The Effect of Degree of Milling on the Nutraceutical Content in Ecofriendly and Conventional Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polished Rice Samples
2.2. Chemicals and Reagents
2.3. Sample Extraction, Analysis, and Quantification
2.3.1. Extraction and Conversion of FAME
2.3.2. FAME Analysis by GC-FID
2.3.3. VitE Extraction
2.3.4. VitE Analysis by GC-FID
2.3.5. Extraction of Phenolic Compounds
2.3.6. Phenolic Compound Analysis by LC-MS/MS
2.3.7. DPPH Free Radical Scavenging Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of DOM on Chemical Composition and Content of Rice
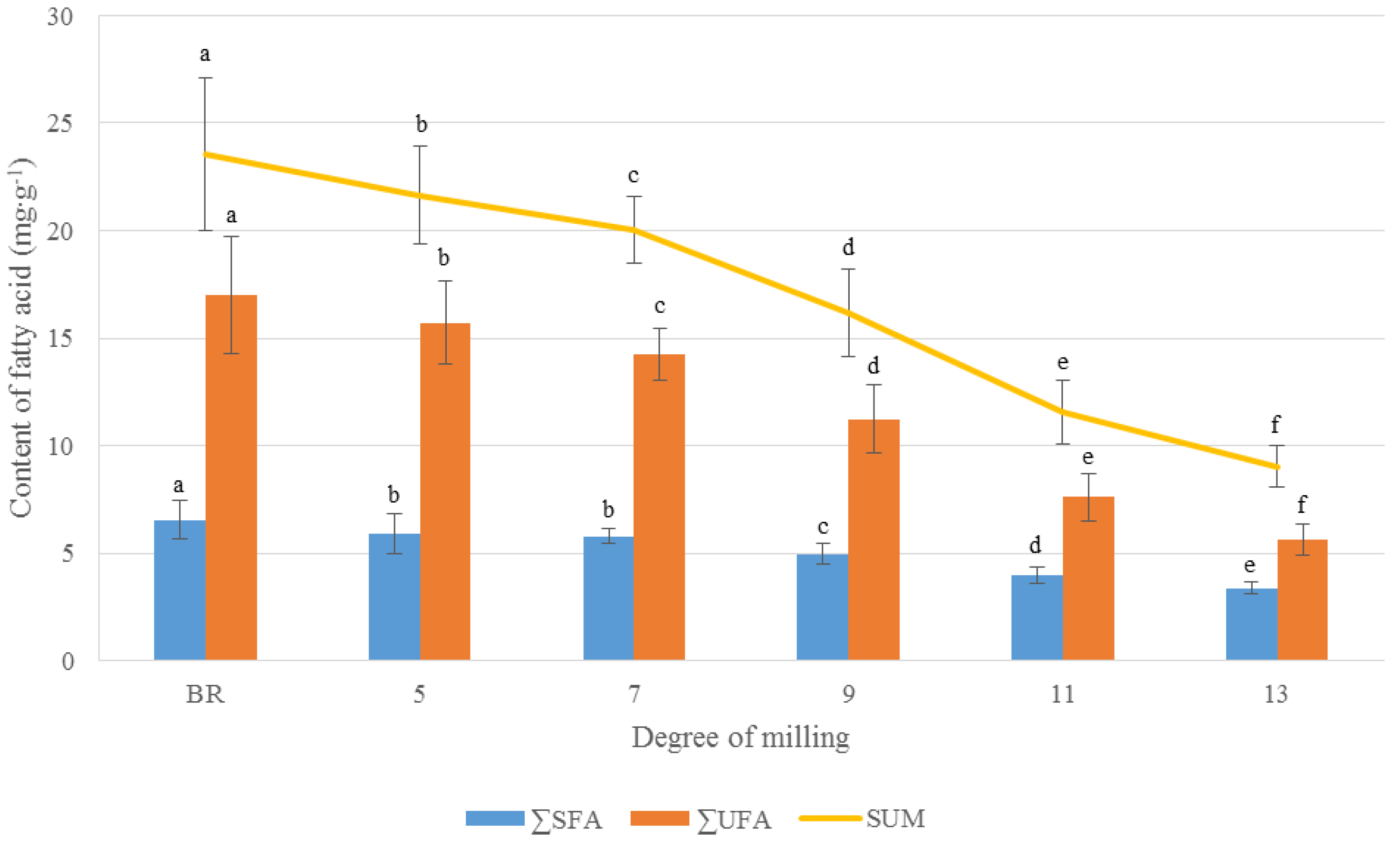
3.1.1. FA Variation in Rice Grains According to the DOM
3.1.2. VitE Variations in Rice Grains According to DOM
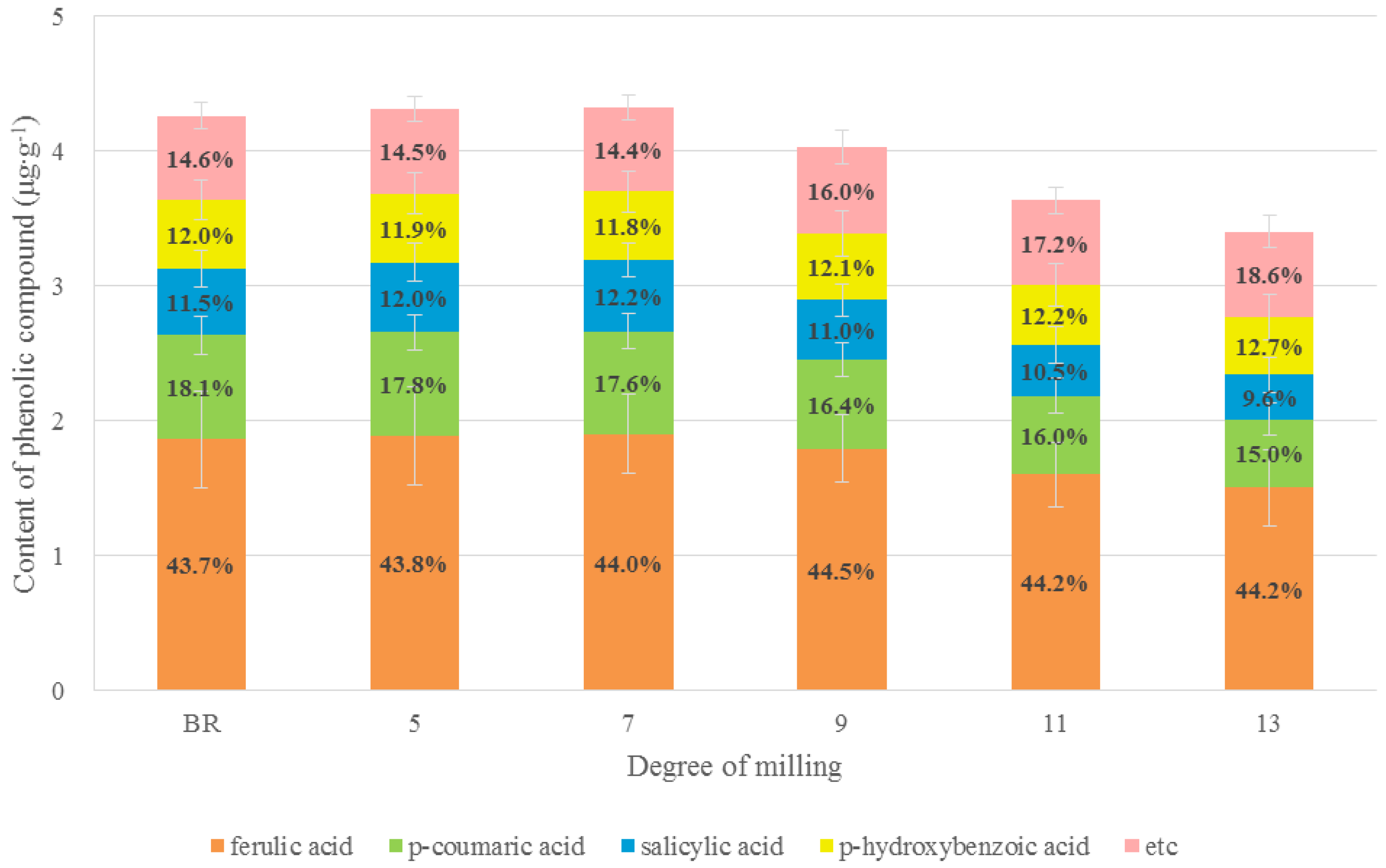
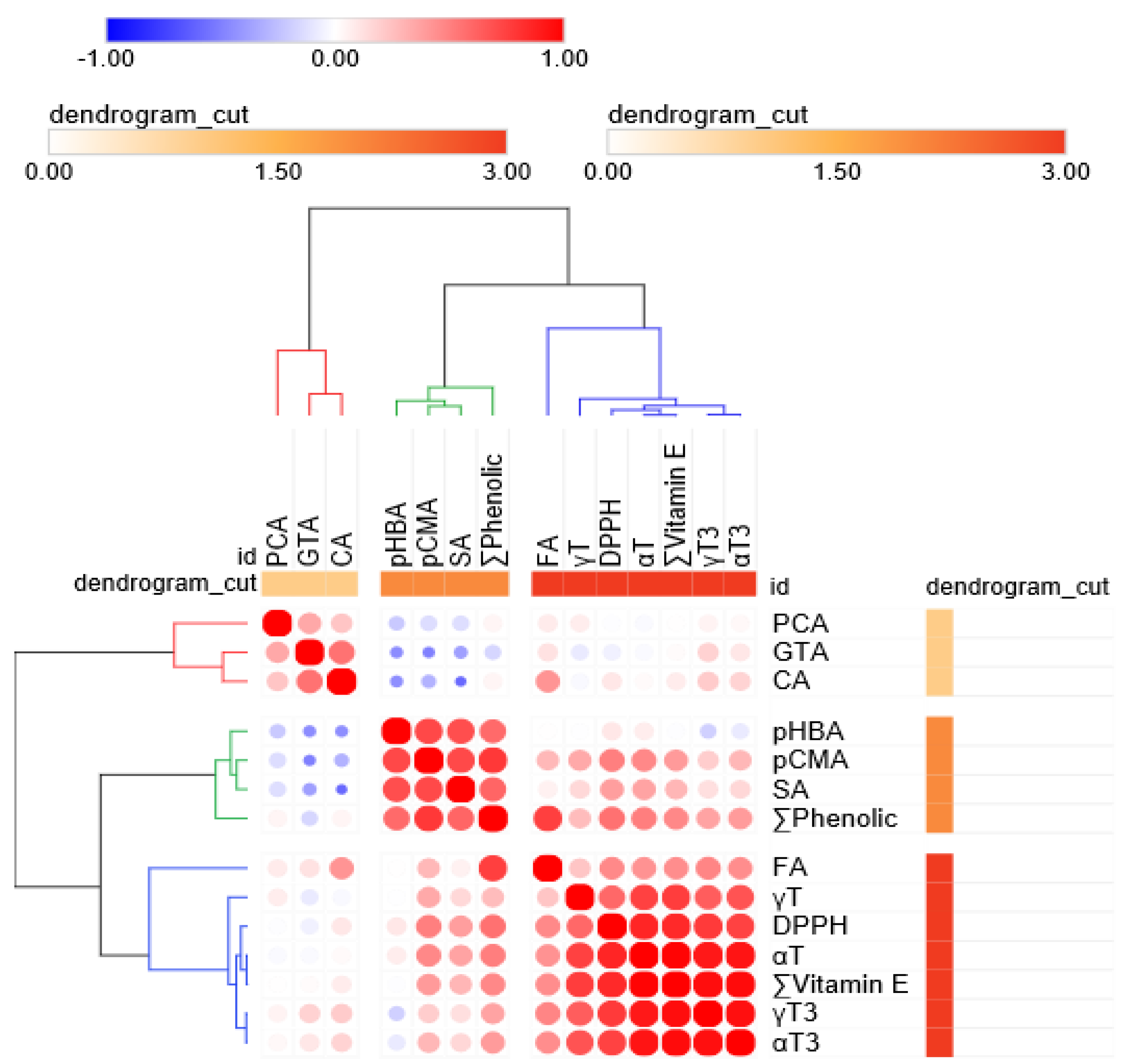
3.1.3. Phenolic Variations in Rice Grains According to DOM
3.2. Effect of Rice Type (Organic, Pesticide-Free, Conventional Rice) on Chemical Composition and Rice Grain Content
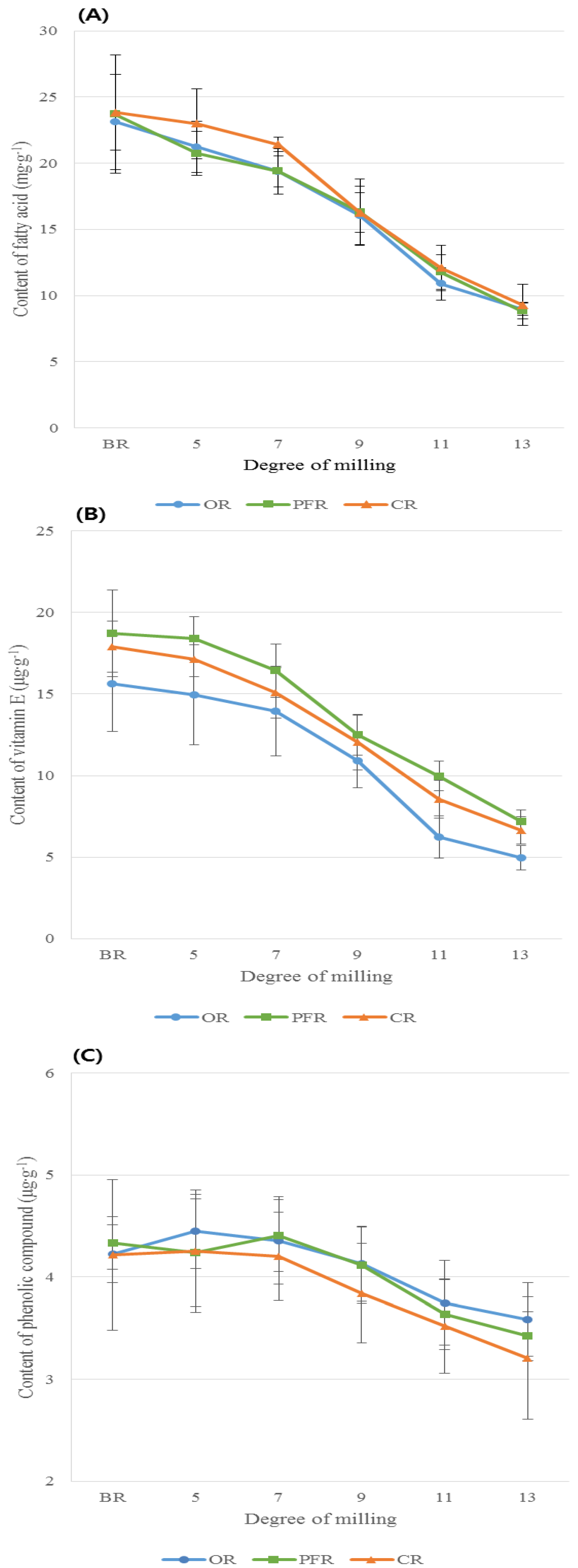
3.2.1. Comparison of FA Content in Rice Types with the Same DOM
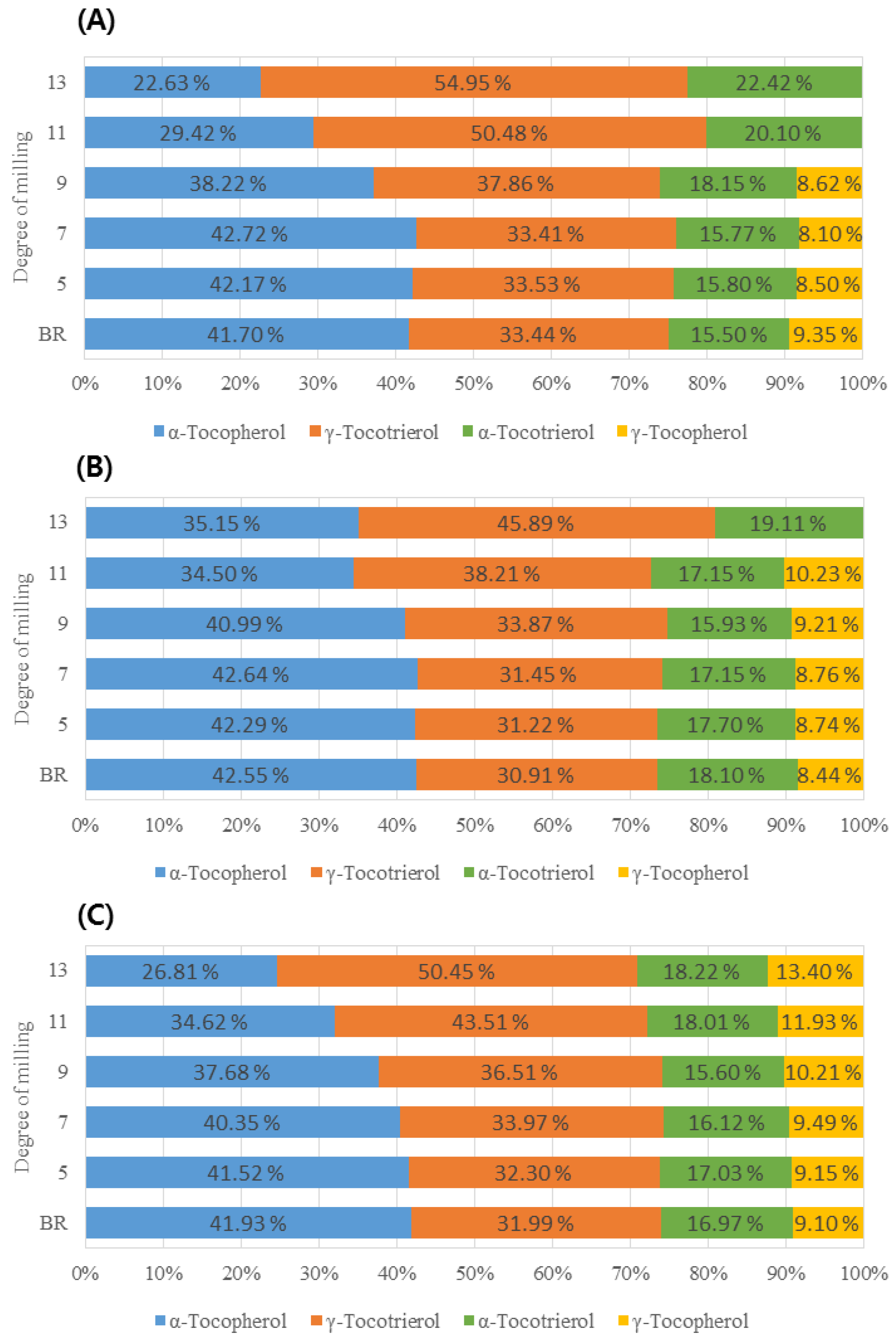
3.2.2. Comparison of VitE Content in Rice Grains According to Rice Types with the Same DOM
3.2.3. Phenolic Level Comparison According to the Rice Types with the Same DOM
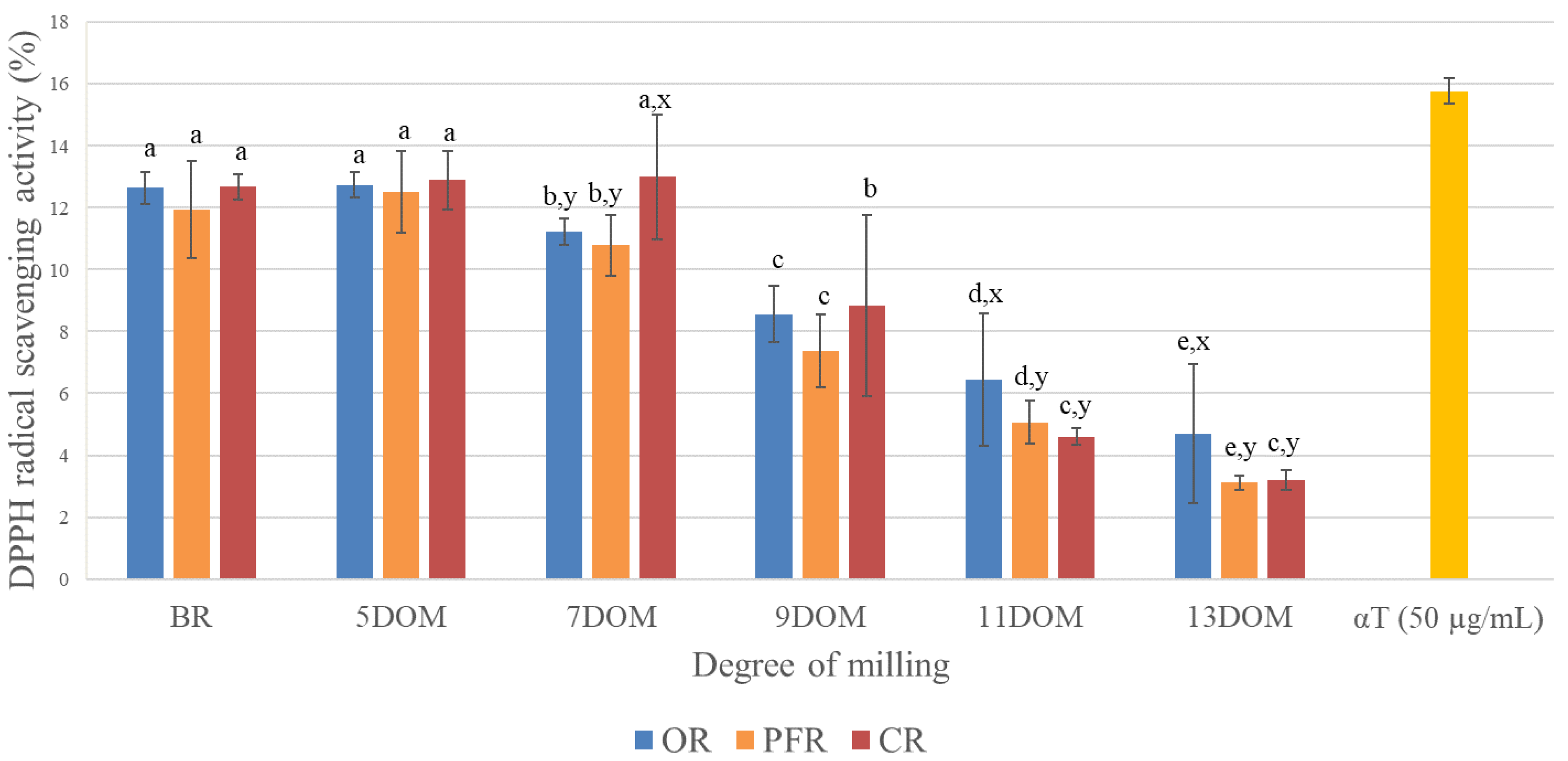
3.3. Comparison of DPPH Free Radical Scavenging Activity According to Rice Type and DOM
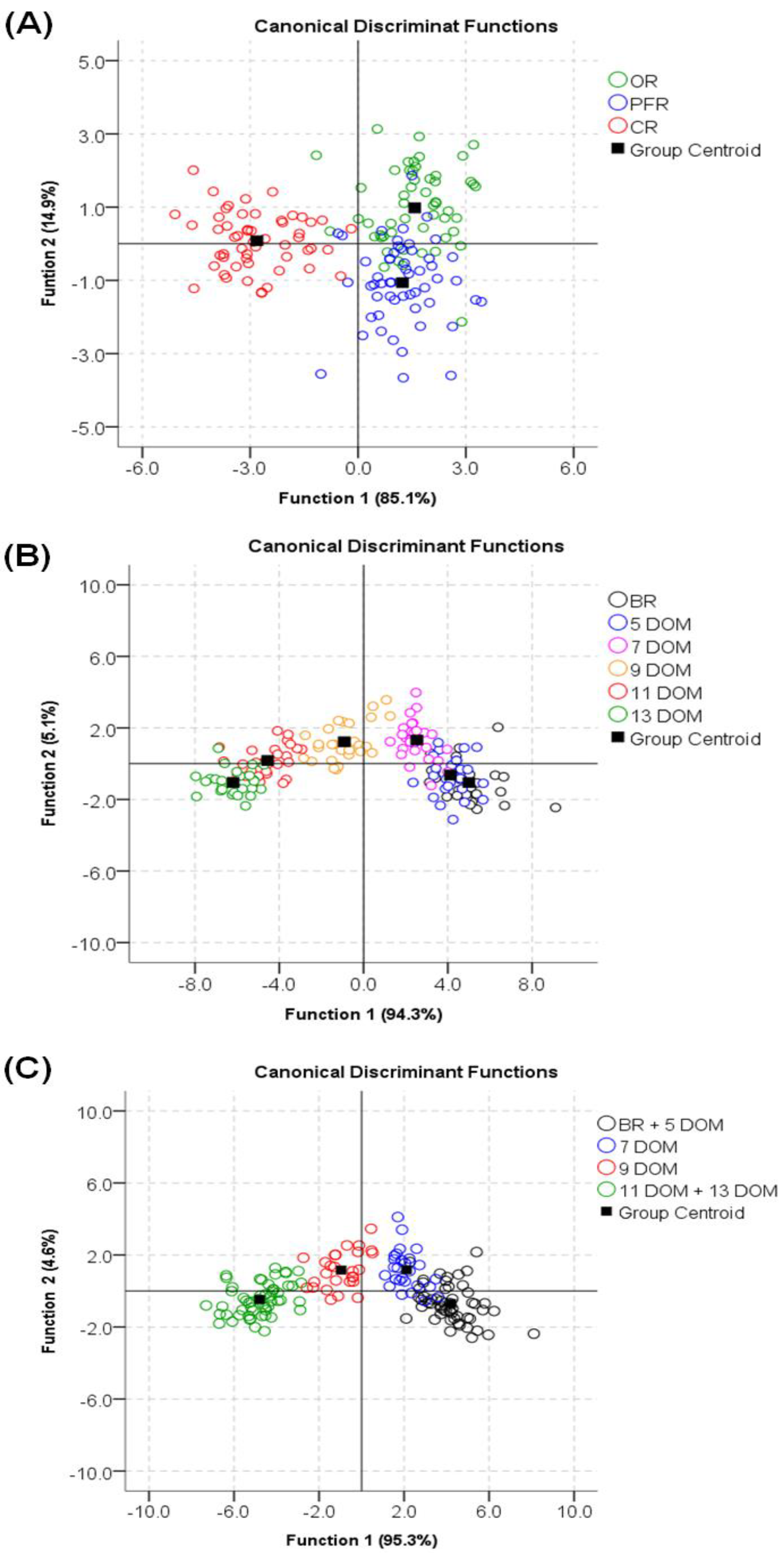
3.4. Discriminant Analysis According to Rice Type and DOM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, R.H. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Vichapong, J.; Sookserm, M.; Srijesdaruk, V.; Swatsitang, P.; Srijaranai, S. High Performance Liquid Chromatographic Analysis of Phenolic Compounds and their Antioxidant Activities in Rice Varieties. LWT 2010, 43, 1325–1330. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, γ-Oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Pascual, C.D.S.C.I.; Massaretto, I.L.; Kawassaki, F.; Barros, R.M.C.; Noldin, J.A.; Marquez, U.M.L.; Kawassaki-Hirashima, F. Effects of Parboiling, Storage and Cooking on the Levels of Tocopherols, Tocotrienols and γ-Oryzanol in Brown Rice (Oryza sativa L.). Food Res. Int. 2013, 50, 676–681. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.E.; Singh, D.; Lee, C.H. Metabolomics Reveal Optimal Grain Preprocessing (Milling) toward Rice Koji Fermentation. J. Agric. Food Chem. 2018, 66, 2694–2703. [Google Scholar] [CrossRef]
- Ko, M.R.; Choi, H.J.; Han, B.K.; Yoo, S.S.; Kim, H.S.; Choi, S.W.; Hur, N.Y.; Kim, C.N.; Kim, B.Y.; Baik, M.Y. Antioxidative Components and Antioxidative Capacity of Brown and Black Rices. Food Eng. Prog. 2011, 15, 195–202. [Google Scholar]
- Park, J.-H.; Nam, S.-H.; Kim, Y.-O.; Kwon, O.-D.; An, K.-N. Comparison of Quality, Physiochemical and Functional Property between Organic and Conventional Rice. J. Korean Soc. Food Sci. Nutr. 2010, 39, 725–730. [Google Scholar] [CrossRef]
- Na, G.S.; Lee, S.K.; Kim, S.Y. Antioxidative Effects and Quality Characteristics of the Rice Cultivated by Organic Farming and Ordinary Farming. J. Korean Soc. Appl. Biol. Chem. 2007, 50, 36–41. [Google Scholar]
- Sirikul, A.; Moongngarm, A.; Khaengkhan, P. Comparison of Proximate Composition, Bioactive Compounds and Antioxidant Activity of Rice Bran and Defatted Rice Bran from Organic Rice and Conventional Rice. Asian J. Food Agro-Ind. 2009, 2, 731–743. [Google Scholar]
- Chung, I.-M.; Kim, J.-K.; Park, I.; Oh, J.-Y.; Kim, S.-H. Effects of Milk Type, Production Month, and Brand on Fatty Acid Composition: A Case Study in Korea. Food Chem. 2016, 196, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Oh, J.-Y.; Kim, S.-H. Comparative Study of Phenolic Compounds, Vitamin E, and Fatty Acids Compositional Profiles in Black Seed-Coated Soybeans (Glycine Max (L.) Merrill) Depending on Pickling Period in Brewed Vinegar. Chem. Cent. J. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Chung, I.-M.; Son, N.-Y.; Chi, H.-Y.; Kim, S.-Y.; Yang, Y.; Kwon, C.; An, Y.; Ahmad, A.; Kim, S.-H. Analysis of Selected Phenolic Compounds in Organic, Pesticide-Free, Conventional Rice (Oryza sativa L.) Using LC-ESI-MS/MS. Molecules 2019, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant Properties of Commercial Wild Rice and Analysis of Soluble and Insoluble Phenolic Acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Lugay, J.C.; Juliano, B.O. Fatty Acid Composition of Rice Lipids by Gas-Liquid Chromatography. J. Am. Oil Chem. Soc. 1964, 41, 273–275. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and Dynamic Membrane Properties of Alpha.-Tocopherol and Alpha.-Tocotrienol: Implication to the Molecular Mechanism of Their Antioxidant Potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
- Butsat, S.; Siriamornpun, S. Antioxidant Capacities and Phenolic Compounds of the Husk, Bran and Endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, Esterified, and Insoluble-Bound Phenolic Acids. 3. Composition of Phenolic Acids in Cereal and Potato Flours. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and Nuture of Ferulic acid Substitution of Cell Wall Polysaccharides in Gramineous Plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Ti, H.; Guo, J.; Zhang, R.; Wei, Z.; Liu, L.; Bai, Y. Phenolic Profiles and Antioxidant Activity in Four Tissue Fractions of Whole Brown Rice. RSC Adv. 2015, 5, 101507–101518. [Google Scholar] [CrossRef]
- Zhou, Z. The Distribution of Phenolic Acids in Rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Wadsworth, J.I. Rice Science and Technology; Marshall, W.E., Wadsworth, J.I., Eds.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Fess, T.; Benedito, V.A. Organic Versus Conventional Cropping Sustainability: A Comparative System Analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant Activity of Tocopherols, Tocotrienols, and γ-Oryzanol Components from Rice Bran against Cholesterol Oxidation Accelerated by 2,2‘-Azobis(2-Methylpropionamidine) Dihydrochloride†. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef]
- Aguilar-Garcia, C.; Gavino, G.; Baragaño-Mosqueda, M.; Hevia, P.; Gavino, V.C. Correlation of Tocopherol, Tocotrienol, γ-Oryzanol and Total Polyphenol Content in Rice Bran with Different Antioxidant Capacity Assays. Food Chem. 2007, 102, 1228–1232. [Google Scholar] [CrossRef]
- Khatoon, S.; Gopalakrishna, A.G. Fat-Soluble Nutraceuticals and Fatty Acid Composition of Selected Indian Rice Varieties. J. Am. Oil Chem. Soc. 2004, 81, 939–943. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Bladenopoulos, K.; Papageorgiou, M. Determination of Tocopherol and Tocotrienol Content of Greek Barley Varieties Under Conventional and Organic Cultivation Techniques Using Validated Reverse Phase High-Performance Liquid Chromatography Method. J. Sci. Food Agric. 2012, 92, 1732–1739. [Google Scholar] [CrossRef]
- Chen, M.-H.; McClung, A.M.A.M. Effects of Cultivars, Organic Cropping Management, and Environment on Antioxidants in Whole Grain Rice. Cereal Chem. J. 2015, 92, 364–369. [Google Scholar] [CrossRef]
- Brandt, K.; Mølgaard, J.P. Organic Agriculture: Does it Enhance or Reduce the Nutritional Value of Plant Foods? J. Sci. Food Agric. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Saha, S.; Pandey, A.K.; Gopinath, K.A.; Bhattacharaya, R.; Kundu, S.; Gupta, H. Nutritional Quality of Organic Rice Grown on Organic Composts. Agron. Sustain. Dev. 2007, 27, 223–229. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, S.; Wang, M.; Feng, X.-Y. Characterization of Free, Conjugated, and Bound Phenolic Acids in Seven Commonly Consumed Vegetables. Molecules 2017, 22, 1878. [Google Scholar] [CrossRef]
- Faraone, I.; Russo, D.; Chiummiento, L.; Cusimamani, E.F.; Choudhary, A.; Monné, M.; Milella, L.; Rai, D.K. Phytochemicals of Minthostachys diffusa Epling and Their Health-Promoting Bioactivities. Foods 2020, 9, 144. [Google Scholar] [CrossRef]
- Faraone, I.; Rai, D.K.; Russo, D.; Chiummiento, L.; Cusimamani, E.F.; Choudhary, A.; Milella, L. Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd. Plants 2019, 8, 265. [Google Scholar] [CrossRef]
- Khalil, I.; Ghani, M.; Khan, R.A.; Akbar, F. Evaluation of Biological Activities and in Vivo Amelioration of CCl4 Induced Toxicity in Lung and Kidney with Abutilon Pannosum (G.Forst.) Schltdl. in Rat. J. Ethnopharmacol. 2020, 249, 112395. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Holtz, R.W. In Vitro Methods to Screen Materials for Anti-aging Effects. In Skin Aging Handbook; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 329–362. [Google Scholar]
- Newmark, H.L. Plant Phenolics as Potential Cancer Prevention Agents. Neurotransm. Interact. Cogn. Funct. 1996, 401, 25–34. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.; Zhang, Y.; Liu, Z.; Shao, S.; Zhou, L.; Rogers, K.M. Differentiating Organically Farmed Rice from Conventional and Green Rice Harvested from an Experimental Field Trial Using Stable Isotopes and Multi-Element Chemometrics. J. Agric. Food Chem. 2018, 66, 2607–2615. [Google Scholar] [CrossRef]
- Mahne Opatić, A.; Nečemer, M.; Lojen, S.; Vidrih, R. Stable Isotope Ratio and Elemental Composition Parameters in Combination with Discriminant Analysis Classification Model to Assign Country of Origin to Commercial Vegetables—A Preliminary Study. Food Control. 2017, 80, 252–258. [Google Scholar] [CrossRef]
- Paolini, M.; Ziller, L.; Laursen, K.H.; Husted, S.; Camin, F. Compound-Specific δ15N and δ13C Analyses of Amino Acids for Potential Discrimination between Organically and Conventionally Grown Wheat. J. Agric. Food Chem. 2015, 63, 5841–5850. [Google Scholar] [CrossRef]


| Rice Type | Predicted Group Membership | Total | ||||
|---|---|---|---|---|---|---|
| OR | PFR | CR | ||||
| Original a | Count | OR | 43 | 9 | 2 | 54 |
| PFR | 8 | 46 | 0 | 54 | ||
| CR | 1 | 1 | 52 | 54 | ||
| % | OR | 79.6 | 16.7 | 3.7 | 100.0 | |
| PFR | 14.8 | 85.2 | 0 | 100.0 | ||
| CR | 1.9 | 1.9 | 96.3 | 100.0 | ||
| Cross-validated b,c | Count | OR | 39 | 13 | 2 | 54 |
| PFR | 11 | 41 | 2 | 54 | ||
| CR | 1 | 1 | 52 | 54 | ||
| % | OR | 72.2 | 24.1 | 3.7 | 100.0 | |
| PFR | 20.4 | 75.9 | 3.7 | 100.0 | ||
| CR | 1.9 | 1.9 | 96.3 | 100.0 | ||
| DOM Level | Predicted Group Membership | Total | |||||
|---|---|---|---|---|---|---|---|
| BR + 5 DOM | 7 DOM | 9 DOM | 11 DOM + 13 DOM | ||||
| Original a | Count | BR + 5 DOM | 50 | 4 | 0 | 0 | 54 |
| 7 DOM | 4 | 23 | 0 | 0 | 27 | ||
| 9 DOM | 0 | 1 | 26 | 0 | 27 | ||
| 11 DOM + 13 DOM | 0 | 0 | 1 | 53 | 54 | ||
| % | BR + 5 DOM | 92.6 | 7.4 | 0 | 0 | 100.0 | |
| 7 DOM | 14.8 | 85.2 | 0 | 0 | 100.0 | ||
| 9 DOM | 0 | 3.7 | 96.3 | 0 | 100.0 | ||
| 11 DOM + 13 DOM | 0 | 0 | 1.9 | 98.1 | 100.0 | ||
| Cross-Validated b,c | Count | BR + 5 DOM | 50 | 4 | 0 | 0 | 54 |
| 7 DOM | 5 | 22 | 0 | 0 | 27 | ||
| 9 DOM | 0 | 2 | 25 | 0 | 27 | ||
| 11 DOM + 13 DOM | 0 | 0 | 1 | 53 | 54 | ||
| % | BR + 5 DOM | 92.6 | 7.4 | 0 | 0 | 100.0 | |
| 7 DOM | 18.5 | 81.5 | 0 | 0 | 100.0 | ||
| 9 DOM | 0 | 7.4 | 92.6 | 0 | 100.0 | ||
| 11 DOM + 13 DOM | 0 | 0 | 1.9 | 98.1 | 100.0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Yang, Y.-J.; Chung, I.-M. The Effect of Degree of Milling on the Nutraceutical Content in Ecofriendly and Conventional Rice (Oryza sativa L.). Foods 2020, 9, 1297. https://doi.org/10.3390/foods9091297
Kim S-H, Yang Y-J, Chung I-M. The Effect of Degree of Milling on the Nutraceutical Content in Ecofriendly and Conventional Rice (Oryza sativa L.). Foods. 2020; 9(9):1297. https://doi.org/10.3390/foods9091297
Chicago/Turabian StyleKim, Seung-Hyun, Yu-Jin Yang, and Ill-Min Chung. 2020. "The Effect of Degree of Milling on the Nutraceutical Content in Ecofriendly and Conventional Rice (Oryza sativa L.)" Foods 9, no. 9: 1297. https://doi.org/10.3390/foods9091297
APA StyleKim, S.-H., Yang, Y.-J., & Chung, I.-M. (2020). The Effect of Degree of Milling on the Nutraceutical Content in Ecofriendly and Conventional Rice (Oryza sativa L.). Foods, 9(9), 1297. https://doi.org/10.3390/foods9091297






