Oxidative Stress and Reproductive Function in the Aging Male
Abstract
1. Introduction
2. Theories of Cellular Aging
2.1. Telomere Theory
2.2. Immunologic Theory
2.3. Free Radical Theory
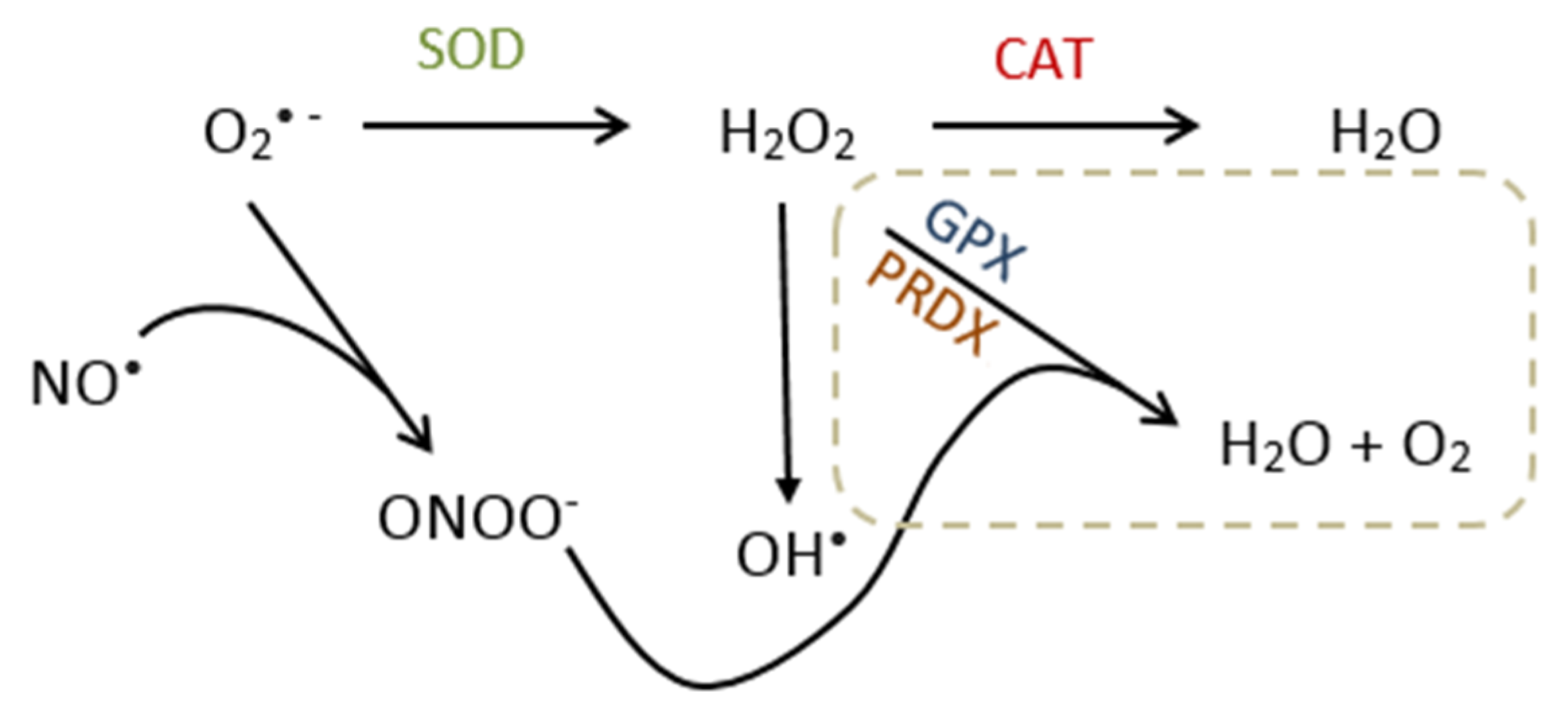
3. Oxidative Stress
3.1. Source of Reactive Oxygen Species
3.2. Molecular Effects/Function of ROS
3.3. Metabolism and Reproductive Function of Nitric Oxide
3.4. Redox Imbalance and Germ Cell Damage
3.4.1. Lipid Peroxidation
3.4.2. DNA Damage
3.4.3. Modifications to Proteins
4. Animal Studies and the Effects of Aging and Oxidative Stress in Male Germ Cells
4.1. Effects of Oxidative Stress in Aged Transgenic Mice
4.2. Effects of Oxidative Stress in Aged Brown Norway Rats
4.3. Treatment of Oxidative Stress in Animals
5. Effects of Aging and Oxidative Stress on Human Spermatozoa
5.1. Effects of Aging on Sperm Quality
5.2. Effects of Aging and Oxidative Stress on Sperm DNA
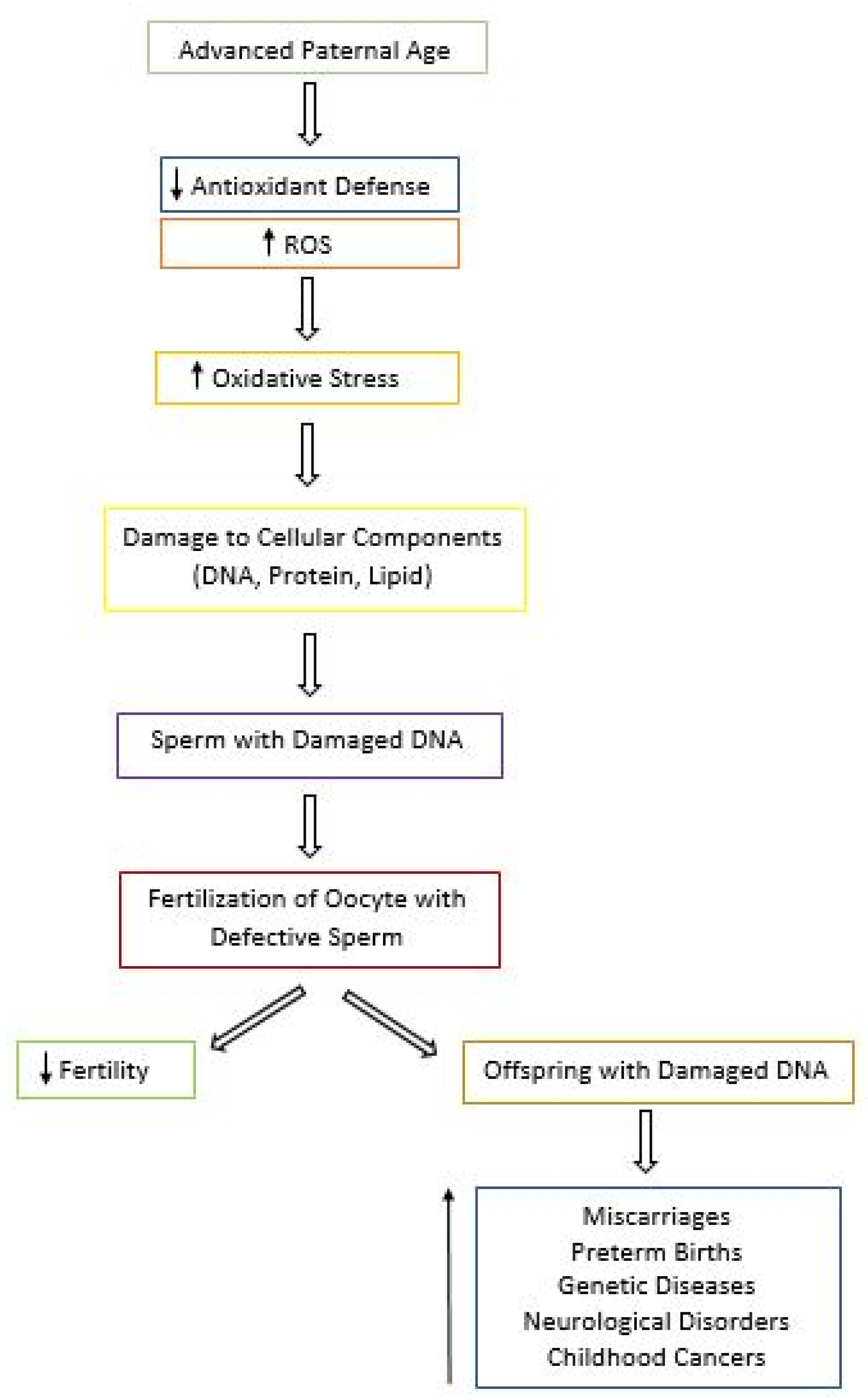
5.3. Effects of Aging and Oxidative Stress on Fertility and Progeny Outcome
5.4. Value of Antioxidant Supplementation in Aging Males
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khandwala, Y.; Zhang, C.; Lu, Y.; Eisenberg, M. The age of fathers in the USA is rising: An analysis of 168 867 480 births from 1972 to 2015. Hum. Reprod. 2017, 32, 2110–2116. [Google Scholar] [CrossRef]
- Schummers, L.; Hacker, M.R.; Williams, P.L.; Hutcheon, J.A.; Vanderweele, T.J.; McElrath, T.F.; Hernandez-Diaz, S. Variation in relationships between maternal age at first birth and pregnancy outcomes by maternal race: A population-based cohort study in the United States. BMJ Open 2019, 9, e033697. [Google Scholar] [CrossRef]
- Lian, Z.H.; Zack, M.M.; Erickson, J.D. Paternal age and the occurrence of birth defects. Am. J. Hum. Genet. 1986, 39, 648–660. [Google Scholar]
- Alio, A.P.; Salihu, H.M.; McIntosh, C.; August, E.M.; Weldeselasse, H.; Sanchez, E.; Mbah, A.K. The effect of paternal age on fetal birth outcomes. Am. J. Mens Health 2012, 6, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care. 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- Fice, H.; Robaire, B. Telomere Dynamics Throughout Spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.B.; et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B. Roy Walford and the immunologic theory of aging. Immun. Ageing A 2005, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Diggs, J. Autoimmune Theory of Aging. Ency. Aging Pub. Health 2008, 143–144. [Google Scholar] [CrossRef]
- Mital, P.; Hinton, B.T.; Dufour, J.M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 2011, 84, 851–858. [Google Scholar] [CrossRef]
- Levy, S.; Serre, V.; Hermo, L.; Robaire, B. The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J. Androl. 1999, 20, 356–365. [Google Scholar]
- Gerschman, R.; Nye, S.W.; Gilbert, D.L.; Dwyer, P.; Fenn, W.O. Studies on oxygen poisoning: Protective effect of beta-mercaptoethylamine. Proc. Soc. Exp. Biol. Med. 1954, 85, 75–77. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antiox. Redox Sign. 2014, 20, 727–731. [Google Scholar]
- Schoneich, C. Reactive oxygen species and biological aging: A mechanistic approach. Exp. Gerontol. 1999, 34, 19–34. [Google Scholar] [CrossRef]
- Mittal, C.K.; Murad, F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: A physiological regulator of guanosine 3’, 5’-monophosphate formation. Proc. Natl. Acad. Sci. USA 1977, 74, 4360–4364. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clinc. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.; Al-Bader, M.; Mousa, A.; Khan, K.M. Molecular effects of chemotherapeutic drugs and their modulation by antioxidants in the testis. Eur. J. Pharmacol. 2012, 674, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Hu, X.Q.; Xiao, L.J.; Hu, Z.Y.; Guo, J.; Zhang, K.Y.; Song, X.X.; Liu, Y.X. An oligonucleotide microarray study on gene expression profile in mouse testis of experimental cryptorchidism. Front. Biosci. 2006, 11, 2465–2482. [Google Scholar] [CrossRef] [PubMed]
- Sandlow, J. Pathogenesis and treatment of varicoceles. BMJ 2004, 328, 967–968. [Google Scholar] [CrossRef]
- Li, J.; Mao, R.; Zhou, Q.; Ding, L.; Tao, J.; Ran, M.M.; Gao, E.S.; Yuan, W.; Wang, J.T.; Hou, L.F. Exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of ERK signal pathway. Toxicol. Mech. Methods. 2016, 26, 180–188. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of Nitric Oxide Concentrations on Human Sperm Motility. J. Adrol. 2004, 25, 245–249. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab. J. Urol. 2017, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, M.; Dubey, R.K.; Imthurn, B.; Macas, E.; Keller, P.J. Effects of nitric oxide on human spermatozoa: Evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum. Reprod. 1995, 10, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.B.; Udekwu, A.O.; Billiar, T.R.; Curran, R.D.; Cerra, F.B.; Simmons, R.L.; Peitzman, A.B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann. Surg. 1991, 214, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J.; Charles, S.L.; Chess-Williams, R. Endothelium-derived relaxing factor release an activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988, 336, 385–388. [Google Scholar] [CrossRef]
- Ignarro, L.I.; Byrns, R.E.; Buga, G.M.; Wood, K.S. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Calver, A.; Collier, J.; Vallance, P. Nitric oxide and cardiovascular control. Exp. Physiol. 1993, 78, 303–332. [Google Scholar] [CrossRef]
- Hibbs, J.B., Jr. Synthesis of nitric oxide from L-arginine: A recently discovered pathway induced by cytokines with anti-tumour and antimicrobial activities. Res. Immunol. 1991, 142, 565–569. [Google Scholar] [CrossRef]
- Karupiah, G.; Xie, Q.W.; Buller, R.M.; Nathan, C.; Duarte, C.; MacMicking, J.D. Inhibition of viral replication by interferon-gamma-induced nitric ox-ide synthase. Science 1993, 261, 1445–1448. [Google Scholar] [CrossRef]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.; Snyder, S.H. Nitric oxide: A physiologic mediator of penile erection. Science 1992, 257, 401–403. [Google Scholar] [CrossRef]
- Ehren, I.; Adolfsson, J.; Wiklund, N.P. Nitric oxide synthase activity in the human urogenital tract. Urol. Res. 1994, 22, 287–290. [Google Scholar] [CrossRef]
- Herrero, M.B.; Viggiano, J.M.; Perez-Martinez, S.; Gimeno, M.F. Evidence that nitric oxide synthase is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Reprod. Fertil. Dev. 1997, 9, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; De Lamirande, E.; Gagnon, C. Low levels of nitric oxide promote sperm capacitation in vitro. J. Androl. 1995, 16, 424–431. [Google Scholar] [PubMed]
- Sengoku, K.; Tamate, K.; Yoshida, T.; Takaoka, Y.; Miyamoto, T.; Ishikawa, M. Effects of low concentrations of nitric oxide on the zona pellucida binding ability of human spermatozoa. Fertil. Steril. 1998, 69, 522–527. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Donnelly, E.T.; Sterling, E.S.L.; Kennedy, M.S.; Thompson, W.; Chakravarthy, U. Nitric oxide synthase and nitrite production in human spermatozoa: Evidence that endogenous nitric oxide is beneficial to sperm motility. Mol. Hum. Reprod. 1996, 2, 873–878. [Google Scholar] [CrossRef]
- Revelli, A.; Bergandi, L.; Massobrio, M.; Lindblom, B.; Bosia, A.; Ghigo, D. The concentration of nitrite in seminal plasma does not correlate with sperm concentration, sperm motility, leukocytospermia, or sperm culture. Fertil. Steril. 2001, 76, 496–500. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox. Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, L.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bohr, V.; Anson, R.M.; Mazur, S.; Dianov, G. Oxidative DNA damage processing and changes with aging. Toxicol. Lett. 1998, 102–103, 47–52. [Google Scholar] [CrossRef]
- Dahl, J.U.; Gray, M.J.; Jakob, U. Protein quality control under oxidative stress conditions. J. Mol. Biol. 2015, 427, 1549–1563. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Rerpod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Taormina, G.; Ferrante, F.; Vieni, S.; Grassi, N.; Russo, A.; Mirisola, M.G. Longevity: Lesson from Model Organisms. Genes 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, J. Aging Male Germ Cells: Responses to Oxidative Stress and the Effects of Altered Antioxidant Status. Ph.D. Thesis, McGill University, Montreal, QC, Canada, April 2016. [Google Scholar]
- Chen, H.; Hardy, M.P.; Huhtaniemi, I.; Zirkin, B.R. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 1994, 15, 551–557. [Google Scholar] [PubMed]
- Jervis, K.M.; Robaire, B. The effects of long-term vitamin E treatment on gene expression and oxidative stress damage in the aging Brown Norway rat epididymis. Biol. Reprod. 2004, 71, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Viger, R.S.; Robaire, B. Gene expression in the aging brown Norway rat epididymis. J. Androl. 1995, 16, 108–117. [Google Scholar]
- Masoro, E.J. Use of rodents as models for the study of “normal aging”: Conceptual and practical issues. Neurobiol. Aging. 1991, 12, 639–643. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Santulli, R.; Strandberg, J.D.; Wright, W.W.; Ewing, L.L. Testicular steroidogenesis in the aging brown Norway rat. J. Androl. 1993, 14, 118–123. [Google Scholar]
- Selvaratnam, J.; Robaire, B. Effects of Aging and Oxidative Stress on Spermatozoa of Superoxide-Dismutase 1- and Catalase-Null Mice. Biol. Reprod. 2016, 95, 60. [Google Scholar] [CrossRef]
- Noblanc, A.; Klaassen, A.; Robaire, B. The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice. Antioxidants 2020, 9, 151. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox. Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Yim, S.H.; Kim, Y.J.; Oh, S.Y.; Fujii, J.; Zhang, Y.; Gladyshev, V.N.; Rhee, S.G. Identification and characterization of alternatively transcribed form of peroxiredoxin IV gene that is specifically expressed in spermatids of postpubertal mouse testis. J. Biol. Chem. 2011, 286, 39002–39012. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, Y.; Okada, F.; Tsunoda, S.; Kibe, N.; Shirasawa, N.; Ikawa, M.; Okabe, M.; Ikeda, Y.; Fujii, J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem. J. 2009, 419, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; San Gabriel, M.C.; Zini, A.; Chan, P.; O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Smith, T.B.; Baker, M.A.; Connaughton, H.S.; Habenicht, U.; Aitken, R.J. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic. Biol. Med. 2013, 65, 872–881. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, F.E.; et al. seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Serre, V.; Robaire, B. Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertil. Steril. 1998, 70, 625–631. [Google Scholar] [CrossRef]
- Oakes, C.C.; Smiraglia, D.J.; Plass, C.; Trasler, J.M.; Robaire, B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc. Natl. Acad. Sci. USA 2003, 100, 1775–1780. [Google Scholar] [CrossRef]
- Paul, C.; Nagano, M.; Robaire, B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway rat. Biol. Reprod. 2011, 85, 1269–1278. [Google Scholar] [CrossRef]
- Selvaratnam, J.; Paul, C.; Robaire, B. Male Rat Germ Cells Display Age-Dependent and Cell-Specific Susceptibility in Response to Oxidative Stress Challenges. Biol. Reprod. 2015, 93, 72. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.P.; Robaire, B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J. Androl. 2007, 28, 229–240. [Google Scholar] [CrossRef]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Carlson, E.J.; Gillespie, A.M.; Shi, Y.; Epstein, C.J. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2000, 55, B5–B9. [Google Scholar]
- Selman, C.; McLaren, J.S.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol. Lett. 2013, 9, 20130432. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Addai, J.; Smith, R.P.; Coward, R.M.; Lamb, D.J.; Lipshultz, L.I. The effects of advanced paternal age on fertility. Asian J. Androl. 2013, 15, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, A.N.; Turek, P.J. Reproductive genetics and the aging male. J. Assist. Reprod. Genet. 2018, 35, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Fisch, H.; Goluboff, E.T.; Olson, J.H.; Feldshuh, J.; Broder, S.J.; Barad, D.H. Semen analyses in 1283 men from the United States over a 25-year period: No decline in quality. Fertil. Steril. 1996, 65, 1009–1014. [Google Scholar] [CrossRef]
- Eskenazi, B.; Wyrobek, A.J.; Sloter, E.; Kidd, S.A.; Moore, L.; Young, S.; Moore, D. The association of age and semen quality in healthy men. Hum. Reprod. 2003, 18, 447–454. [Google Scholar] [CrossRef]
- Homonnai, Z.T.; Fainman, N.; David, M.P.; Paz, G.F. Semen quality and sex hormone pattern of 29 middle aged men. Andrologia 1982, 14, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, W.J.; Overstreet, J.W.; Sikka, S.C.; Denne, J.; Ahuja, S.; Hoover, A.M.; Sides, G.D.; Cordell, W.H.; Harrison, L.M.; Whitaker, J.S. Semen and sperm reference ranges for men 45 years of age and older. J. Androl. 2006, 27, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Levitas, E.; Lunenfeld, E.; Weisz, N.; Friger, M.; Potashnik, G. Relationship between age and semen parameters in men with normal sperm concentration: Analysis of 6022 semen samples. Andrologia 2007, 39, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Berling, S.; Wolner-Hanssen, P. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: A study of males from Southern Sweden. Hum. Reprod. 1997, 12, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Spandorfer, S.D.; Avrech, O.M.; Colombero, L.T.; Palermo, G.D.; Rosenwaks, Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 334–338. [Google Scholar] [CrossRef]
- Auger, J.; Kunstmann, J.M.; Czyglik, F.; Jouannet, P. Decline in semen quality among fertile men in Paris during the past 20 years. N. Engl. J. Med. 1995, 332, 281–285. [Google Scholar] [CrossRef]
- Dondero, F.; Mazzilli, F.; Giovenco, P.; Lenzi, A.; Cerasaro, M. Fertility in elderly men. J. Endocrinol. Investig. 1985, 8, 87–91. [Google Scholar]
- Centola, G.M.; Eberly, S. Seasonal variations and age-related changes in human sperm count, motility, motion parameters, morphology, and white blood cell concentration. Fertil. Steril. 1999, 72, 803–808. [Google Scholar] [CrossRef]
- Haidl, G.; Jung, A.; Schill, W.B. Ageing and sperm function. Hum. Reprod. 1996, 11, 558–560. [Google Scholar] [CrossRef][Green Version]
- Schwartz, D.; Mayaux, M.J.; Spira, A.; Moscato, M.L.; Jouannet, P.; Czyglik, F.; David, G. Semen characteristics as a function of age in 833 fertile men. Fertil. Steril. 1983, 39, 530–535. [Google Scholar] [CrossRef]
- Rolf, C.; Behre, H.M.; Nieschlag, E. Reproductive parameters of older compared to younger men of infertile couples. Int. J. Androl. 1996, 19, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chan, S.Y.; Leung, A.; Ng, R.P.; Ng, M.; Tang, L.C.; Ma, L.C.; Tsoi, W.L.; Kwan, M. Cross-sectional study of semen parameters in a large group of normal Chinese men. Int. J. Androl. 1985, 8, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.; Cawood, E.; Richardson, D.; MacDonald, E.; Aitken, J. Evidence of deteriorating semen quality in the United Kingdom: Birth cohort study in 577 men in Scotland over 11 years. BMJ 1996, 312, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Muller, C.H.; Berger, R.E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 2003, 80, 1420–1430. [Google Scholar] [CrossRef]
- Plastira, K.; Msaouel, P.; Angelopoulou, R.; Zanioti, K.; Plastiras, A.; Pothos, A.; Bolaris, S.; Paparisteidis, N.; Mantas, D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J. Assist. Reprod. Genet. 2007, 24, 437–443. [Google Scholar] [CrossRef]
- Vagnini, L.; Baruffi, R.L.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Pontes, A.; Oliveira, J.B.A.; Franco Jr, J.G. The effects of male age on sperm DNA damage in an infertile population. Reprod. Biomed. Online 2007, 15, 514–519. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef]
- Rolf, C.; Kenkel, S.; Nieschlag, E. Age-related disease pattern in infertile men: Increasing incidence of infections in older patients. Andrologia 2002, 34, 209–217. [Google Scholar] [CrossRef]
- Hassan, M.A.; Killick, S.R. Effect of male age on fertility: Evidence for the decline in male fertility with increasing age. Fertil. Steril. 2003, 79, 1520–1527. [Google Scholar] [CrossRef]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Sharma, R.; Pagani, R.; Lucon, A.M.; Srougi, M.; Hallak, J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008, 71, 490–494. [Google Scholar] [CrossRef]
- Bray, I.; Gunnell, D.; Davey Smith, G. Advanced paternal age: How old is too old? J. Epidemiol. Comm. H. 2006, 60, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.S.; Cruz Ithier, M.A.; Rosen, T.; Ashkinadze, E. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat. Diagn. 2018, 39, 81–87. [Google Scholar] [CrossRef]
- D’Onofrio, B.M.; Rickert, M.E.; Frans, E.; Kuja-Halkola, R.; Almqvist, C.; Sjolander, A.; Larsson, H.; Lichtenstein, P. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry 2014, 71, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, 12, CD007411. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.E.; Eskenazi, B.; Marchetti, F.; Young, S.; Weldon, R.H.; Baumgartner, A.; Anderson, D.; Wyrobek, A.J. Micronutrients intake is associated with improved sperm DNA quality in older men micronutrient. Fertil. Steril. 2012, 5, 1130–1136. [Google Scholar] [CrossRef]
- Silver, E.W.; Eskenazi, B.; Evenson, D.P.; Block, G.; Young, S.; Wyrobek, A.J. Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J. Androl. 2005, 26, 550–556. [Google Scholar] [CrossRef]
- Lewin, A.; Lavon, H. The effect of coenzyme Q10 on sperm motility and function. Mol. Aspects Med. 1997, 18, 213–219. [Google Scholar] [CrossRef]
- Alahmar, A.T. The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2019, 46, 112–118. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, I.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef]
| Authors/Study | Antioxidant Treatment | Sample | Findings |
|---|---|---|---|
| Cochrane Review [105] | Dietary antioxidant supplementation | Subfertile men | No effect on live birth rates and clinical pregnancy. |
| Greco et al. [106] | Oral vitamin C and E | Infertile men | Reduced DNA fragmentation. |
| Schmid et al. [107] | Vitamin C and E, zinc | Aged men (>44 years) | 20% less sperm DNA damage. |
| Silver et al. [108] | Vitamin C and E, beta | Healthy men | No improvement in sperm chromatin integrity and no benefit for fertility issues. |
| Alahmar et al. [110] | CoQ10 | Men with oligoasthenoteratozoospermia (OAT) | Increase in sperm concentration and motility, antioxidant capacity, and SOD and CAT activity. |
| Lafuente et al. [111] | CoQ10 | Infertile men | Improvement in sperm parameters. No change in pregnancy rates. No data on live births. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Powanda, P.; Robaire, B. Oxidative Stress and Reproductive Function in the Aging Male. Biology 2020, 9, 282. https://doi.org/10.3390/biology9090282
Nguyen-Powanda P, Robaire B. Oxidative Stress and Reproductive Function in the Aging Male. Biology. 2020; 9(9):282. https://doi.org/10.3390/biology9090282
Chicago/Turabian StyleNguyen-Powanda, Paulina, and Bernard Robaire. 2020. "Oxidative Stress and Reproductive Function in the Aging Male" Biology 9, no. 9: 282. https://doi.org/10.3390/biology9090282
APA StyleNguyen-Powanda, P., & Robaire, B. (2020). Oxidative Stress and Reproductive Function in the Aging Male. Biology, 9(9), 282. https://doi.org/10.3390/biology9090282





