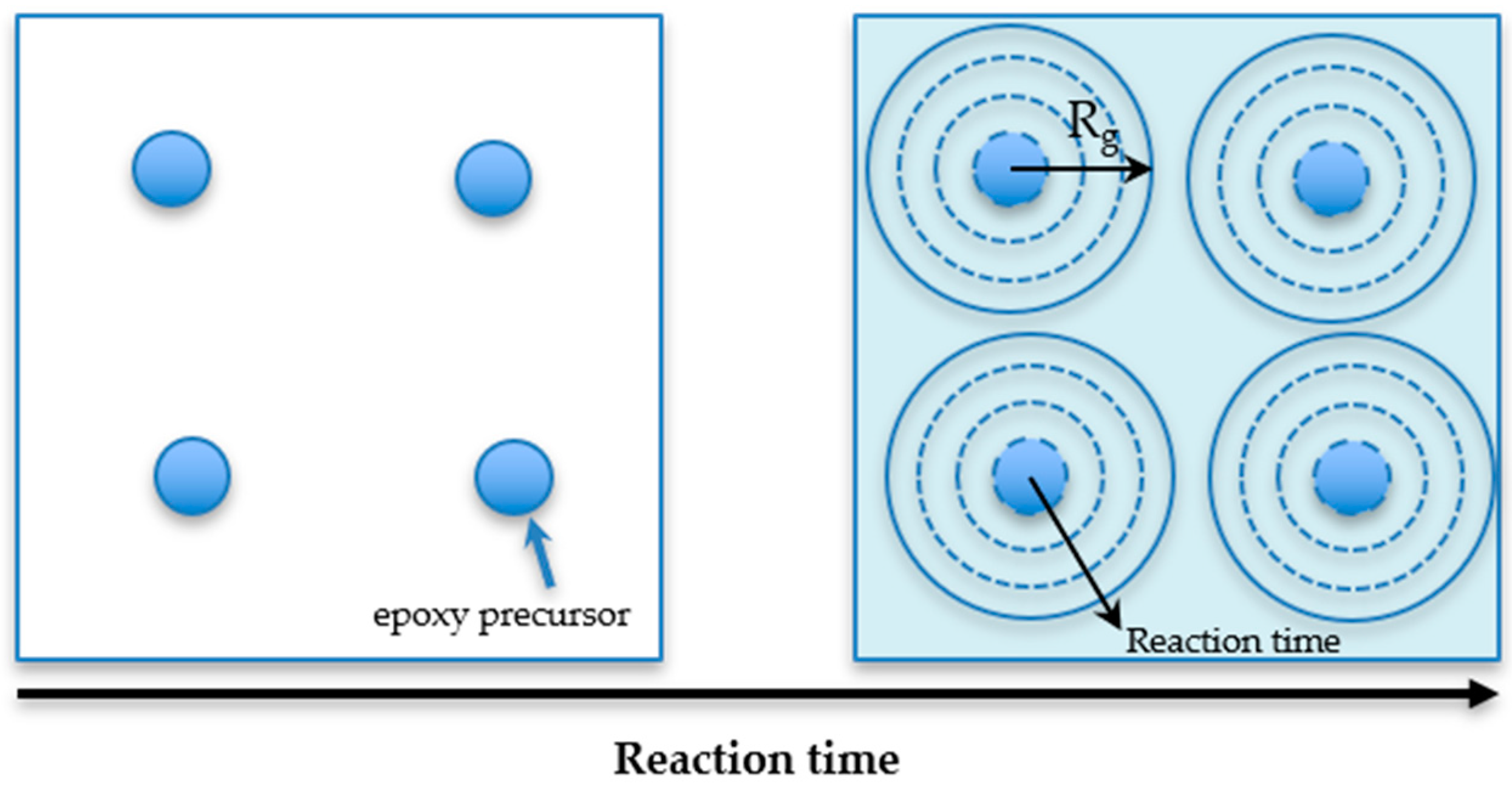
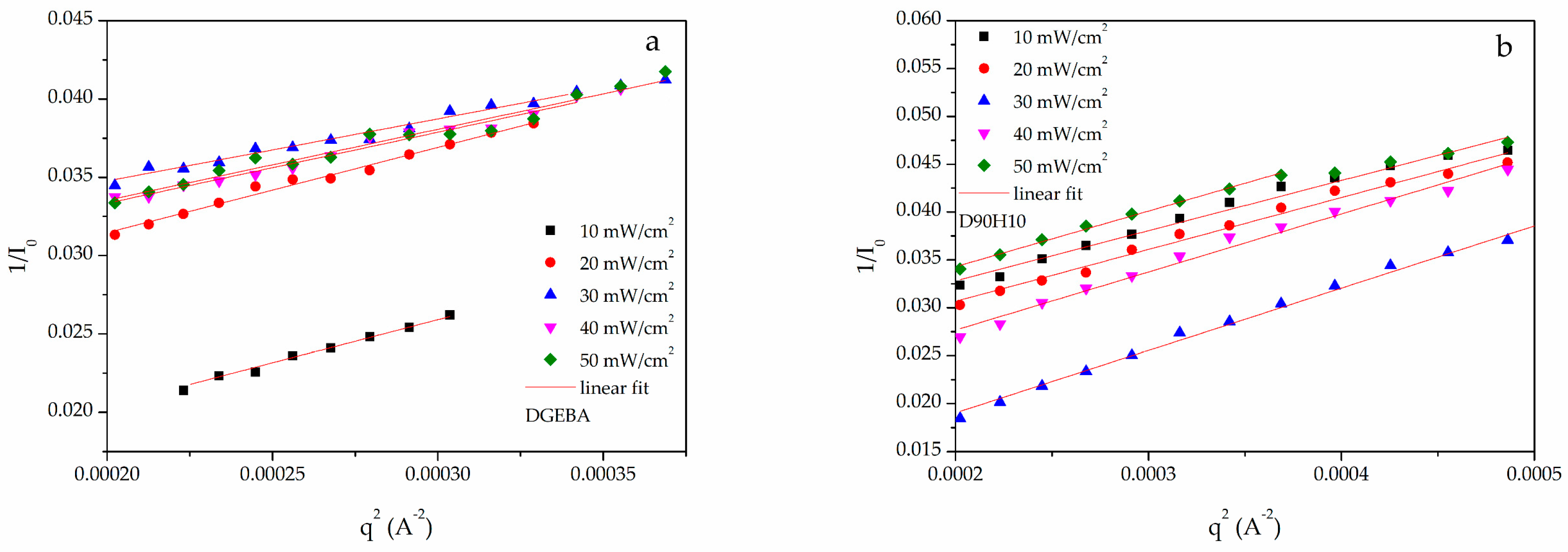
2.6. Radius of Gyration of the Network Segment
2.7. Molecular Weight Between Crosslinking Points
3. Results and Discussion
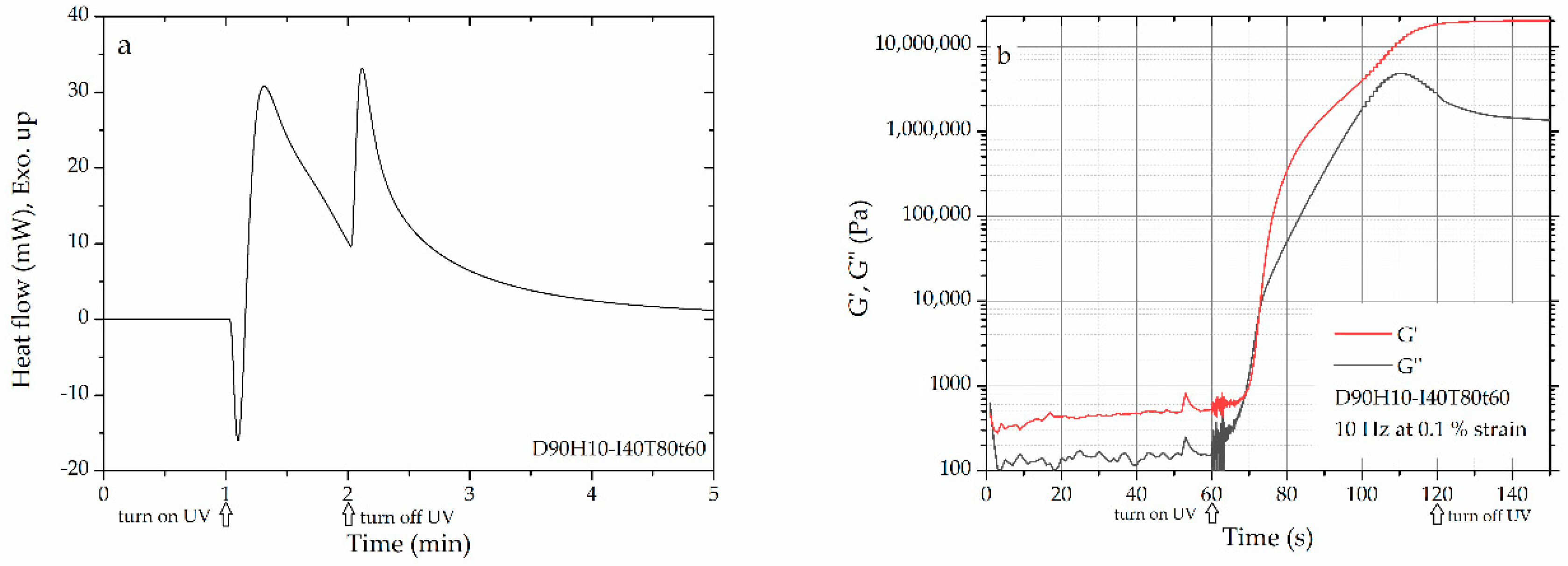
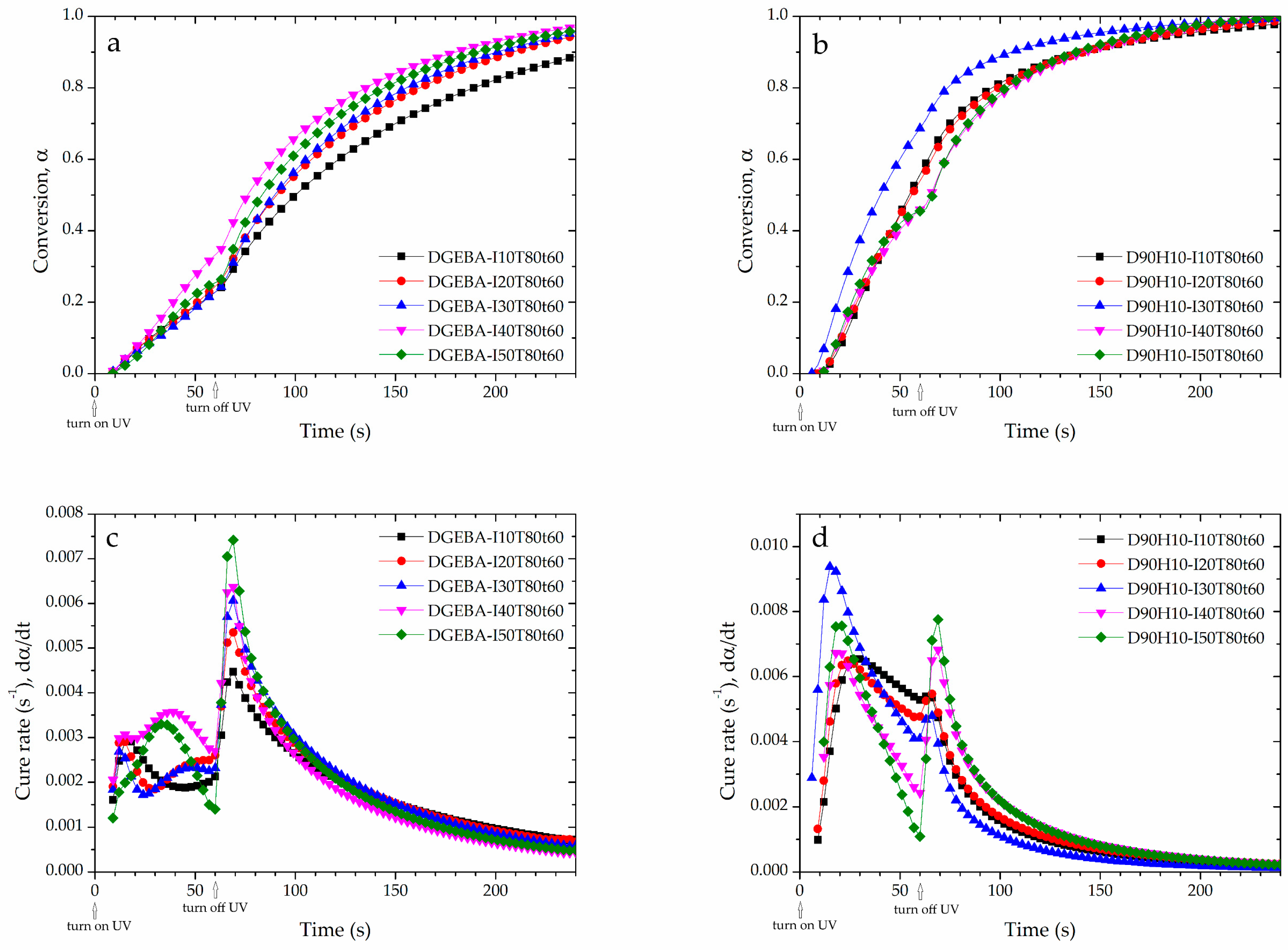
3.1. Curing Behavior and Rheological Property at Various Photo-Curing Conditions
3.2. The Thermal Properties and Radius of Gyration of the Cured Samples at Various Curing Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-A. UV intensity, temperature and dark-curing effects in cationic photo-polymerization of a cycloaliphatic epoxy resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
- Moon, J.; Shul, Y.-G.; Han, H.; Hong, S.; Choi, Y.; Kim, H. A study on UV-curable adhesives for optical pick-up: I. Photo-initiator effects. Int. J. Adhes. Adhes. 2005, 25, 301–312. [Google Scholar] [CrossRef]
- Crivello, J.V. UV and electron beam-induced cationic polymerization. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 8–21. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated crosslinking polymerisation. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Kinetic study of the cationic photopolymerization of epoxy monomers. J. Polym. Sci. Part A: Polym. Chem. 1990, 28, 3429–3443. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef] [Green Version]
- Penczek, S.; Kubisa, P.; Szymanski, R. Activated monomer propagation in cationic polymerizations. Makromol. Chemie. Macromol. Symp. 1986, 3, 203–220. [Google Scholar] [CrossRef]
- Ficek, B.A.; Thiesen, A.M.; Scranton, A.B. Cationic photopolymerizations of thick polymer systems: Active center lifetime and mobility. Eur. Polym. J. 2008, 44, 98–105. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hong, S.; Sun, H.; Kim, M.G.; Choi, K.; Cho, J.; Choi, H.R.; Koo, J.C.; Moon, H.; Byun, D.; et al. UV-curing kinetics and performance development of in situ curable 3D printing materials. Eur. Polym. J. 2017, 93, 140–147. [Google Scholar] [CrossRef]
- Halley, P.J.; George, G.A. Chemorheology of Polymers; Cambridge University Press (CUP): Cambridge, UK, 2009. [Google Scholar]
- Taki, K.; Nakamura, T.; Taki, K.; Tomomi, N. Effects of Curing Conditions and Formulations on Residual Monomer Contents and Temperature Increase of a Model UV Gel Nail Formulation. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Decker, C. UV-Radiation Curing of Adhesives. In Handbook of Adhesives and Surface Preparation; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 221–243. [Google Scholar]
- Peeters, S. Overview of dual-cure and hybrid-cure systems in radiation curing. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Springer Science & Business Media: Berlin, Germany, 1993; Volume 3, pp. 177–217. [Google Scholar]
- Kotch, T.G.; Lees, A.J.; Fuerniss, S.J.; Papathomas, K.I. Luminescent organometallic complexes as visible probes in the isothermal curing of epoxy resins. Chem. Mater. 1992, 4, 675–683. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wang, L.; Peng, P.; Wu, C.; Wu, X.; Jiang, P. Preparation of hyperbranched aromatic polyamide grafted nanoparticles for thermal properties reinforcement of epoxy composites. Polym. Chem. 2011, 2, 1380. [Google Scholar] [CrossRef]
- Guo, Q.; Harrats, C.; Groeninckx, G.; Koch, M. Miscibility, crystallization kinetics and real-time small-angle X-ray scattering investigation of the semicrystalline morphology in thermosetting polymer blends of epoxy resin and poly(ethylene oxide). Polymer 2001, 42, 4127–4140. [Google Scholar] [CrossRef]
- Horng, T.J.; Woo, E.M. Effects of network segment structure on the phase homogeneity of crosslinked poly (ethylene oxide)/epoxy networks. Polymer 1998, 39, 4115–4122. [Google Scholar] [CrossRef]
- Kalogeras, I.M.; Roussos, M.; Christakis, I.; Spanoudaki, A.; Pietkiewicz, D.; Brostow, W.; Vassilikou-Dova, A. Dielectric properties of cured epoxy resin+poly(ethylene oxide) blends. J. Non Crystalline Solids 2005, 351, 2728–2734. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, S.; Zhang, N.; Ma, D. Miscibility of epoxy resins/poly(ethylene oxide) blends cured with phthalic anhydride. Polymer 1994, 35, 2619–2623. [Google Scholar] [CrossRef]
- Gong, W.; Mai, Y.; Zhou, Y.; Qi, N.; Wang, B.; Yan, D. Effect of the Degree of Branching on Atomic-Scale Free Volume in Hyperbranched Poly[3-ethyl-3-(hydroxymethyl)oxetane]. A Positron Study. Macromolecules 2005, 38, 9644–9649. [Google Scholar] [CrossRef]
- Boonlert-Uthai, T.; Samthong, C.; Somwangthanaroj, A. Synthesis, Thermal Properties and Curing Kinetics of Hyperbranched BPA/PEG Epoxy Resin. Polymer 2019, 11, 1545. [Google Scholar] [CrossRef] [Green Version]
- Sixun, Z. Epoxy resin/poly(ethylene oxide) blends cured with aromatic amine. Polymer 1995, 36, 3609–3613. [Google Scholar] [CrossRef]
- ASTM D1652-97. Standard Test Methods for Epoxy Content of Epoxy Resins; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Hammouda, B. A Tutorial on Small-Angle Neutron Scattering from Polymers; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Rubinstein, M. Polymer physics-The ugly duckling story: Will polymer physics ever become a part of “proper” physics? J. Polym. Sci. Part B: Polym. Phys. 2010, 48, 2548–2551. [Google Scholar] [CrossRef]
- Ganjaee-Sari, M.; Stribeck, A.; Moradian, S.; Zeinolebadi, A.; Bastani, S.; Botta, S.; Bakhshandeh, E. Dynamic mechanical behavior and nanostructure morphology of hyperbranched-modified polypropylene blends. Polym. Int. 2013, 63, 195–205. [Google Scholar] [CrossRef]
- Jafarifard, S.; Bastani, S.; Soleimani-Gorgani, A.; Ganjaee-Sari, M. The chemo-rheological behavior of an acrylic based UV-curable inkjet ink: Effect of surface chemistry for hyperbranched polymers. Prog. Org. Coat. 2016, 90, 399–406. [Google Scholar] [CrossRef]
- Ganjaee-Sari, M.; Moradian, S.; Bastani, S.; Stribeck, N. Modification of poly(propylene) by grafted polyester-amide-based dendritic nanostructures with the aim of improving its dyeability. J. Appl. Polym. Sci. 2011, 124, 2449–2462. [Google Scholar] [CrossRef]
- Luo, X.; Xie, S.; Liu, J.; Hu, H.; Jiang, J.; Huang, W.; Gao, H.; Zhou, D.; Lu, Z.; Yan, D. The relationship between the degree of branching and glass transition temperature of branched polyethylene: Experiment and simulation. Polym. Chem. 2014, 5, 1305–1312. [Google Scholar] [CrossRef]
- Luo, X.; Xie, S.-J.; Huang, W.; Dai, B.-N.; Lu, Z.; Yan, D.-Y. Effect of branching architecture on glass transition behavior of hyperbranched copolystyrenes: The experiment and simulation studies. Chin. J. Polym. Sci. 2015, 34, 77–87. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, X.; Luo, S.; Wang, X.; Graff, R.W.; Hu, D.; Guo, R.; Gao, H. Investigate the Glass Transition Temperature of Hyperbranched Copolymers with Segmented Monomer Sequence. Macromolecules 2016, 49, 4416–4422. [Google Scholar] [CrossRef]
- Pongsa, U.; Samthong, C.; Somwangthanaroj, A. Direct functionalization with 3,5-substituted benzoic acids of multiwalled carbon nanotube/epoxy composites. Polym. Eng. Sci. 2013, 53, 2194–2204. [Google Scholar] [CrossRef]
- Pongsa, U.; Somwangthanaroj, A. Effective thermal conductivity of 3,5-diaminobenzoyl-functionalized multiwalled carbon nanotubes/epoxy composites. J. Appl. Polym. Sci. 2013, 130, 3184–3196. [Google Scholar] [CrossRef]
- Boey, F.Y.C.; Qiang, W. Glass-transition temperature–conversion relationship for an epoxy–hexahydro-4-methylphthalic anhydride system. J. Appl. Polym. Sci. 2000, 78, 511–516. [Google Scholar] [CrossRef]
- Rath, S.; Boey, F.; Abadie, M. Cationic electron-beam curing of a high-functionality epoxy: Effect of post-curing on glass transition and conversion. Polym. Int. 2004, 53, 857–862. [Google Scholar] [CrossRef]




| System | EEW (g eq−1) | DGEBA Resin | HBE10P Resin | Photoinitiator | ||
|---|---|---|---|---|---|---|
| wt % | Mass (g) | wt % | Mass (g) | Mass (g) | ||
| DGEBA | 170.21 | 100 | 10.0 | - | - | 0.5 |
| D90H10 | 182.98 | 90 | 9.0 | 10 | 1.0 | 0.5 |
| UV Intensity (mW/cm2) | Photo-Rheometer | Photo DSC | SAXS | |||||
|---|---|---|---|---|---|---|---|---|
| tgel (s) | Mc (g mol−1) | Number of Cross-Links Per Molecule | Tg (°C) | Rg (nm) | ||||
| DGEBA | ||||||||
| 10 | 41.0 ± 0.3 | 2410 ± 10 | 1,028,532 ± 245 | 0.0003 ± 1 × 10−6 | 0.23 ± 0.01 | 0.90 ± 0.01 | 140.15 ± 0.35 | 13.42 ± 0.14 |
| 20 | 31.6 ± 0.3 | 5025 ± 30 | 492,469 ± 141 | 0.0007 ± 3 × 10−6 | 0.25 ± 0.01 | 0.95 ± 0.01 | 120.72 ± 0.57 | 8.90 ± 0.00 |
| 30 | 23.8 ± 0.3 | 4080 ± 30 | 608,043 ± 345 | 0.0006 ± 4 × 10−6 | 0.23 ± 0.01 | 0.95 ± 0.00 | 114.22 ± 0.32 | 6.67 ± 0.01 |
| 40 | 17.7 ± 0.3 | 3890 ± 10 | 636,658 ± 940 | 0.0005 ± 1 × 10−6 | 0.32 ± 0.01 | 0.97 ± 0.01 | 114.33 ± 0.26 | 7.51 ± 0.04 |
| 50 | 14.5 ± 0.3 | 3412 ± 30 | 727,820 ± 8110 | 0.0005 ± 5 × 10−6 | 0.25 ± 0.01 | 0.96 ± 0.01 | 105.07 ± 0.59 | 7.45 ± 0.00 |
| D90H10 | ||||||||
| 10 | 22.3 ± 0.2 | 2643 ± 50 | 942,497 ± 10 | 0.0004 ± 8 × 10−6 | 0.56 ± 0.01 | 0.98 ± 0.01 | 132.70 ± 0.72 | 8.39 ± 0.00 |
| 20 | 18.8 ± 0.2 | 1897 ± 6 | 1,311,499 ± 7 | 0.0003 ± 9 × 10−7 | 0.54 ± 0.01 | 0.99 ± 0.00 | 121.41 ± 0.49 | 9.06 ± 0.00 |
| 30 | 11.4 ± 0.4 | 1880 ± 10 | 1,325,515 ± 6 | 0.0003 ± 2 × 10−6 | 0.69 ± 0.01 | 0.99 ± 0.00 | 102.93 ± 0.68 | 18.74 ± 0.65 |
| 40 | 12.3 ± 0.2 | 7050 ± 50 | 354,106 ± 2 | 0.0011 ± 8 × 10−6 | 0.45 ± 0.01 | 1.00 ± 0.00 | 103.89 ± 0.38 | 10.82 ± 0.00 |
| 50 | 12.0 ± 0.2 | 9160 ± 20 | 270,073 ± 90 | 0.0014 ± 2 × 10−6 | 0.69 ± 0.01 | 1.00 ± 0.00 | 107.59 ± 0.80 | 8.77 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonlert-uthai, T.; Taki, K.; Somwangthanaroj, A. Curing Behavior, Rheological, and Thermal Properties of DGEBA Modified with Synthesized BPA/PEG Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization. Polymers 2020, 12, 2240. https://doi.org/10.3390/polym12102240
Boonlert-uthai T, Taki K, Somwangthanaroj A. Curing Behavior, Rheological, and Thermal Properties of DGEBA Modified with Synthesized BPA/PEG Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization. Polymers. 2020; 12(10):2240. https://doi.org/10.3390/polym12102240
Chicago/Turabian StyleBoonlert-uthai, Tossapol, Kentaro Taki, and Anongnat Somwangthanaroj. 2020. "Curing Behavior, Rheological, and Thermal Properties of DGEBA Modified with Synthesized BPA/PEG Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization" Polymers 12, no. 10: 2240. https://doi.org/10.3390/polym12102240
APA StyleBoonlert-uthai, T., Taki, K., & Somwangthanaroj, A. (2020). Curing Behavior, Rheological, and Thermal Properties of DGEBA Modified with Synthesized BPA/PEG Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization. Polymers, 12(10), 2240. https://doi.org/10.3390/polym12102240







