Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice
Simple Summary
Abstract
1. Introduction
2. Results
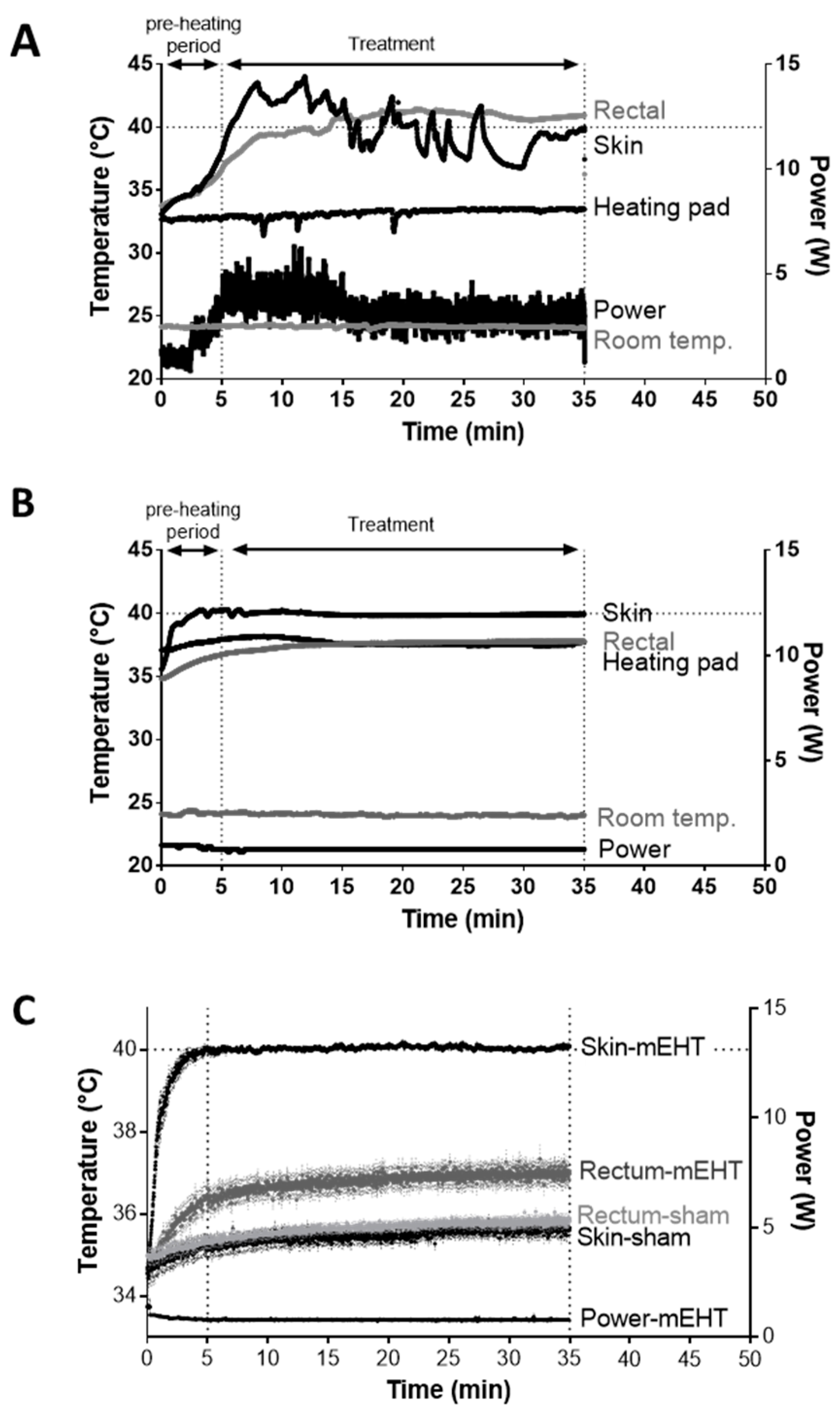
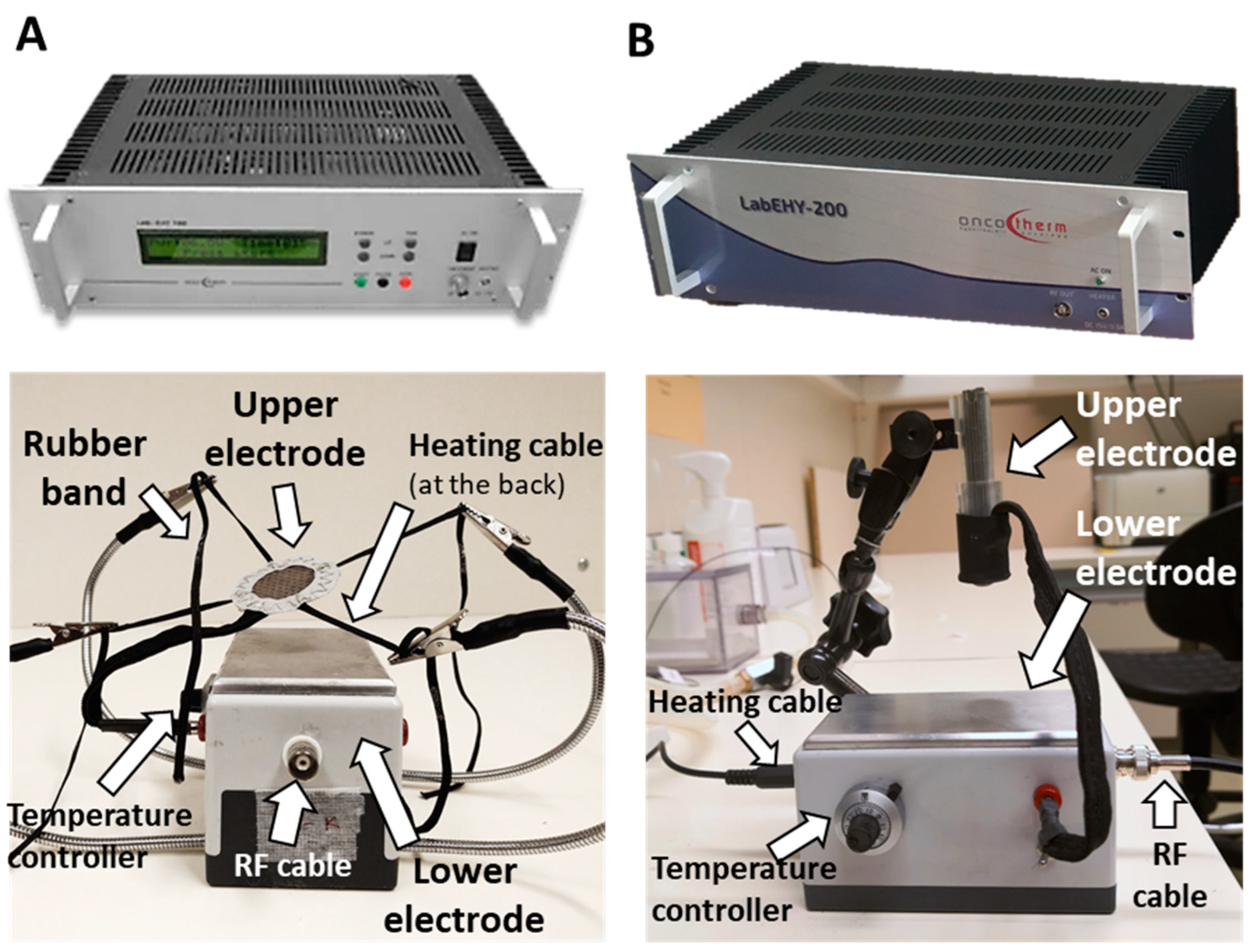
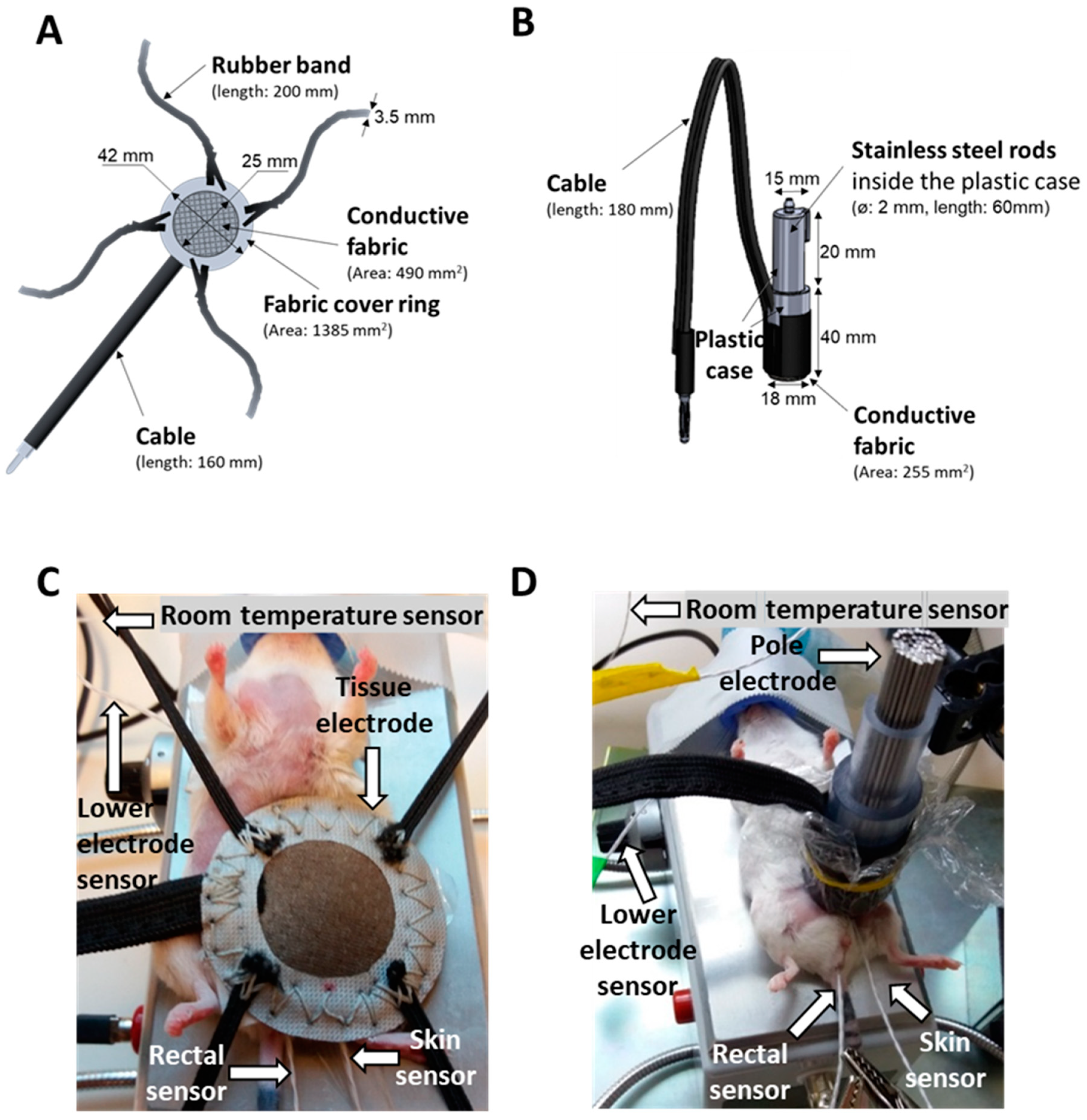
2.1. New LabEHY-200 with Pole Electrode Significantly Improved Treatment Feasibility and Accuracy
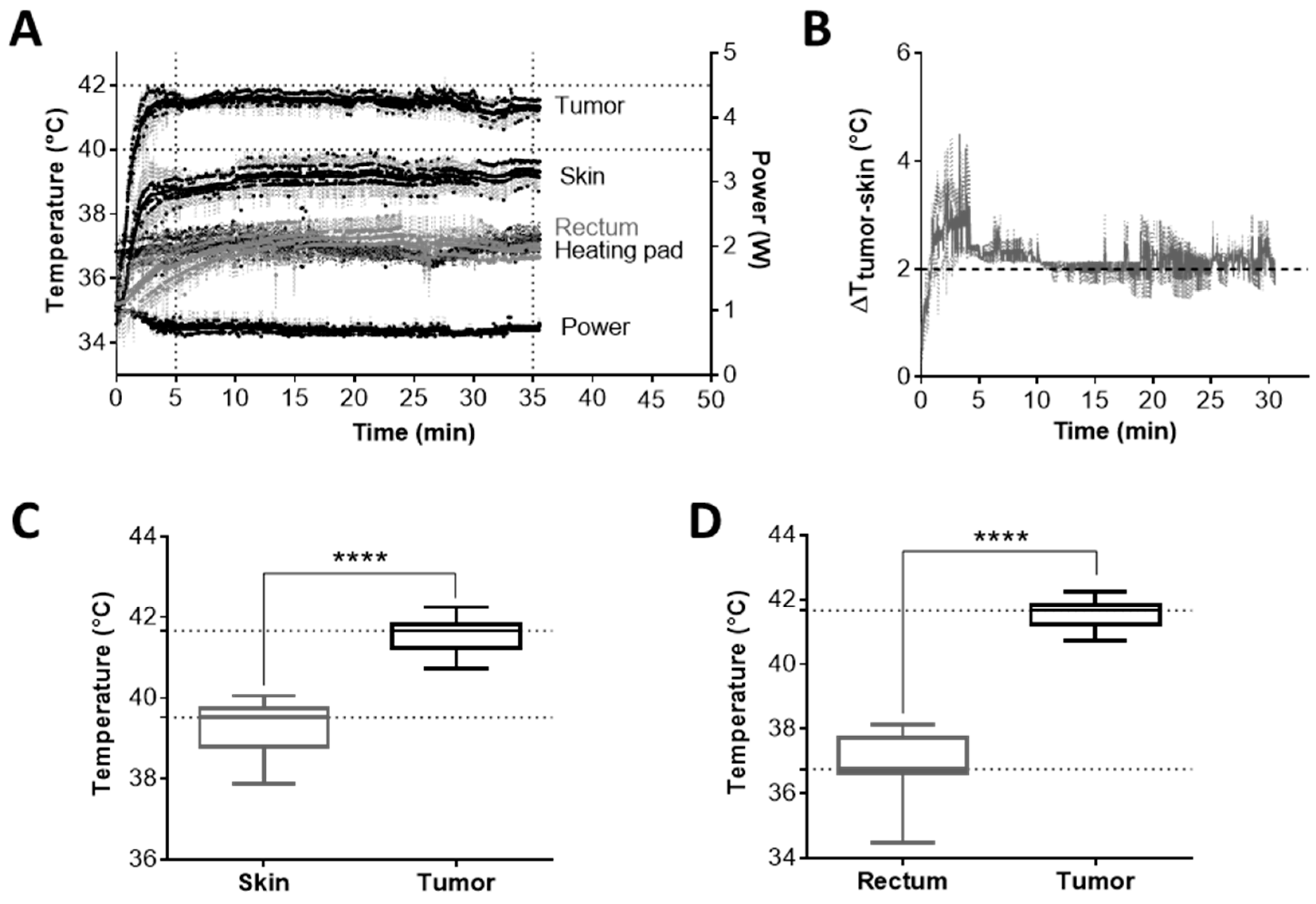
2.2. mEHT Induced Local Temperature Increase in the Tumor
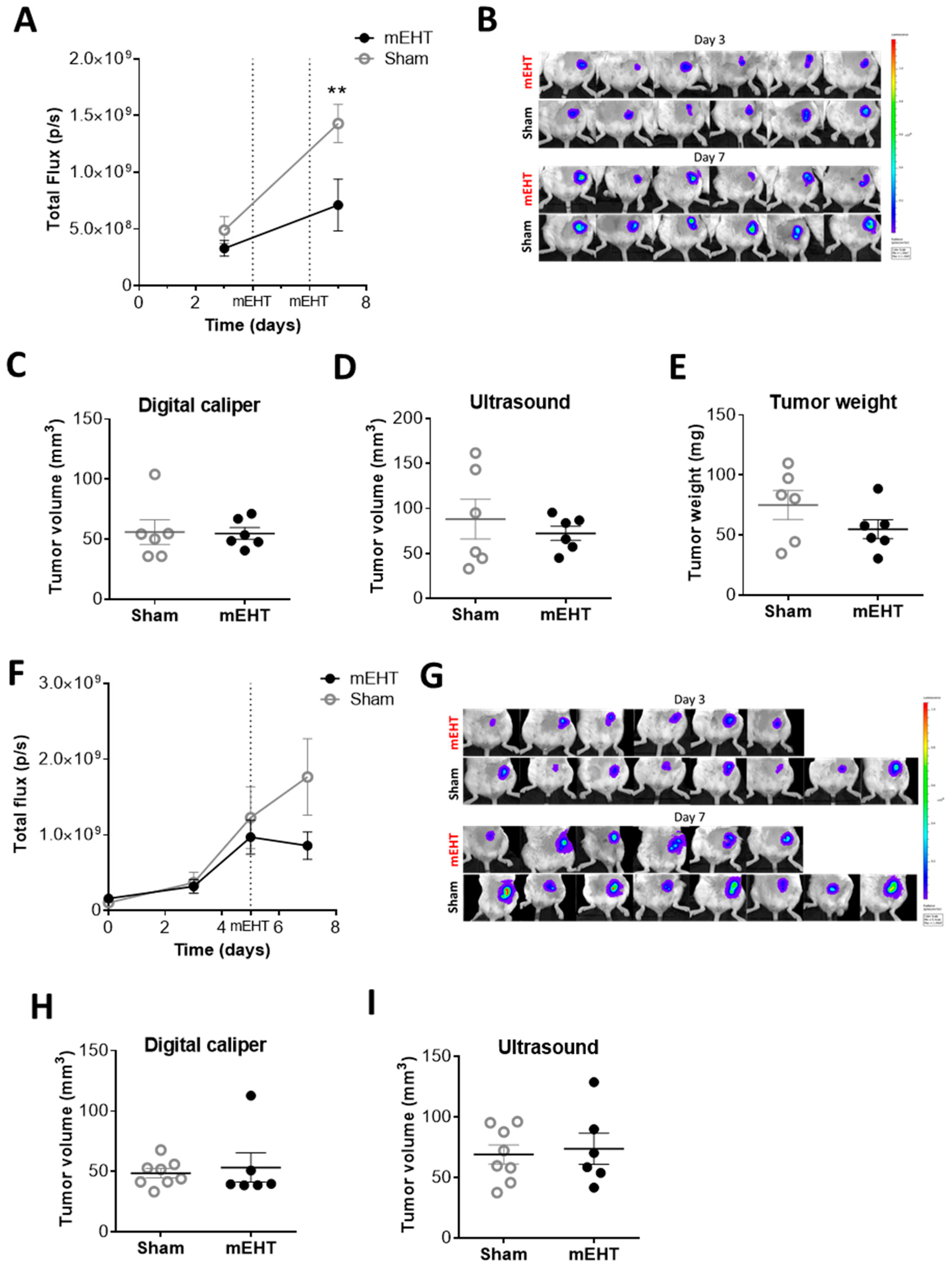
2.3. mEHT Decreased the Number of Viable Tumor Cells as Detected by In Vivo Fluorescence but did not Influence Tumor Size after Only Two Treatments
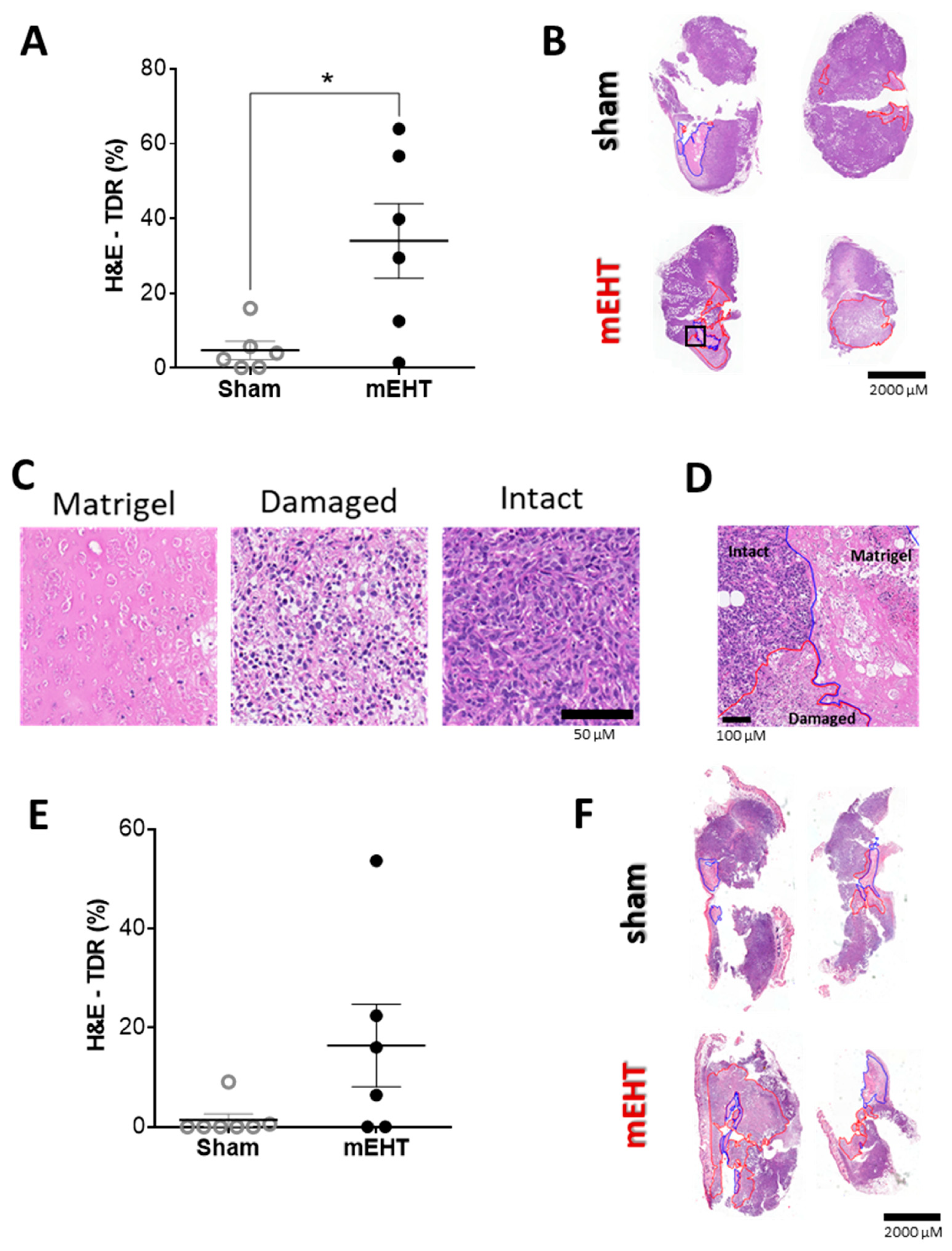
2.4. mEHT Treatment Induced Tumor Destruction
2.5. Repeated mEHT Induced Hypocellularity of Treated Tumors
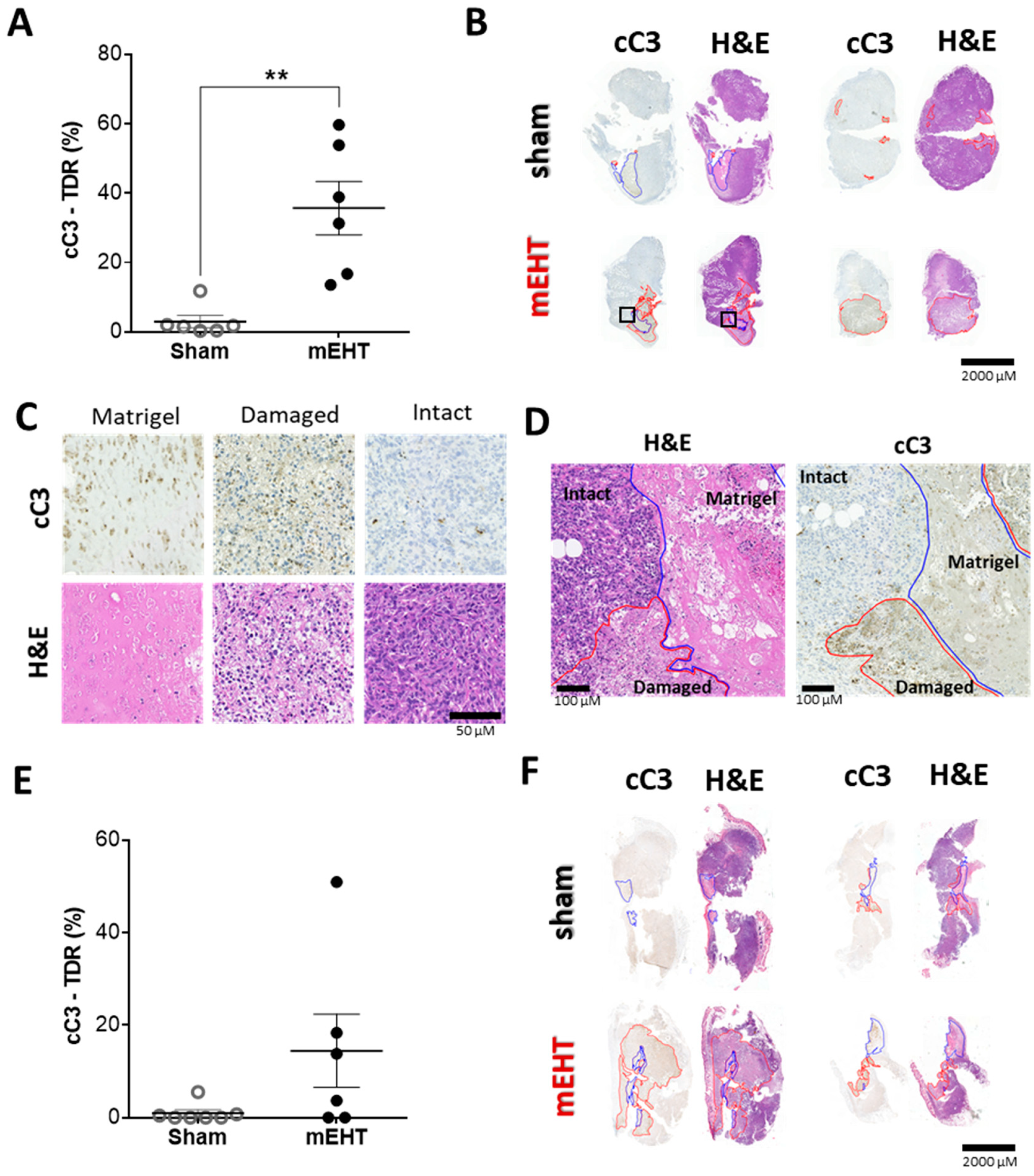
2.6. mEHT-Treatment Induced Caspase-Dependent Apoptosis
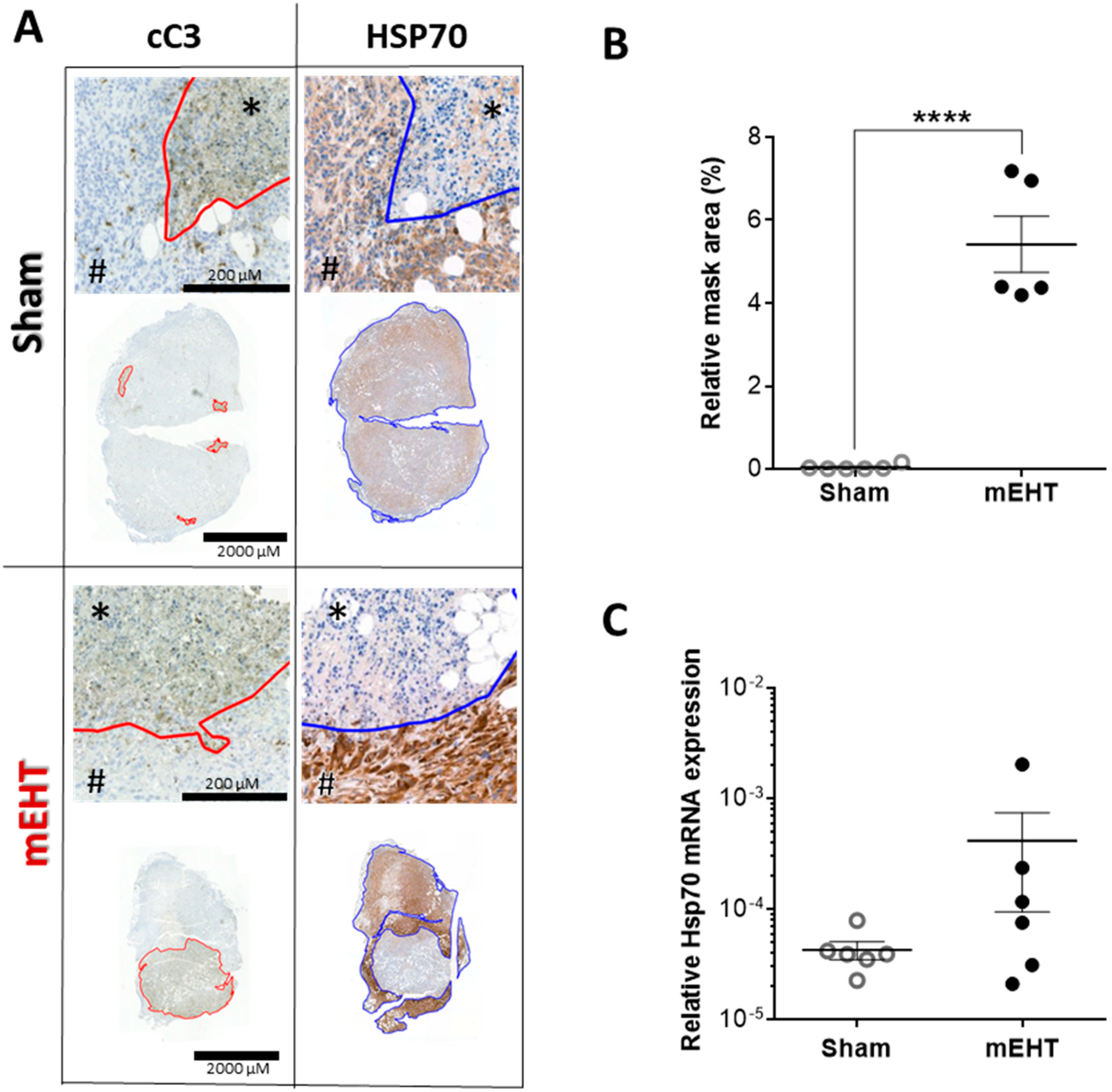
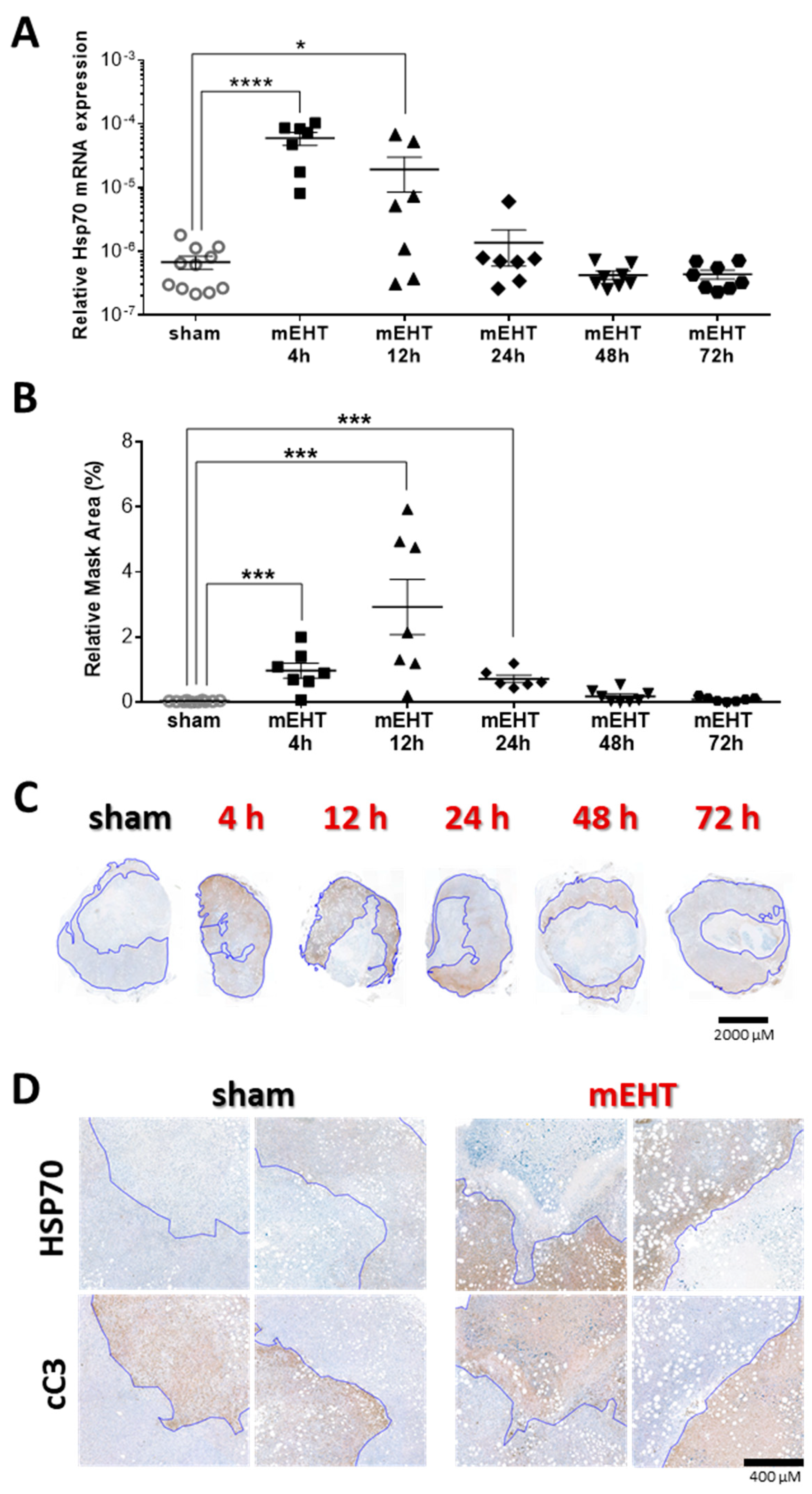
2.7. Repeated mEHT Induced Heat Shock Protein 70 (Hsp70) in Viable Tumor Cells
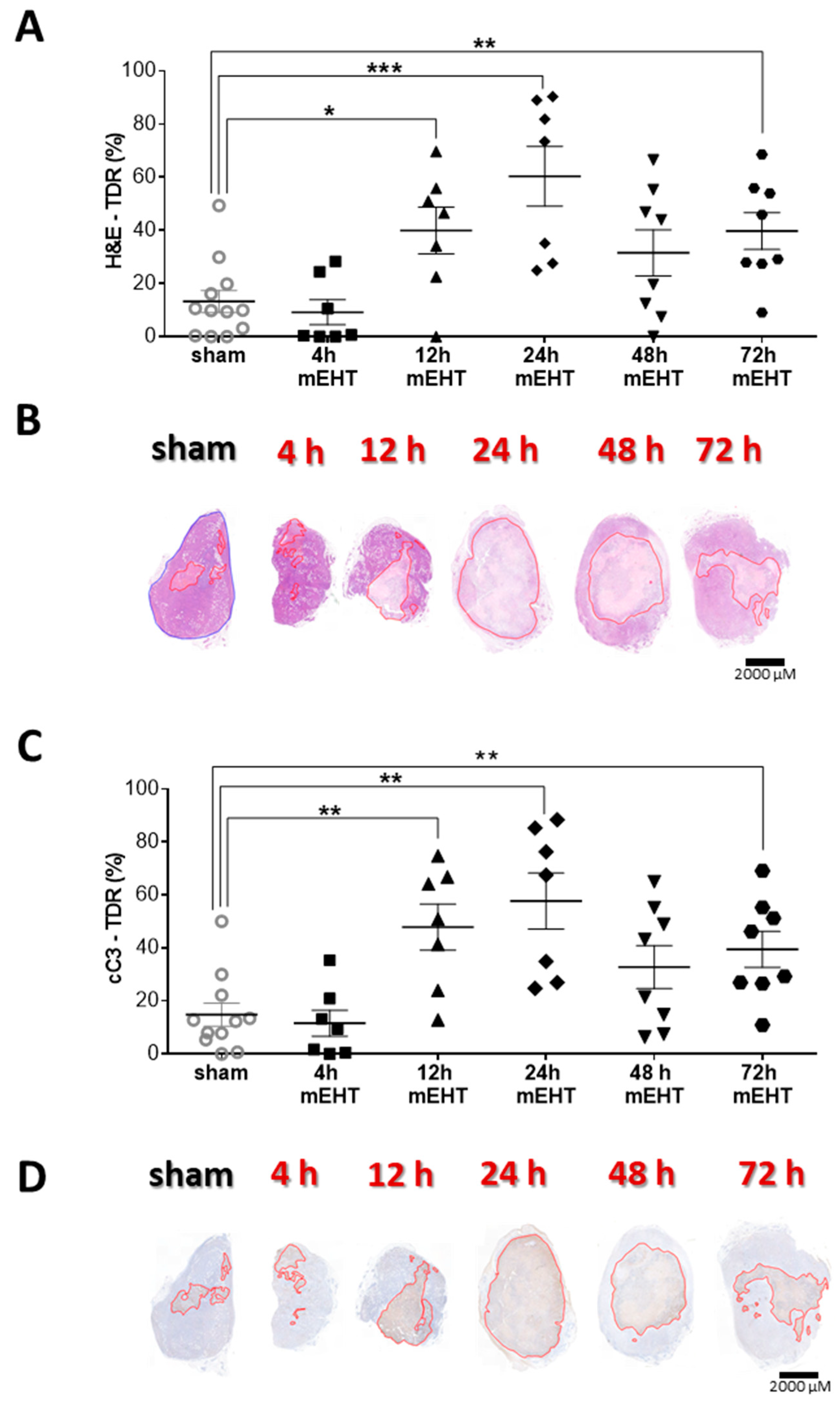
2.8. Tumor-Destructive Effect of mEHT Was Time-Dependent
2.9. Tumor-Cell-Killing Effect of mEHT Was Enhanced Synergistically by Combination with Heat Shock Inhibitors
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. In Vivo Model
4.3. Tumor Size Measurement
4.4. In Vivo Treatments
4.5. In Vitro Treatments
4.6. Histopathology and Immunohistochemistry
4.7. RNA Isolation and mRNA RT-PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | Triple-negative breast cancer |
| HER2 | Human epidermal growth factor receptor 2 |
| mEHT | Modulated electro-hyperthermia |
| Hsp70 | Heat shock protein 70 |
| DAMP | Damage-associated molecular pattern |
| MMTV+ | Mouse mammary tumor virus positive |
| cC3 | Cleaved caspase-3 |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| IVIS | In vivo imaging system |
| RF | Radio frequency |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
| TDR | Tissue damage ratio |
References
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Den Brok, W.D.; Speers, C.H.; Gondara, L.; Baxter, E.; Tyldesley, S.K.; Lohrisch, C.A. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res. Treat. 2017, 161, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Vancsik, T.; Kovago, C.; Kiss, E.; Papp, E.; Forika, G.; Benyo, Z.; Meggyeshazi, N.; Krenacs, T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J. Cancer 2018, 9, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Meggyeshazi, N.; Andocs, G.; Balogh, L.; Balla, P.; Kiszner, G.; Teleki, I.; Jeney, A.; Krenacs, T. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther. Onkol. 2014, 190, 815–822. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Bio. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Jeon, T.-W.; Yang, H.; Lee, C.G.; Oh, S.T.; Seo, D.; Baik, I.H.; Lee, E.H.; Yun, I.; Park, K.R.; Lee, Y.-H. Electro-hyperthermia up-regulates tumour suppressor Septin 4 to induce apoptotic cell death in hepatocellular carcinoma. Int. J. Hyperthermia 2016, 32, 648–656. [Google Scholar] [CrossRef]
- Cha, J.; Jeon, T.-W.; Lee, C.G.; Oh, S.T.; Yang, H.-B.; Choi, K.-J.; Seo, D.; Yun, I.; Baik, I.H.; Park, K.R.; et al. Electro-hyperthermia inhibits glioma tumorigenicity through the induction of E2F1-mediated apoptosis. Int. J. Hyperthermia 2015, 31, 784–792. [Google Scholar] [CrossRef]
- Andocs, G.; Meggyeshazi, N.; Balogh, L.; Spisak, S.; Maros, M.E.; Balla, P.; Kiszner, G.; Teleki, I.; Kovago, C.; Krenacs, T. Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell Stress Chaperones 2015, 20, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Besztercei, B.; Vancsik, T.; Benedek, A.; Major, E.; Thomas, M.J.; Schvarcz, C.A.; Krenacs, T.; Benyo, Z.; Balogh, A. Stress-Induced, p53-Mediated Tumor Growth Inhibition of Melanoma by Modulated Electrohyperthermia in Mouse Models without Major Immunogenic Effects. Int. J. Mol. Sci. 2019, 20, 4019. [Google Scholar] [CrossRef] [PubMed]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 Breast Tumor Model. In Cur. Protoc. Immunol. 2001, 20, 20.2. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef]
- Le, M.T.; Hamar, P.; Guo, C.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Invest. 2014, 124, 5109–5128. [Google Scholar] [CrossRef]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, W.C.; JH, S.P.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1beta-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- De Billy, E.; Powers, M.V.; Smith, J.R.; Workman, P. Drugging the heat shock factor 1 pathway: Exploitation of the critical cancer cell dependence on the guardian of the proteome. Cell Cycle 2009, 8, 3806–3808. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hirayoshi, K.; Kudo, H.; Takechi, H.; Aoike, A.; Kawai, K.; Nagata, K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol. Cell Biol. 1992, 12, 3490–3498. [Google Scholar] [CrossRef]
- Zheng, X.; Krakowiak, J.; Patel, N.; Beyzavi, A.; Ezike, J.; Khalil, A.S.; Pincus, D. Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. Elife 2016, 5, e18638. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, J.A.; Shin, K.D.; Shin, D.S.; Han, Y.M.; Lee, Y.J.; Lee, J.S.; Kwon, B.M.; Han, D.C. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J. Biol. Chem. 2011, 286, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Lonial, S.; Boise, L.H. When Cancer Fights Back: Multiple Myeloma, Proteasome Inhibition, and the Heat-Shock Response. Mol. Cancer Res. 2015, 13, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Akutsu, Y.; Andocs, G.; Suganami, A.; Hu, X.; Yusup, G.; Komatsu-Akimoto, A.; Hoshino, I.; Hanari, N.; Mori, M.; et al. Modulated electro-hyperthermia enhances dendritic cell therapy through an abscopal effect in mice. Oncol. Rep. 2014, 32, 2373–2379. [Google Scholar] [CrossRef]
- Yang, W.; Han, G.H.; Shin, H.-Y.; Lee, E.-J.; Cho, H.; Chay, D.B.; Kim, J.-H. Combined treatment with modulated electro-hyperthermia and an autophagy inhibitor effectively inhibit ovarian and cervical cancer growth. Int. J. Hyperthermia 2019, 36, 9–20. [Google Scholar] [CrossRef]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther. Onkol. 2009, 185, 120–126. [Google Scholar] [CrossRef]
- Hiraoka, M.; Jo, S.; Akuta, K.; Nishimura, Y.; Takahashi, M.; Abe, M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer 1987, 60, 121–127. [Google Scholar] [CrossRef]
- Roussakow, S. The History of Hyperthermia Rise and Decline. Conf. Pap. Sci. 2013, 22, 1–40. [Google Scholar] [CrossRef]
- Wust, P.; Kortum, B.; Strauss, U.; Nadobny, J.; Zschaeck, S.; Beck, M.; Stein, U.; Ghadjar, P. Non-thermal effects of radiofrequency electromagnetic fields. Sci. Rep. 2020, 10, 13488. [Google Scholar] [CrossRef]
- Wust, P.; Ghadjar, P.; Nadobny, J.; Beck, M.; Kaul, D.; Winter, L.; Zschaeck, S. Physical analysis of temperature-dependent effects of amplitude-modulated electromagnetic hyperthermia. Int. J. Hyperthermia 2019, 36, 1246–1254. [Google Scholar] [CrossRef]
- Szasz, A. Challenges and Solutions in Oncological Hyperthermia. Therm. Med. 2013, 29, 1–23. [Google Scholar] [CrossRef][Green Version]
- Andocs, G.; Rehman, M.U.; Zhao, Q.L.; Tabuchi, Y.; Kanamori, M.; Kondo, T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discov. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Kok, H.P.; Schooneveldt, G.; Bakker, A.; de Kroon-Oldenhof, R.; Korshuize-van Straten, L.; de Jong, C.E.; Steggerda-Carvalho, E.; Geijsen, E.D.; Stalpers, L.J.A.; Crezee, J. Predictive value of simulated SAR and temperature for changes in measured temperature after phase-amplitude steering during locoregional hyperthermia treatments. Int. J. Hyperthermia 2018, 35, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Ahmed, A.; Smith, C.W.; Moore, J.L.; Kerby, I.J.; Davies, R.M. Specific absorption rate and tissue temperature in local hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1997–2002. [Google Scholar] [CrossRef]
- Papp, E.; Vancsik, T.s.; Kiss, E.; Szasz, O. Energy Absorption by the Membrane Rafts in the Modulated Electro-Hyperthermia (mEHT). Open J. Biophys. 2017, 7, 216–229. [Google Scholar] [CrossRef][Green Version]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperthermia 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.; Issels, R. Physiological consequences of hyperthermia: Heat, heat shock proteins and the immune response. Int. J. Hyperthermia 2002, 18, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, K.; Lipp, A.M.; Nimmervoll, B.; Sonnleitner, A.; Hesse, J.; Haselgruebler, T.; Balogi, Z. The complex function of hsp70 in metastatic cancer. Cancers (Basel) 2013, 6, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Nylandsted, J.; Gyrd-Hansen, M.; Fau-Danielewicz, A.; Danielewicz, A.; Fau-Fehrenbacher, N.; Fehrenbacher, N.; Fau-Lademann, U.; Lademann, U.; Fau-Høyer-Hansen, M.; Høyer-Hansen, M.; et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 2004, 200, 425–435. [Google Scholar] [CrossRef]
- Danics, L.; Schvarcz, C.A.; Kaucsár, T.; Benyó, Z.; Hamar, P.; Institute of Translational Medicine, Budapest, Hungary. Unpublished work. 2019.
- Yang, K.-L.; Huang, C.-C.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Hsia, C.-C.; Andocs, G.; Wang, H.-E.; Chi, K.-H.; Yang, K.-L.; et al. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget 2016, 7, 84082–84092. [Google Scholar] [CrossRef]
- Vancsik, T.; Forika, G.; Balogh, A.; Kiss, E.; Krenacs, T. Modulated electro-hyperthermia induced p53 driven apoptosis and cell cycle arrest additively support doxorubicin chemotherapy of colorectal cancer in vitro. Cancer Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.-W.; Huang, C.-C.; Yang, K.-L.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Andocs, G.; Szasz, A.; Li, W.-T.; Chi, K.-H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.-W.; Chi, K.-H.; Huang, C.-C.; Chi, M.-S.; Chiang, H.-C.; Yang, K.-L.; Li, W.-T.; Wang, Y.-S. Modulated electro-hyperthermia-enhanced liposomal drug uptake by cancer cells. Int. J. Nanomed. 2019, 14, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Hammarsten, P.; Josefsson, A.; Thysell, E.; Lundholm, M.; Hägglöf, C.; Iglesias-Gato, D.; Flores-Morales, A.; Stattin, P.; Egevad, L.; Granfors, T.; et al. Immunoreactivity for prostate specific antigen and Ki67 differentiates subgroups of prostate cancer related to outcome. Mod. Pathol. 2019. [Google Scholar] [CrossRef]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef]
- Melling, N.; Kowitz, C.M.; Simon, R.; Bokemeyer, C.; Terracciano, L.; Sauter, G.; Izbicki, J.R.; Marx, A.H. High Ki67 expression is an independent good prognostic marker in colorectal cancer. J. Clin. Pathol. 2016, 69, 209–214. [Google Scholar] [CrossRef]
- Gabai, V.L.; Yaglom, J.A.; Wang, Y.; Meng, L.; Shao, H.; Kim, G.; Colvin, T.; Gestwicki, J.; Sherman, M.Y. Anticancer Effects of Targeting Hsp70 in Tumor Stromal Cells. Cancer Res. 2016, 76, 5926–5932. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Alfieri, A.A.; Young, C.W. Quercetin, an inhibitor of lactate transport and a hyperthermic sensitizer of HeLa cells. Cancer Res. 1984, 44, 102–106. [Google Scholar]
- Larocca, L.M.; Ranelletti, F.O.; Maggiano, N.; Rutella, S.; La Barbera, E.O.; Rumi, C.; Serra, F.; Voso, M.T.; Piantelli, M.; Teofili, L.; et al. Differential sensitivity of leukemic and normal hematopoietic progenitors to the killing effect of hyperthermia and quercetin used in combination: Role of heat-shock protein-70. Int. J. Cancer 1997, 73, 75–83. [Google Scholar] [CrossRef]
- Piantelli, M.; Tatone, D.; Castrilli, G.; Savini, F.; Maggiano, N.; Larocca, L.M.; Ranelletti, F.O.; Natali, P.G. Quercetin and tamoxifen sensitize human melanoma cells to hyperthermia. Melanoma Res. 2001, 11, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Ara, G.; Teicher, B.A.; Stevenson, M.A.; Calderwood, S.K. Effects of the flavonoid drug quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int. J. Hyperthermia 2001, 17, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lu, Z.; Du, H.; Yang, X.; Zhai, G. Hyaluronic acid-quercetin conjugate micelles: Synthesis, characterization, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2014, 123, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Shanely, R.A.; Knab, A.M.; Austin, M.D.; Sha, W. The variable plasma quercetin response to 12-week quercetin supplementation in humans. Eur. J. Clin. Nutr. 2010, 64, 692–697. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; de Takats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Zhang, G.L.; Zhang, Y.; Cao, K.X.; Wang, X.M. Orthotopic Injection of Breast Cancer Cells into the Mice Mammary Fat Pad. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Gilboa-Geffen, A.; Hamar, P.; Le, M.T.; Wheeler, L.A.; Trifonova, R.; Petrocca, F.; Wittrup, A.; Lieberman, J. Gene Knockdown by EpCAM Aptamer-siRNA Chimeras Suppresses Epithelial Breast Cancers and Their Tumor-Initiating Cells. Mol. Cancer Ther. 2015, 14, 2279–2291. [Google Scholar] [CrossRef]



| Antigen | Type | Reference No. | Dilution | Vendor 1 |
|---|---|---|---|---|
| Cleaved caspase-3 | Rabbit, pAb | #9664 | 1:300 | Cell Signaling |
| Hsp70 | Rabbit, pAb | #4872 | 1:200 | Cell Signaling |
| Ki67 | Rabbit, pAb | #RM-9106 | 1:400 | Thermo |
| Gene Symbol | Gene Name | Primer Pairs |
|---|---|---|
| HSPA1A | heat shock protein 70 [Mus musculus] | Fwd: ATGGACAAGGCGCAGATCC Rev: CTCCGACTTGTCCCCCAT |
| GAPDH | glyceraldehyde-dehydrogenase [Mus musculus] | Fwd: CCAGAATGAGGATCCCAGAA Rev: ACCACCTGAAACATGCAACA |
| MKI67 | Ki67 [Mus musculus] | Fwd: GAAGTCAAAGAGCAAGAGGCAA Rev: TTCTGTTGGCTTGCTTCCATC |
| 18S | 18S [Mus musculus] | Fwd: CTCAACACGGGAAACCTCAC Rev: CGCTCCACCAACTAAGAACG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danics, L.; Schvarcz, C.A.; Viana, P.; Vancsik, T.; Krenács, T.; Benyó, Z.; Kaucsár, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581. https://doi.org/10.3390/cancers12092581
Danics L, Schvarcz CA, Viana P, Vancsik T, Krenács T, Benyó Z, Kaucsár T, Hamar P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers. 2020; 12(9):2581. https://doi.org/10.3390/cancers12092581
Chicago/Turabian StyleDanics, Lea, Csaba András Schvarcz, Pedro Viana, Tamás Vancsik, Tibor Krenács, Zoltán Benyó, Tamás Kaucsár, and Péter Hamar. 2020. "Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice" Cancers 12, no. 9: 2581. https://doi.org/10.3390/cancers12092581
APA StyleDanics, L., Schvarcz, C. A., Viana, P., Vancsik, T., Krenács, T., Benyó, Z., Kaucsár, T., & Hamar, P. (2020). Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers, 12(9), 2581. https://doi.org/10.3390/cancers12092581







