Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Juice Production
2.3. Design of Experiments
2.4. Statistical Analysis
2.5. Authentication of Optimized Conditions
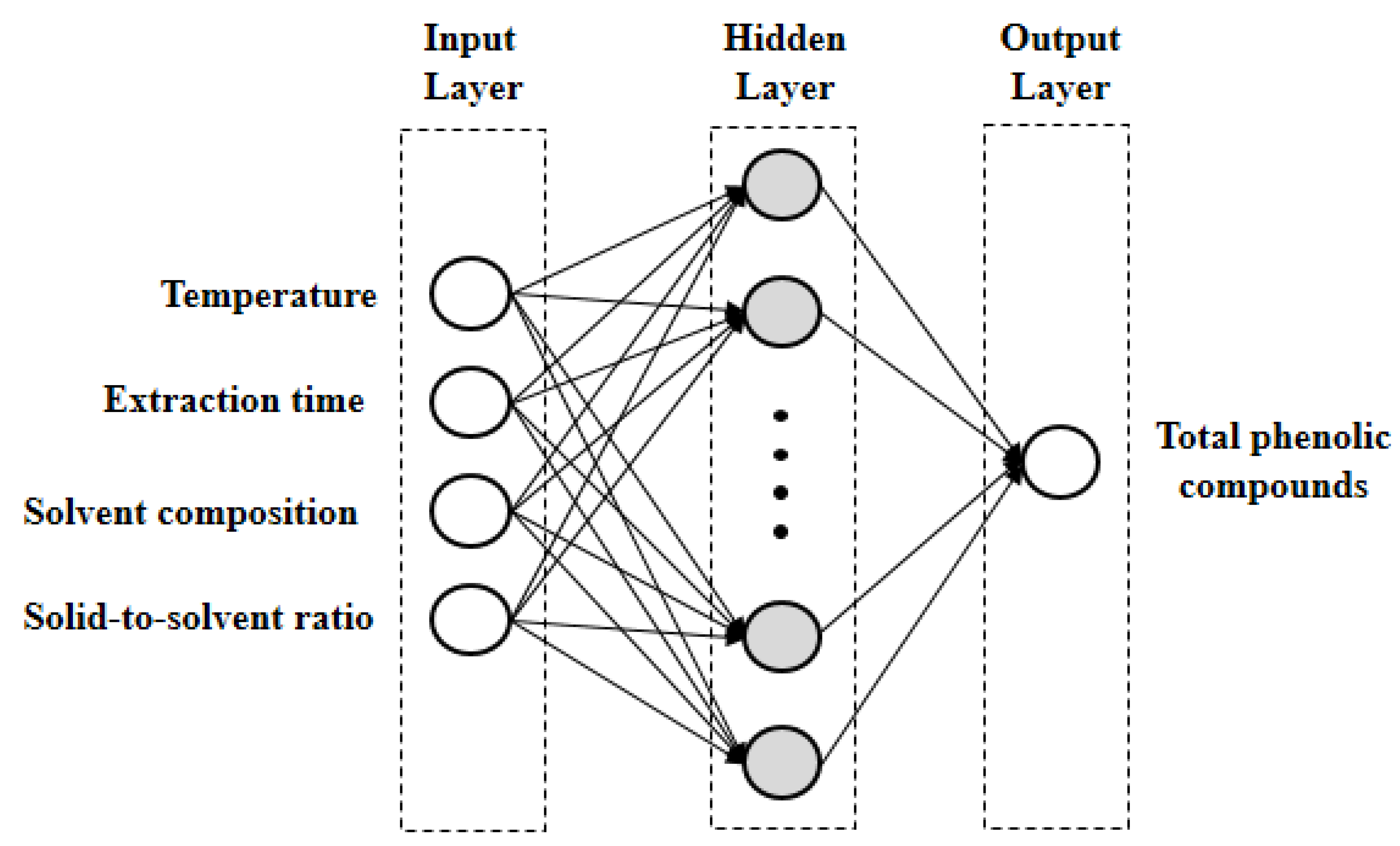
2.6. Artificial Neural Network Modelling
2.7. Microwave-Assisted Extraction of Total Polyphenolic Compounds from Kiwifruit Pomace
2.8. Phytochemical Analysis
3. Results and Discussion
3.1. Fractional Factorial Design and Analysis
3.2. Response Optimization Using the Desirability Function Approach
3.3. ANN Modelling
3.4. A Comparison between RSM and ANN Statistical Models
3.5. Preliminary Characterization of Optimized KP Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.W.; Ferguson, A.R. Actinidia in China: Natural diversity, phylogeographical evolution, interspecific gene flow and kiwifruit cultivar improvement. Acta Hortic. 2007, 753, 31–40. [Google Scholar] [CrossRef]
- Guroo, I.; Wani, S.A.; Wani, S.M.; Ahmad, M.; Mir, S.A.; Masoodi, F.A. A review of production and processing of kiwifruit. J. Food Proc. Technol. 2017, 8, 8. [Google Scholar]
- Faostat. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 30 December 2018).
- Drummond, L. The composition and nutritional value of kiwifruit. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2013; pp. 33–57. [Google Scholar]
- Padmanabhan, P.; Paliyath, G. Kiwifruit. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 490–494. [Google Scholar] [CrossRef]
- Martin-Cabrejas, M.A.; Esteban, R.M.; Lopez-Andreu, F.J.; Waldron, K.; Selvendran, R. Dietary fiber content of pear and kiwi pomaces. J. Agric. Food Chem. 1995, 43, 662–666. [Google Scholar] [CrossRef]
- Carbone, K.; Garrigos, M.C.; Jimenez, A. Polyphenols: From wastes to high added value bio-products. In Frontiers in Natural Product Chemistry; Rahman, A., Ed.; Bentham Books: Sharjah, UAE, 2016; Volume 2, pp. 115–178. [Google Scholar]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly) phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Stefanello, F.S.; da Mota Huerta, K.; Monteiro, S.S.; da Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Sarve, A.; Sonawane, S.S.; Varma, M.N. Ultrasound assisted biodiesel production from sesame (Sesamum indicum L.) oil using barium hydroxide as a heterogeneous catalyst: Comparative assessment of prediction abilities between response surface methodology (RSM) and artificial neural network (ANN). Ultrason. Sonochem. 2015, 26, 218–228. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef]
- Zheng, N.; Chen, F.; Wang, Z.; Lin, J. Modelling and optimization of artificial neural network and response surface methodology in ultra-high-pressure extraction of Artemisia argyi Levl. et Vant and its antifungal activity. Food Anal. Methods 2013, 6, 421–431. [Google Scholar] [CrossRef]
- Dahmoune, F.; Remini, H.; Dairi, S.; Aoun, O.; Moussi, K.; Bouaoudia-Madi, N.; Mouni, L. Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crops Prod. 2015, 77, 251–261. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Paliotta, M.; Centioni, L.; Mencarelli, F.; Carbone, K. The effect of genotype and drying condition on the bioactive compounds of sour cherry pomace. Eur. Food Res. Technol. 2017, 244, 635–645. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Yılmaz, M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crops Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Piotrowski, A.P.; Napiorkowsk, J.J. A comparison of methods to avoid overfitting in neural networks training in the case of catchment runoff modelling. J. Hydrol. 2013, 476, 97–111. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Nishiyama, I.; Fukuda, T.; Oota, T. Genotypic differences in chlorophyll, lutein, and β-carotene contents in the fruits of Actinidia species. J. Agric. Food. Chem. 2005, 53, 6403–6407. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Buenger, J.; Ackermann, H.; Jentzsch, A.; Mehling, A.; Pfitzner, I.; Reiffen, K.A.; Wollenweber, U. An interlaboratory comparison of methods used to assess antioxidant potentials. Int. J. Cosmet. Sci. 2006, 28, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]



| Coded Factors | Uncoded Factors | TPC (mg GAE g−1 dw) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | X4 | Solvent Composition, % (X1) | Solid-to-Solvent Ratio (X2) | Temperature, °C (X3) | Extraction Time, min (X4) | Experimental | PredictedRSM | PredictedANN |
| 1 | −1 | −1 | −1 | −1 | 0 | 1:10 | 25 | 5 | 3.6 ± 0.2 f | 3.40 | 3.59 |
| 2 | −1 | −1 | 0 | 0 | 0 | 1:10 | 50 | 10 | 2.40 ± 0.06 d | 3.16 | 2.41 |
| 3 | −1 | −1 | 1 | 1 | 0 | 1:10 | 75 | 15 | 4.87 ± 0.02 k | 4.13 | 4.90 |
| 4 | −1 | 0 | −1 | 0 | 0 | 1:20 | 25 | 10 | 3.3 ± 0.5 f | 3.31 | 3.38 |
| 5 | −1 | 0 | 0 | 1 | 0 | 1:20 | 50 | 15 | 3.40 ± 0.08 f | 3.21 | 3.39 |
| 6 | −1 | 0 | 1 | −1 | 0 | 1:20 | 75 | 5 | 3.33 ± 0.08 f | 3.40 | 3.31 |
| 7 | −1 | 1 | −1 | 1 | 0 | 1:30 | 25 | 15 | 3.49 ± 0.04 f | 3.77 | 3.40 |
| 8 | −1 | 1 | 0 | −1 | 0 | 1:30 | 50 | 5 | 3.3 ± 0.7 f | 3.37 | 3.30 |
| 9 | −1 | 1 | 1 | 0 | 0 | 1:30 | 75 | 10 | 3.1 ± 0.1 f | 3.13 | 3.09 |
| 10 | 0 | −1 | −1 | 1 | 50 | 1:10 | 25 | 15 | 3.83 ± 0.06 f | 3.98 | 3.83 |
| 11 | 0 | −1 | 0 | −1 | 50 | 1:10 | 50 | 5 | 4.25 ± 0.02 h | 4.25 | 4.28 |
| 12 | 0 | −1 | 1 | 0 | 50 | 1:10 | 75 | 10 | 4.57 ± 0.02 i | 4.90 | 4.53 |
| 13 | 0 | 0 | −1 | −1 | 50 | 1:20 | 25 | 5 | 4.70 ± 0.08 j | 4.32 | 4.63 |
| 14 | 0 | 0 | 0 | 0 | 50 | 1:20 | 50 | 10 | 4.12 ± 0.07 g | 3.90 | 4.07 |
| 15 | 0 | 0 | 1 | 1 | 50 | 1:20 | 75 | 15 | 4.0 ± 0.2 fg | 4.70 | 4.00 |
| 16 | 0 | 1 | −1 | 0 | 50 | 1:30 | 25 | 10 | 4.69 ± 0.06 j | 4.37 | 4.79 |
| 17 | 0 | 1 | 0 | 1 | 50 | 1:30 | 50 | 15 | 4.20 ± 0.08 gh | 4.10 | 4.27 |
| 18 | 0 | 1 | 1 | −1 | 50 | 1:30 | 75 | 5 | 4.23 ± 0.05 gh | 4.10 | 4.24 |
| 19 | 1 | −1 | −1 | 0 | 100 | 1:10 | 25 | 10 | 1.36 ± 0.06 a | 1.34 | 1.40 |
| 20 | 1 | −1 | 0 | 1 | 100 | 1:10 | 50 | 15 | 2.01 ± 0.03 c | 1.95 | 2.01 |
| 21 | 1 | −1 | 1 | −1 | 100 | 1:10 | 75 | 5 | 2.86 ± 0.07 e | 2.62 | 2.90 |
| 22 | 1 | 0 | −1 | 1 | 100 | 1:20 | 25 | 15 | 1.5 ± 0.1 a | 1.45 | 1.56 |
| 23 | 1 | 0 | 0 | −1 | 100 | 1:20 | 50 | 5 | 1.3 ± 0.2 a | 1.53 | 1.30 |
| 24 | 1 | 0 | 1 | 0 | 100 | 1:20 | 75 | 10 | 2.06 ± 0.07 c | 2.00 | 2.09 |
| 25 | 1 | 1 | −1 | −1 | 100 | 1:30 | 25 | 5 | 1.3 ± 0.1 a | 1.93 | 1.32 |
| 26 | 1 | 1 | 0 | 0 | 100 | 1:30 | 50 | 10 | 1.80 ± 0.02 b | 1.33 | 1.80 |
| 27 | 1 | 1 | 1 | 1 | 100 | 1:30 | 75 | 15 | 1.86 ± 0.05 b | 1.95 | 1.86 |
| Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | Significance | |
|---|---|---|---|---|---|---|
| X1 | 48.5769 | 1 | 48.5769 | 317.3026 | <0.0001 | *** |
| X2 | 0.6476 | 1 | 0.6476 | 4.2502 | 0.0425 | * |
| X3 | 2.1135 | 1 | 2.1135 | 13.8055 | 0.0003 | *** |
| X4 | 0.0219 | 1 | 0.0219 | 0.1429 | 0.7062 | ns |
| X1 X2 | 0.3115 | 1 | 0.3115 | 2.0349 | 0.1570 | ns |
| X1 X3 | 0.8212 | 1 | 0.8212 | 5.3643 | 0.0227 | * |
| X1 X4 | 0.0221 | 1 | 0.0221 | 0.1442 | 0.7050 | ns |
| X2 X3 | 4.0416 | 1 | 4.0416 | 26.3994 | <0.0001 | *** |
| X2 X4 | 0.0185 | 1 | 0.0185 | 0.1211 | 0.7286 | ns |
| X3 X4 | 0.7193 | 1 | 0.7193 | 4.6984 | 0.0327 | * |
| X12 | 68.9947 | 1 | 68.9947 | 450.6707 | <0.0001 | *** |
| X22 | 0.3384 | 1 | 0.3384 | 2.2103 | 0.1404 | ns |
| X32 | 2.0114 | 1 | 2.0114 | 13.1383 | 0.0005 | *** |
| X42 | 0.8040 | 1 | 0.8040 | 5.2519 | 0.0242 | * |
| Residual | 14.3908 | 94 | 0.1531 | |||
| Total SS | 144.1879 | 108 | ||||
| R2 | 0.900 | |||||
| Radj2 | 0.885 |
| Parameter | Experimental Values | Literature Values a |
|---|---|---|
| TPC | 4.8 ± 0.1 | <2.0 |
| FLC | 1.38 ± 0.01 | <1.0 |
| AAC | 120.6 ± 0.5 | 59.80 |
| TCC | 5.9 ± 0.1 | 2.02 |
| ACDPPH | 5.49 ± 0.02 | 39.45 b |
| ACABTS | 560 ± 1 | n.d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction. Agriculture 2020, 10, 435. https://doi.org/10.3390/agriculture10100435
Carbone K, Amoriello T, Iadecola R. Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction. Agriculture. 2020; 10(10):435. https://doi.org/10.3390/agriculture10100435
Chicago/Turabian StyleCarbone, Katya, Tiziana Amoriello, and Rosamaria Iadecola. 2020. "Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction" Agriculture 10, no. 10: 435. https://doi.org/10.3390/agriculture10100435
APA StyleCarbone, K., Amoriello, T., & Iadecola, R. (2020). Exploitation of Kiwi Juice Pomace for the Recovery of Natural Antioxidants through Microwave-Assisted Extraction. Agriculture, 10(10), 435. https://doi.org/10.3390/agriculture10100435






