Dietary Advanced Glycation Endproducts and the Gastrointestinal Tract
Abstract
:1. Introduction
2. The Maillard Reaction
3. Terminology
4. Dietary AGEs in the Gastro-Intestinal Tract: Digestion, Absorption, Formation, and Degradation
4.1. Digestion of AGEs
4.2. Absorption
4.3. Formation of AGEs in the GI Tract
4.4. Degradation and Effect in the Colon
5. Local Health Effect in the Intestinal Tract
5.1. Pro-Inflammatory Effect of dAGEs
5.2. Other Local Effects
6. Mechanisms
6.1. Receptor Mediated Effects
6.2. Redox Modulation and Loss of Protein Function
7. Dietary AGEs and Inflammatory Bowel Diseases
Evidence of AGE and RAGE-Involvement in IBD
8. What Are the Characteristics of AGE-Rich Food Products?
8.1. Factors Influencing the MR
8.2. Assessment of Dietary AGEs in Food Products
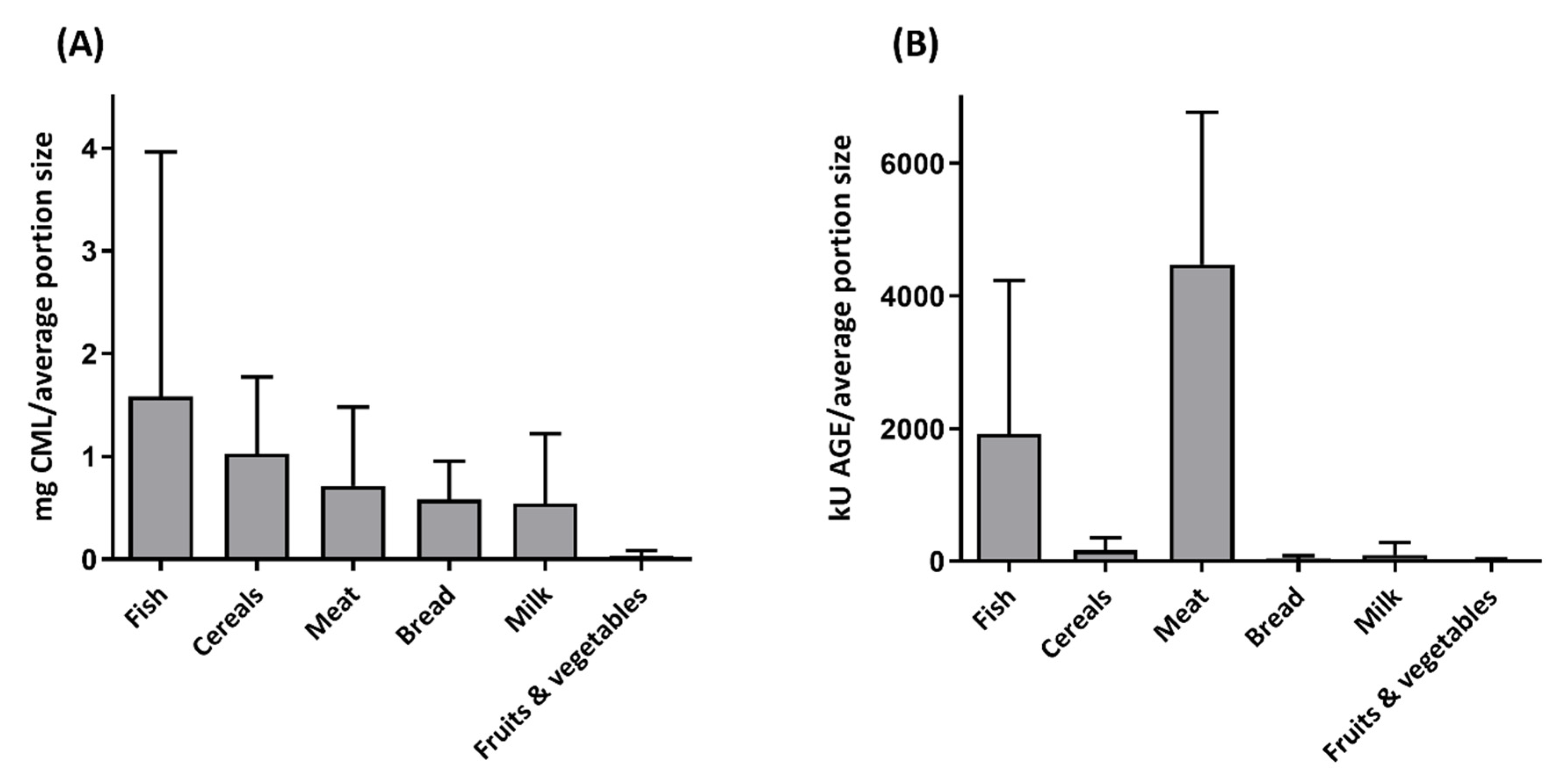
8.3. Exposure to dAGEs
8.4. Can This Be Clarified by the Maillard Reaction?
8.5. Populations at Risk
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.A.; Wu, C.H.; Yen, G.C. Perspective of Advanced Glycation End Products on Human Health. J. Agric. Food Chem. 2018, 66, 2065–2070. [Google Scholar] [CrossRef]
- Bastos, D.M.; Monaro, É.; Siguemoto, É.; Séfora, M. Maillard Reaction Products in Processed Food: Pros and Cons; Intech Open Access Publisher: London, UK, 2012. [Google Scholar]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Henle, T. Dietary advanced glycation end products—A risk to human health? A call for an interdisciplinary debate. Mol. Nutr. Food Res. 2007, 51, 1075–1078. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Hofmann, T.; Ames, J.; Krome, K.; Faist, V. Determination of the molecular weight distribution of non-enzymatic browning products formed by roasting of glucose and glycine and studies on their effects on NADPH-cytochrome c-reductase and glutathione-S-transferase in Caco-2 cells. Nahrung Food 2001, 45, 189–194. [Google Scholar] [CrossRef]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef]
- Hofmann, T. Studies on the relationship between molecular weight and the color potency of fractions obtained by thermal treatment of glucose amino acid and glucose/protein solutions by using ultracentrifugation and color dilution techniques. J. Agric. Food Chem. 1998, 46, 3891–3895. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. [Google Scholar] [CrossRef]
- Finot, P.A.; Magnenat, E. Metabolic transit of early and advanced Maillard products. Prog. Food Nutr. Sci. 1981, 5, 193–207. [Google Scholar]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef]
- Sams, L.; Paume, J.; Giallo, J.; Carriere, F. Relevant pH and lipase for in vitro models of gastric digestion. Food Funct. 2016, 7, 30–45. [Google Scholar] [CrossRef]
- Hellwig, M.; Matthes, R.; Peto, A.; Lobner, J.; Henle, T. N-epsilon-fructosyllysine and N-epsilon-carboxymethyllysine, but not lysinoalanine, are available for absorption after simulated gastrointestinal digestion. Amino Acids 2014, 46, 289–299. [Google Scholar] [CrossRef]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef]
- Read, N.W.; Miles, C.A.; Fisher, D.; Holgate, A.M.; Kime, N.D.; Mitchell, M.A.; Reeve, A.M.; Roche, T.B.; Walker, M. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology 1980, 79, 1276–1282. [Google Scholar] [CrossRef]
- Joubran, Y.; Moscovici, A.; Lesmes, U. Antioxidant activity of bovine alpha lactalbumin Maillard products and evaluation of their in vitro gastro-duodenal digestive proteolysis. Food Funct. 2015, 6, 1229–1240. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of Glyoxal-Glycated beta-Casein and beta-Lactoglobulin and Distribution of Peptide-Bound Advanced Glycation End Products in Gastrointestinal Digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Leonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of beta-lactoglobulin and beta-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Moscovici, A.M.; Joubran, Y.; Briard-Bion, V.; Mackie, A.; Dupont, D.; Lesmes, U. The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gastrointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef]
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [Green Version]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef]
- Wada, Y.; Lonnerdal, B. Effects of Different Industrial Heating Processes of Milk on Site-Specific Protein Modifications and Their Relationship to in Vitro and in Vivo Digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef]
- Joubran, Y.; Moscovici, A.; Portmann, R.; Lesmes, U. Implications of the Maillard reaction on bovine alpha-lactalbumin and its proteolysis during in vitro infant digestion. Food Funct. 2017, 8, 2295–2308. [Google Scholar] [CrossRef]
- Nyakayiru, J.; van Lieshout, G.A.A.; Trommelen, J.; van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; van Loon, L.J.C. The glycation level of milk protein strongly modulates post-prandial lysine availability in humans. Br. J. Nutr. 2020, 123, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Faist, V.; Erbersdobler, H.F. Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction. Ann. Nutr. Metab. 2001, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Somoza, V. Five years of research on health risks and benefits of Maillard reaction products: An update. Mol. Nutr. Food Res. 2005, 49, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of free and peptide-bound glycated amino acids: Synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem 2011, 12, 1270–1279. [Google Scholar] [CrossRef]
- Grunwald, S.; Krause, R.; Bruch, M.; Henle, T.; Brandsch, M. Transepithelial flux of early and advanced glycation compounds across Caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. Br. J. Nutr. 2006, 95, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Geissler, S.; Hellwig, M.; Markwardt, F.; Henle, T.; Brandsch, M. Synthesis and intestinal transport of the iron chelator maltosine in free and dipeptide form. Eur. J. Pharm. Biopharm. 2011, 78, 75–82. [Google Scholar] [CrossRef]
- Geissler, S.; Hellwig, M.; Zwarg, M.; Markwardt, F.; Henle, T.; Brandsch, M. Transport of the advanced glycation end products alanylpyrraline and pyrralylalanine by the human proton-coupled peptide transporter hPEPT1. J. Agric. Food Chem. 2010, 58, 2543–2547. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Niquet-Leridon, C.; Strauch, C.; Monnie, V.M.; Tessier, F.J.; Navarro, M.P.; Delgado-Andrade, C. An Advanced Glycation End Product (AGE)-Rich Diet Promotes N epsilon-Carboxymethyl-lysine Accumulation in the Cardiac Tissue and Tendons of Rats. J. Agric. Food Chem. 2014, 62, 6001–6006. [Google Scholar] [CrossRef]
- Xu, H.Z.; Wang, Z.Q.; Wang, Y.; Hu, S.D.; Liu, N.F. Biodistribution and Elimination Study of Fluorine-18 Labeled N-epsilon-Carboxymethyl-Lysine following Intragastric and Intravenous Administration. PLoS ONE 2013, 8, e57897. [Google Scholar]
- Degen, J.; Beyer, H.; Heymann, B.; Hellwig, M.; Henle, T. Dietary influence on urinary excretion of 3-deoxyglucosone and its metabolite 3-deoxyfructose. J. Agric. Food Chem. 2014, 62, 2449–2456. [Google Scholar] [CrossRef]
- Foerster, A.; Henle, T. Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): Studies on the urinary excretion of pyrraline. Biochem. Soc. Trans. 2003, 31, 1383–1385. [Google Scholar] [CrossRef]
- FÖRster, A.; KÜHne, Y.; Henle, T.O. Studies on Absorption and Elimination of Dietary Maillard Reaction Products. Ann. N. Y. Acad. Sci. 2005, 1043, 474–481. [Google Scholar] [CrossRef]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; He, C.; Vlassara, H. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J. Am. Soc. Nephrol. 2003, 14, 728–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebekova, K.; Saavedra, G.; Zumpe, C.; Somoza, V.; Klenovicsova, K.; Birlouez-Aragon, I. Plasma concentration and urinary excretion of N-epsilon-(carboxymethyl)lysine in breast milk- and formula-fed infants. Mail. React. Recent Adv. Food Biomed. Sci. 2008, 1126, 177–180. [Google Scholar]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Adams-Huet, B.; Imrhan, V. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: Role of dietary fat. Br. J. Nutr. 2015, 114, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.; Hanssen, N.M.J.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Fernandez-Gomez, B.; Cai, W.; Uribarri, J.; Del Castillo, M.D. In vitro formation of Maillard reaction products during simulated digestion of meal-resembling systems. Food Res. Int. 2019, 118, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Bains, Y.; Gugliucci, A.; Caccavello, R. Advanced glycation endproducts form during ovalbumin digestion in the presence of fructose: Inhibition by chlorogenic acid. Fitoterapia 2017, 120, 1–5. [Google Scholar] [CrossRef]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. The link between soda intake and asthma: Science points to the high-fructose corn syrup, not the preservatives: A commentary. Nutr. Diabetes 2016, 6, e234. [Google Scholar] [CrossRef]
- DeChristopher, L.R. Perspective: The Paradox in Dietary Advanced Glycation End Products Research-The Source of the Serum and Urinary Advanced Glycation End Products Is the Intestines, Not the Food. Adv. Nutr. 2017, 8, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef]
- Jones, H.F.; Butler, R.N.; Brooks, D.A. Intestinal fructose transport and malabsorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G202–G206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J. Physiol. 2003, 552 Pt 3, 823–832. [Google Scholar] [CrossRef]
- Oimomi, M.; Nakamichi, T.; Ohara, T.; Sakai, M.; Igaki, N.; Hata, F.; Baba, S. Fructose-related glycation. Diabetes Res. Clin. Pract. 1989, 7, 137–139. [Google Scholar] [CrossRef]
- Helou, C.; Denis, S.; Spatz, M.; Marier, D.; Rame, V.; Alric, M.; Tessier, F.J.; Gadonna-Widehem, P. Insights into bread melanoidins: Fate in the upper digestive tract and impact on the gut microbiota using in vitro systems. Food Funct. 2015, 6, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, L.; Zhang, X.; Yao, H.; Yang, M.; Gai, Z.; Li, B.; Zhao, D. Degradation of Peptide-Bound Maillard Reaction Products in Gastrointestinal Digests of Glyoxal-Glycated Casein by Human Colonic Microbiota. J. Agric. Food Chem. 2019, 67, 12094–12104. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Fogliano, V. Bread crust melanoldins as potential prebiotic ingredients. Mol. Nutr. Food Res. 2005, 49, 673–678. [Google Scholar] [CrossRef]
- Deppe, V.M.; Bongaerts, J.; O’Connell, T.; Maurer, K.H.; Meinhardt, F. Enzymatic deglycation of Amadori products in bacteria: Mechanisms, occurrence and physiological functions. Appl. Microbiol. Biotechnol. 2011, 90, 399–406. [Google Scholar] [CrossRef]
- Wiame, E.; Delpierre, G.; Collard, F.; Van Schaftingen, E. Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J. Biol. Chem. 2002, 277, 42523–42529. [Google Scholar] [CrossRef] [Green Version]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Hinton, D.J.; Davies, S.J.; Crabbe, M.J.; Gibson, G.R.; Ames, J.M. Metabolism of Maillard reaction products by the human gut microbiota—Implications for health. Mol. Nutr. Food Res. 2006, 50, 847–857. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor alpha-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snelson, M.; Coughlan, M.T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Burillo, S.; Pastoriza, S.; Jimenez-Hernandez, N.; D’Auria, G.; Francino, M.P.; Rufian-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 58, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Zheng, F.; Striker, G.E.; Vlassara, H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am. J. Pathol. 2008, 173, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Shangari, N.; Depeint, F.; Furrer, R.; Bruce, W.R.; Popovic, M.; Zheng, F.; O’Brien, P.J. A thermolyzed diet increases oxidative stress, plasma alpha-aldehydes and colonic inflammation in the rat. Chem. Biol. Interact. 2007, 169, 100–109. [Google Scholar] [CrossRef]
- Buetler, T.; Henle, T. The effects of AGEing on diet. Am. J. Pathol. 2009, 174, 351; author reply 352–353. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Van der Lugt, T.; Weseler, A.; Gebbink, W.; Vrolijk, M.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Gao, Q.D.; Zhu, L.; Peppa, M.; He, C.; Vlassara, H. Oxidative stress-inducing carbonyl compounds from common foods: Novel mediators of cellular dysfunction. Mol. Med. 2002, 8, 337–346. [Google Scholar] [CrossRef]
- Chun, S.H.; Lee, H.A.; Lee, K.B.; Kim, S.H.; Park, K.Y.; Lee, K.W. Effects of Glycated Whey Protein Concentrate on Pro-inflammatory Cytokine Expression and Phagocytic Activity in RAW264.7 Macrophages. Biol. Pharm. Bull. 2016, 39, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R. Acrylamide in Crisps (Reducing Acrylamide in Crisps). J. Cell Sci. Apoptosis 2017, 1, 104. [Google Scholar]
- Spergel, J.M. Epidemiology of atopic dermatitis and atopic march in children. Immunol. Allergy Clin. N. Am. 2010, 30, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. The increased prevalence of allergy and the hygiene hypothesis: Missing immune deviation, reduced immune suppression, or both? Immunology 2004, 112, 352–363. [Google Scholar] [CrossRef]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food Processing: The Influence of the Maillard Reaction on Immunogenicity and Allergenicity of Food Proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Patrignani, M.; Rinaldi, G.J.; Rufian-Henares, J.A.; Lupano, C.E. Antioxidant capacity of Maillard reaction products in the digestive tract: An in vitro and in vivo study. Food Chem. 2019, 276, 443–450. [Google Scholar] [CrossRef]
- Chuyen, N.V.; Ijichi, K.; Umetsu, H.; Moteki, K. Antioxidative properties of products from amino acids or peptides in the reaction with glucose. Adv. Exp. Med. Biol. 1998, 434, 201–212. [Google Scholar] [PubMed]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Xie, J.; Mendez, J.D.; Mendez-Valenzuela, V.; Aguilar-Hernandez, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Zhao, J.B.; Gregersen, H. Up-Regulated Expression of Advanced Glycation End-Products and Their Receptor in the Small Intestine and Colon of Diabetic Rats. Dig. Dis. Sci. 2012, 57, 48–57. [Google Scholar] [CrossRef]
- Xue, J.; Rai, V.; Singer, D.; Chabierski, S.; Xie, J.; Reverdatto, S.; Burz, D.S.; Schmidt, A.M.; Hoffmann, R.; Shekhtman, A. Advanced glycation end product recognition by the receptor for AGEs. Structure 2011, 19, 722–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Reverdatto, S.; Frolov, A.; Hoffmann, R.; Burz, D.S.; Shekhtman, A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 2008, 283, 27255–27269. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Bongarzone, S.; Savickas, V.; Luzi, F.; Gee, A.D. Targeting the Receptor for Advanced Glycation Endproducts (RAGE): A Medicinal Chemistry Perspective. J. Med. Chem. 2017, 60, 7213–7232. [Google Scholar] [CrossRef] [Green Version]
- Kislinger, T.; Fu, C.; Huber, B.; Qu, W.; Taguchi, A.; Du Yan, S.; Hofmann, M.; Yan, S.F.; Pischetsrieder, M.; Stern, D.; et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999, 274, 31740–31749. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Ray, R.; Singer, D.; Bohme, D.; Burz, D.S.; Rai, V.; Hoffmann, R.; Shekhtman, A. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry 2014, 53, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Buetler, T.M.; Leclerc, E.; Baumeyer, A.; Latado, H.; Newell, J.; Adolfsson, O.; Parisod, V.; Richoz, J.; Maurer, S.; Foata, F.; et al. N-epsilon-carboxymethyllysine-modified proteins are unable to bind to RAGE and activate an inflammatory response. Mol. Nutr. Food Res. 2008, 52, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. (Berl.) 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFkappaB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.; Hwang, I.; Han, S.H.; Shin, J.S.; Shin, O.S.; Yu, J.W. Advanced glycation end products impair NLRP3 inflammasome-mediated innate immune responses in macrophages. J. Biol. Chem. 2017, 292, 20437–20448. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; He, J.C.; Zhu, L.; Lu, C.; Vlassara, H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 13801–13806. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Torreggiani, M.; Zhu, L.; Chen, X.; He, J.C.; Striker, G.E.; Vlassara, H. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: Implications for vascular disease. Am. J. Physiol. Cell Physiol. 2010, 298, C624–C634. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; He, J.C.; Cai, W.; Liu, H.; Zhu, L.; Vlassara, H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11767–11772. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Uribarri, J.; Cai, W.J.; Striker, G. Advanced glycation end product homeostasis—Exogenous oxidants and innate defenses. Mail. React. Recent Adv. Food Biomed. Sci. 2008, 1126, 46–52. [Google Scholar]
- Bansal, S.; Siddarth, M.; Chawla, D.; Banerjee, B.D.; Madhu, S.V.; Tripathi, A.K. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol. Cell Biochem. 2012, 361, 289–296. [Google Scholar] [CrossRef]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Bork, K.; Gnanapragassam, V.S.; Bennmann, D.; Jacobs, K.; Navarette-Santos, A.; Hofmann, B.; Simm, A.; Danker, K.; Horstkorte, R. Novel insights in the dysfunction of human blood-brain barrier after glycation. Mech. Ageing Dev. 2016, 155, 48–54. [Google Scholar] [CrossRef]
- Bucala, R.; Makita, Z.; Vega, G.; Grundy, S.; Koschinsky, T.; Cerami, A.; Vlassara, H. Modification of Low-Density-Lipoprotein by Advanced Glycation End-Products Contributes to the Dyslipidemia of Diabetes and Renal-Insufficiency. Proc. Natl. Acad. Sci. USA 1994, 91, 9441–9445. [Google Scholar] [CrossRef] [Green Version]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Tung, J.; Loftus, E.V., Jr.; Freese, D.K.; El-Youssef, M.; Zinsmeister, A.R.; Melton, L.J., 3rd; Harmsen, W.S.; Sandborn, W.J.; Faubion, W.A., Jr. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2006, 12, 1093–1100. [Google Scholar] [CrossRef]
- Munkholm, P.; Langholz, E.; Davidsen, M.; Binder, V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 1994, 35, 360–362. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Itoh, K.; Ochiai, M.; Iwai, A.; Park, Y.; Hata, S.; Takeuchi, K.; Ito, M.; Imaki, J.; Miura, S.; et al. Increased pentosidine, an advanced glycation end-product, in urine and tissue reflects disease activity in inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2008, 23, S140–S145. [Google Scholar] [CrossRef]
- Andrassy, M.; Igwe, J.; Autschbach, F.; Volz, C.; Remppis, A.; Neurath, M.F.; Schleicher, E.; Humpert, P.M.; Wendt, T.; Liliensiek, B.; et al. Posttranslationally modified proteins as mediators of sustained intestinal inflammation. Am. J. Pathol. 2006, 169, 1223–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccocioppo, R.; Vanoli, A.; Klersy, C.; Imbesi, V.; Boccaccio, V.; Manca, R.; Betti, E.; Cangemi, G.C.; Strada, E.; Besio, R.; et al. Role of the advanced glycation end products receptor in Crohn’s disease inflammation. World J. Gastroenterol. 2013, 19, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Zen, K.; Chen, C.X.J.; Chen, Y.T.; Wilton, R.; Liu, Y. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J. Immunol. 2007, 178, 2483–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Imbesi, V.; Betti, E.; Boccaccio, V.; Kruzliak, P.; Gallia, A.; Cangemi, G.C.; Maffe, G.C.; Vanoli, A.; Merante, S.; et al. The Circulating Level of Soluble Receptor for Advanced Glycation End Products Displays Different Patterns in Ulcerative Colitis and Crohn’s Disease: A Cross-Sectional Study. Dig. Dis. Sci. 2015, 60, 2327–2337. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bozzini, S.; Betti, E.; Imbesi, V.; Klersy, C.; Lakyova, L.S.; Sukovsky, L.; Benacka, J.; Kruzliak, P.; Corazza, G.R.; et al. Functional polymorphisms of the receptor for the advanced glycation end product promoter gene in inflammatory bowel disease: A case-control study. Clin. Exp. Med. 2019, 19, 367–375. [Google Scholar] [CrossRef]
- Dabritz, J.; Friedrichs, F.; Weinhage, T.; Hampe, J.; Kucharzik, T.; Lugering, A.; Broeckel, U.; Schreiber, S.; Spieker, T.; Stoll, M.; et al. The functional -374T/A polymorphism of the receptor for advanced glycation end products may modulate Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G823–G832. [Google Scholar] [CrossRef]
- Yuan, X.J.; Zhao, J.S.; Qu, W.T.; Zhang, Y.X.; Jia, B.P.; Fan, Z.Y.; He, Q.H.; Li, J.X. Accumulation and effects of dietary advanced glycation end products on the gastrointestinal tract in rats. Int. J. Food Sci. Technol. 2018, 53, 2273–2281. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61, 1700118. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Nass, N.; Bayreuther, K.; Simm, A. Systemic activation of NF-kappaB driven luciferase activity in transgenic mice fed advanced glycation end products modified albumin. Glycoconj. J. 2017, 34, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.M.; Craus, A.; Niquet-Leridon, C.; Tessier, F.J. Highly heated food rich in Maillard reaction products limit an experimental colitis in mice. Food Funct. 2012, 3, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Al Amir, I.; Dubayle, D.; Heron, A.; Delayre-Orthez, C.; Anton, P.M. Maillard reaction products from highly heated food prevent mast cell number increase and inflammation in a mouse model of colitis. Nutr. Res. 2017, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Nesreen, A.; Gadonna-Widehem, P.; Delayre-Orthez, C.; Marier, D.; Garnier, B.; Carbonero, F.; Anton, P.M. Repeated Oral Exposure to N (epsilon)-Carboxymethyllysine, a Maillard Reaction Product, Alleviates Gut Microbiota Dysbiosis in Colitic Mice. Dig. Dis. Sci. 2017, 62, 3370–3384. [Google Scholar]
- Mills, D.J.S.; Tuohy, K.M.; Booth, J.; Buck, M.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J. Appl. Microbiol. 2008, 105, 706–714. [Google Scholar] [CrossRef]
- Munch, G.; Schicktanz, D.; Behme, A.; Gerlach, M.; Riederer, P.; Palm, D.; Schinzel, R. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat. Biotechnol. 1999, 17, 1006–1010. [Google Scholar] [CrossRef]
- Pratt, C.W.; Cornely, K. Essential Biochemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; p. 704. [Google Scholar]
- Ashoor, S.H.; Zent, J.B. Maillard Browning of Common Amino Acids and Sugars. J. Food Sci. 1984, 49, 1206–1207. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Puigserver, A. Nonenzymatic browning reaction of essential amino acids: Effect of pH on caramelization and Maillard reaction kinetics. J. Agric. Food Chem. 1999, 47, 1786–1793. [Google Scholar] [CrossRef]
- Kwak, E.J.; Lim, S.I. The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the Chemistry of Non-Enzymatic Browning Reactions in Different Ribose-Amino Acid Model Systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helou, C.; Gadonna-Widehem, P.; Robert, N.; Branlard, G.; Thebault, J.; Librere, S.; Jacquot, S.; Mardon, J.; Piquet-Pissaloux, A.; Chapron, S.; et al. The impact of raw materials and baking conditions on Maillard reaction products, thiamine, folate, phytic acid and minerals in white bread. Food Funct. 2016, 7, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Milkovska-Stamenova, S.; Hoffmann, R. Influence of storage and heating on protein glycation levels of processed lactose-free and regular bovine milk products. Food Chem. 2017, 221, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Prosser, C.G.; Carpenter, E.A.; Hodgkinson, A.J. N(epsilon)-carboxymethyllysine in nutritional milk formulas for infants. Food Chem. 2019, 274, 886–890. [Google Scholar] [CrossRef]
- Klenovics, K.S.; Boor, P.; Somoza, V.; Celec, P.; Fogliano, V.; Sebekova, K. Advanced glycation end products in infant formulas do not contribute to insulin resistance associated with their consumption. PLoS ONE 2013, 8, e53056. [Google Scholar] [CrossRef]
- Oldfield, M.D.; Bach, L.A.; Forbes, J.M.; Nikolic-Paterson, D.; McRobert, A.; Thallas, V.; Atkins, R.C.; Osicka, T.; Jerums, G.; Cooper, M.E. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J. Clin. Investig. 2001, 108, 1853–1863. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- Wagner, Z.; Wittmann, I.; Mazak, I.; Schinzel, R.; Heidland, A.; Kientsch-Engel, R.; Nagy, J. N(epsilon)-(carboxymethyl)lysine levels in patients with type 2 diabetes: Role of renal function. Am. J. Kidney Dis. 2001, 38, 785–791. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Hellwig, M.; Degen, J.; Henle, T. 3-deoxygalactosone, a “new” 1,2-dicarbonyl compound in milk products. J. Agric. Food Chem. 2010, 58, 10752–10760. [Google Scholar] [CrossRef]
- Helou, C.; Jacolot, P.; Niquet-Leridon, C.; Gadonna-Widehem, P.; Tessier, F.J. Maillard reaction products in bread: A novel semi-quantitative method for evaluating melanoidins in bread. Food Chem. 2016, 190, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D. Analytical strategies to depict the fate of the Maillard reaction in foods. Curr. Opin. Food Sci. 2018, 19, 15–22. [Google Scholar] [CrossRef]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. N-epsilon-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Niquet-Leridon, C.; Jacolot, P.; Niamba, C.N.; Grossin, N.; Boulanger, E.; Tessier, F.J. The rehabilitation of raw and brown butters by the measurement of two of the major Maillard products, N(epsilon)-carboxymethyl-lysine and 5-hydroxymethylfurfural, with validated chromatographic methods. Food Chem. 2015, 177, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Delatour, T.; Hegele, J.; Parisod, V.; Richoz, J.; Maurer, S.; Steven, M.; Buetler, T. Analysis of advanced glycation endproducts in dairy products by isotope dilution liquid chromatography–electrospray tandem mass spectrometry. The particular case of carboxymethyllysine. J. Chromatogr. A 2009, 1216, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, B.; Wu, Y.; Li, H.; Xu, D.; Nian, Y.; Mao, S.; Li, C.; Xu, X.; Zhou, G. Comparison of Free and Bound Advanced Glycation End Products in Food: A Review on the Possible Influence on Human Health. J. Agric. Food Chem. 2019, 67, 14007–14018. [Google Scholar] [CrossRef]
- Hegele, J.; Buetler, T.; Delatour, T. Comparative LC-MS/MS profiling of free and protein-bound early and advanced glycation-induced lysine modifications in dairy products. Anal. Chim. Acta 2008, 617, 85–96. [Google Scholar] [CrossRef]
- Henle, T.; Zehetner, G.; Klostermeyer, H. Fast and sensitive determination of furosine. Z. Lebensm. Unters. Forsch. 1995, 200, 235–237. [Google Scholar] [CrossRef]
- Zhu, Y.; Snooks, H.; Sang, S. Complexity of Advanced Glycation End Products in Foods: Where Are We Now? J. Agric. Food Chem. 2018, 66, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment (RIVM). Dutch National Food Consumption Survey 2009; Version 11-2009; RIVM: The Hague, The Netherlands, 2009; Available online: www.voedselconsumptiepeiling.nl (accessed on 18 September 2018).
- Hou, J.K.; Lee, D.; Lewis, J. Diet and inflammatory bowel disease: Review of patient-targeted recommendations. Clin. Gastroenterol. Hepatol. 2014, 12, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 2002, 51 (Suppl. S1), i41–i44. [Google Scholar] [CrossRef] [PubMed]
- Catalioto, R.M.; Maggi, C.A.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Nederland, Z. Farmacotherapeutisch Kompas. 2020. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/indicatieteksten/functionele_maagklachten (accessed on 1 March 2020).
- Penndorf, I.; Biedermann, D.; Maurer, S.V.; Henle, T. Studies on N-terminal glycation of peptides in hypoallergenic infant formulas: Quantification of alpha-N-(2-furoylmethyl) amino acids. J. Agric. Food Chem. 2007, 55, 723–727. [Google Scholar] [CrossRef]
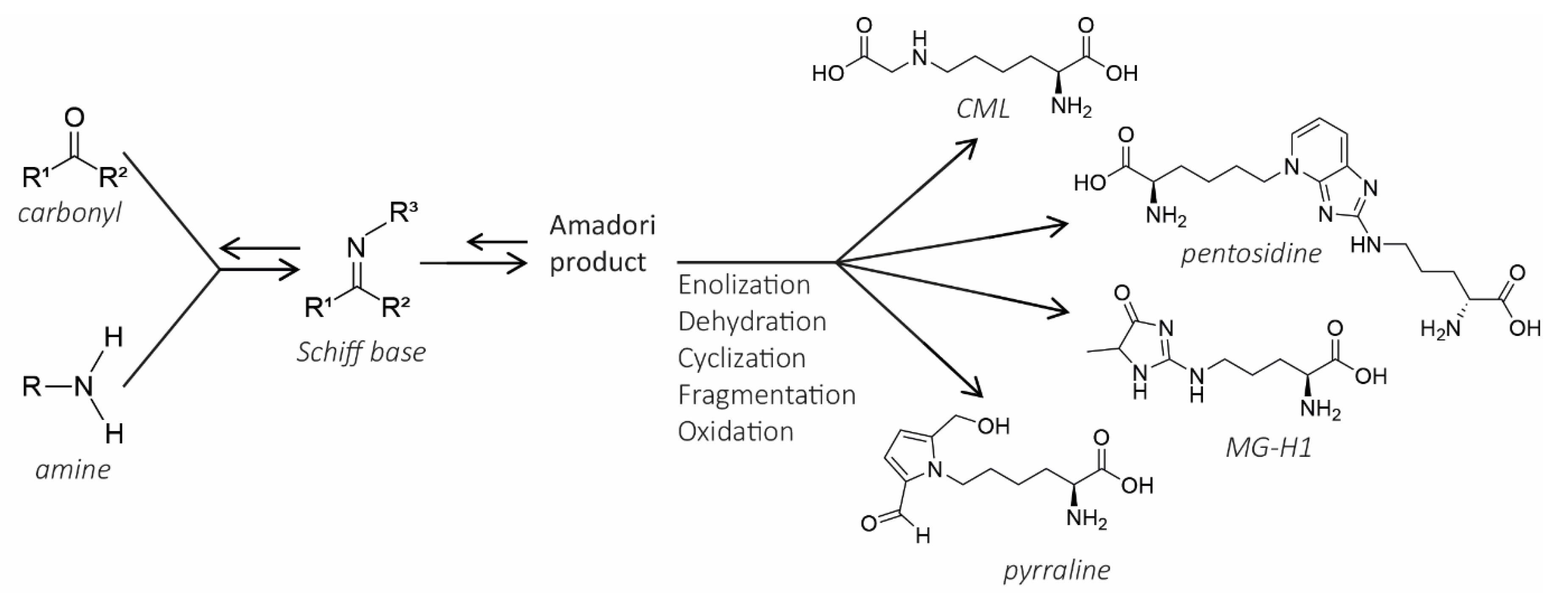
| Compound | Evidence | Notes | Reference(s) |
|---|---|---|---|
| Nε-carboxymethyl-lysine (CML) | Strongly retained inside Caco-2 cells. | Diffused into gastrointestinal epithelial (Caco-2) cells, but was not able to cross the basolateral membrane. Accumulation in intestinal cells. Not likely transported by amino acid and peptide carriers and the transepithelial flux measured for the compounds occurs most probably by simple diffusion. | [34,35] |
| Nε-carboxyethyl-lysine (CEL) | Strongly retained inside Caco-2 cells. | - | [34] |
| methylglyoxal-derived hydroimidazolone-1 (MG-H1) | Strongly retained inside Caco-2 cells. | - | [34] |
| Maltosine | Absorbed as dipeptide into Caco-2 cells by PEPT1 and strongly retained in cells. Not absorbed in free form. | Free maltosine permeates the basolateral cell membrane by simple diffusion down its concentration gradient and possibly by the action of basolateral amino acid transporters. | [34,36] |
| Glycated dipeptides | Absorbed into Caco-2 cells by PEPT1. | Intracellularly hydrolysed by peptidases to the free modified amino acids and alanine. | [34] |
| Pyrraline | Not free pyrraline, but the dipeptide with alanine is absorbed by PEPT1 in HeLa cells (cervical cancer cells). | After intracellular hydrolysation free pyrraline diffused through the basolateral membrane. | [34,37] |
| fructoselysine | Simple diffusion to a small extent in Caco-2 cells. | Not likely transported by amino acid and peptide carriers and the transepithelial flux measured for the compounds occurs most probably by simple diffusion. | [35] |
| Food Product | CML (mg)/100 g | CEL (mg)/100 g | MG-H1 (mg)/100 g |
|---|---|---|---|
| Blood sausages | 4.8 | 7.7 | 63.0 |
| Peanut butter | 3.1 | 6.7 | 44.5 |
| Cereals | 2.0 | 1.6 | 41.6 |
| Ginger biscuit | 2.5 | 2.0 | 28.3 |
| Salted peanuts | 1.7 | 3.4 | 25.7 |
| Rusk | 2.0 | 1.4 | 23.1 |
| Red cooked beef | 2.0 | 5.6 | 13.5 |
| Chocolate sprinkles | 5.1 | 2.0 | 9.3 |
| Canned salmon | 1.2 | 2.8 | 11.0 |
| Fried tofu | 0.9 | 1.2 | 10.9 |
| Food Product | dAGEs Content (mg/100 g) [147] | Average Intake per Day (g) | Daily Exposure to dAGEs (mg/day) |
|---|---|---|---|
| Blood sausages | 75.5 | 32 | 24.2 |
| Beef steak (canned) | 18.7 | 78 | 14.6 |
| Cereals (frosted flakes) | 27.1 | 39 | 10.5 |
| Fried rice | 10.9 | 91 | 9.9 |
| Peanut butter (Calve) | 51.5 | 18 | 9.3 |
| Brown bread | 6.7 | 100 | 6.7 |
| Peanuts | 31.7 | 21 | 6.7 |
| Meat ball | 10.1 | 60 | 6.1 |
| Chicken Wings | 4.6 | 127 | 5.9 |
| Ginger biscuit | 32.8 | 12 | 3.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Lugt, T.; Opperhuizen, A.; Bast, A.; Vrolijk, M.F. Dietary Advanced Glycation Endproducts and the Gastrointestinal Tract. Nutrients 2020, 12, 2814. https://doi.org/10.3390/nu12092814
van der Lugt T, Opperhuizen A, Bast A, Vrolijk MF. Dietary Advanced Glycation Endproducts and the Gastrointestinal Tract. Nutrients. 2020; 12(9):2814. https://doi.org/10.3390/nu12092814
Chicago/Turabian Stylevan der Lugt, Timme, Antoon Opperhuizen, Aalt Bast, and Misha F. Vrolijk. 2020. "Dietary Advanced Glycation Endproducts and the Gastrointestinal Tract" Nutrients 12, no. 9: 2814. https://doi.org/10.3390/nu12092814





