Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer, Part 2: A Review on Cytotoxic, Antiproliferative, and Antitumor Phytochemicals, and Future Perspective
Abstract
:1. Introduction
2. Traditional Uses of Selected Plants
3. Phytochemistry of Ethiopian Anticancer Plants
3.1. Terpenoids
| Plant Family | Class of Compounds | Cell Lines | IC50 | Pharmacology | Isolated Active Compounds | Reference |
|---|---|---|---|---|---|---|
| Ferula communis L. (Apiaceae) | Daucane Sesquiterpene | Jurkat T-cells | Ionotropism | Ferutinin | [34] | |
| Vernonia amygdalina Delile (Asteraceae) | Sesquiterpene lactones | KB | - | - | Vernodalin and Vernomygdin | [77] |
| Vernonia hymenolepis A. Rich. (Asteraceae) | Sesquiterpene Dilactone | - | - | Vernolepin | ||
| Zehneria scabra (L.F. Sond) (Cucurbitaceae) | Triterpenoid | Brine shrimp | 10 μg/mL | Sonhafouonicacid (8) | [66] | |
| Croton macrostachyus Hochst. ex Delile* (Euphorbiaceae) | Diterpenoid | HCT116 | 50 µg/mL | Caspase mediated apoptosis | methyl 2-(furan-3-yl)-6α,10β-dimethy-l4-oxo-2,4,4α,5,6,6α,10α,10β--octahydro-1H-benzo[f]isochromene-7-carboxylate) | [64] |
| Euphorbia tirucalli L. (Euphorbiaceae) | Triterpenoid | CS12 | 12.8 µg/mL | Apoptosis | Euphol (9) | [67] |
| AGS | 14.7 µg/mL | |||||
| MKN45 | 14.4 µg/mL | |||||
| Ricinus communis L. (Euphorbiaceae) | Monoterpenoid | SK-MEL-28 | 21.67 ± 4.74 µg/mL | Appoptosis | 1,8-Cineole, camphor, α-pinene, β-Caryophyllene | [70] |
| K-562 | 24.49 ± 1.61 µg/mL | |||||
| COLO 679 | 20.14 ± 2.99 µg/mL | |||||
| OAW42 | 13.52 ± 0.20 µg/mL | |||||
| HT-29 | 19.86 ± 5.94 µg/mL | |||||
| MCF-7 | 37.87 ± 3.36 µg/mL | |||||
| PBMC | 13.55 ± 0.85 µg/mL | |||||
| Jatropha curcas L. (Euphorbiaceae) | Diterpenoid | HL-60 | 8.5 µM | Jatrophalactone (11) | [72] | |
| SMMC-7721 | 20.6 µM | |||||
| A-549 | 19.7 µM | |||||
| MCF-7 | 20.1 µM | |||||
| SW480 | 19.2 µM | |||||
| HL-60 | >40 µM | Curcusecon A-J, 4-epi-curcusecon E, Curcusone E | ||||
| SMMC-7721 | >40 µM | |||||
| A-549 | >40 µM | |||||
| MCF-7 | >40 µM | |||||
| SW480 | >40 µM | |||||
| HL-60 | 2.86 µM | 3-Dehydroxy-2-epi-Caniojane (12) | ||||
| SMMC-7721 | 3.94 µM | |||||
| A-549 | 3.49 µM | |||||
| MCF-7 | 11.69 µM | |||||
| SW480 | 14.05 µM | |||||
| HL-60 | 1.63 µM | Curcusone A (13) | ||||
| SMMC-7721 | 3.10 µM | |||||
| A-549 | 3.35 µM | |||||
| MCF-7 | 2.47 µM | |||||
| SW480 | 2.10 µM | |||||
| HL-60 | 2.64 µM | Curcusone B (14) | ||||
| SMMC-7721 | 3.30 µM | |||||
| A-549 | 3.88 µM | |||||
| MCF-7 | 3.14 µM | |||||
| SW480 | 2.91 µM | |||||
| HL-60 | 1.36 µM | Curcusone C (15) | ||||
| SMMC-7721 | 2.17 µM | |||||
| A-549 | 3.88 µM | |||||
| MCF-7 | 1.61 µM | |||||
| SW480 | 1.99 µM | |||||
| HL-60 | 2.81 µM | Curcusone D (16) | ||||
| SMMC-7721 | 3.58 µM | |||||
| A-549 | 4.70 µM | |||||
| MCF-7 | 2.77 µM | |||||
| SW480 | 2.83 µM | |||||
| HL-60 | 22.80 µM | Jatrogrosidone | ||||
| SMMC-7721 | 19.49 µM | |||||
| A-549 | 34.93 µM | |||||
| MCF-7 | 21.83 µM | |||||
| SW480 | 20.06 µM | |||||
| HL-60 | 23.30 µM | 2-epi-Jatrogrossidone | ||||
| SMMC-7721 | 18.36 µM | |||||
| A-549 | 36.53 µM | |||||
| MCF-7 | 22.72 µM | |||||
| SW480 | 21.08 µM | |||||
| HEPG2 | 0.084 µM | Curcusone C (15) | [73] | |||
| 0.153 µM | Curcusone D (16) | |||||
| 0.183 µM | 4E-Jatrogrossidentadion (17) | |||||
| Premna schimperi Engl.* (Verbenaceae) | Clerodane diterpene | L929 | 11 ± 2.3 µg/mL | - | (5R,8R,9S, I OR)-12-Oxo-ent-3,13(16)-clerodjen-15-oic acid | [57] |
| RAW264.7 | 10 ± 2.3 µg/mL | |||||
| SK.N.SH | 1.5 ± 0.3 µg/mL | |||||
| Ekebergia capensis Sparrm. (Meliaceae) | Triterpenoids | HEp2 | 1.4 µM | - | Oleanonic acid (18) | [74] |
| 4T1 | 13.3 µM | |||||
| Olea europaea subsp. Cuspidata (Wall. ex. G. Don) Cif. (Oleaceae) | Triterpenoids | HT-29 | 28.8 ± 0.9 µg/mL | Apoptosis | Maslinic acid (10) | [69] |
| Podocarpus falcatus* (Podocarpaceae) | Terpenoids-Nagilactones (diterpenoids) | HT-29 | 0.6 ± 0.4 µM | 16-Hydroxynagilactone F (1) | [63] | |
| 1.1 ± 0.5 µM | 2β,16-Dihydroxynagilactone F (2) | |||||
| 0.3 ± 0.1 µM | 2β-Hydroxynagilactone F | |||||
| >10 µM | 7β-Hydroxymacrophyllic acid | |||||
| >10 µM | Macrophyllic acid | |||||
| 0.9 ± 0.3 µM | Nagilactone D (3) | |||||
| 5.1 ± 0.8 µM | 15-Hydroxynagilactone (4) | |||||
| 0.5 ± 0.1 µM | Nagilactone I (5) | |||||
| >10 µM | Inumakiol D | |||||
| >10 µM | Ponasterone A | |||||
| Cucumis prophetarum (Cucurbitaceae) | Triterpenoids | MCF-7 | 7.2 µM | Cucurbitacin E (20) | [76] | |
| MDA MB 231 | 2.1 µM | |||||
| A2780 | 5.4 µM | |||||
| A2780 CP | 15.9 µM | |||||
| HepG2 | 3.4 µM | |||||
| HCT-116 | 3.4 µM | |||||
| MCF-7 | 16.0 µM | Cucurbitacin B (21) | ||||
| MDA MB 231 | 0.96 µM | |||||
| A2780 | 7.6 µM | |||||
| A2780 CP | 14.2 µM | |||||
| HepG2 | 1.7 µM | |||||
| HCT-116 | 1.7 µM | |||||
| MCF-7 | 47.9 µM | Hexanor-Cucurbitacin D | ||||
| MDA MB 231 | 12.0 µM | |||||
| A2780 | >100 µM | |||||
| A2780 CP | >100 µM | |||||
| HepG2 | 37.8 µM | |||||
| HCT-116 | 30.7 µM | |||||
| MCF-7 | 26.7 µM | Cucurbitacin D (22) | ||||
| MDA MB 231 | 4.0 µM | |||||
| A2780 | 21.6 µM | |||||
| A2780 CP | 6.9 µM | |||||
| HepG2 | 5.0 µM | |||||
| HCT-116 | 7.6 µM | |||||
| MCF-7 | 18.4 µM | Cucurbitacin F 25-O-acetate | ||||
| MDA MB 231 | 3.4 µM | |||||
| A2780 | 15.8 µM | |||||
| A2780 CP | 15.2 µM | |||||
| HepG2 | 10.2 µM | |||||
| HCT-116 | 11.2 µM | |||||
| MDA MB 231 | >100 µM | Dihydrocucurbitacin D | ||||
| 27.3 µM | Cucurbitacin E glucoside (23) | |||||
| 1 µM | Isocucurbitacin D (24) | |||||
| Centella asiatica | Triterpenoids | SW480 | 20 µg/mL (80% growth inhibition) | Growth inhibition and apoptosis | Asiatic Acid (19) | [75] |
| SNU668 | ||||||
| CT26 | ||||||
| Plumbago zeylanica | Triterpenoids | MDA-MB-231 | 5 µg/mL | Inhibits proliferation and migration | 3β-Hydroxylup-20(29)-ene-27,28-dioic acid (7) | [65] |
3.2. Phenolic Compounds
3.3. Alkaloids
3.4. Steroids and Lignans
4. Preclinical, In Vivo, and Clinical Studies on Ethiopian Anticancer Plants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Rishton, G.M. Natural products as a robust source of new drugs and drug leads: Past successes and present day issues. Am. J. Cardiol. 2008, 101, S43–S49. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelbessa, E.; Demissew, S. Diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiop. J. Biol. Sci. 2014, 13, 37–45. [Google Scholar]
- Tuasha, N.; Petros, B.; Asfaw, Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bussa, N.F.; Belayneh, A. Traditional medicinal plants used to treat cancer, tumors and inflammatory ailments in Harari Region, Eastern Ethiopia. S. Afr. J. Bot. 2019, 122, 360–368. [Google Scholar] [CrossRef]
- Atlabachew, M.; Chandravanshi, B.S.; Redi, M. Selected secondary metabolites and antioxidant activity of khat (Catha edulis Forsk) chewing leaves extract. Int. J. Food Prop. 2014, 17, 45–64. [Google Scholar] [CrossRef] [Green Version]
- Worku, N.; Mossie, A.; Stich, A.; Daugschies, A.; Trettner, S.; Hemdan, N.Y.; Birkenmeier, G. Evaluation of the in vitro efficacy of Artemisia annua, Rumex abyssinicus, and Catha edulis Forsk extracts in cancer and Trypanosoma brucei cells. ISRN Biochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tauchen, J.; Doskocil, I.; Caffi, C.; Lulekal, E.; Marsik, P.; Havlik, J.; Van Damme, P.; Kokoska, L. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Ind. Crops Prod. 2015, 74, 671–679. [Google Scholar] [CrossRef]
- Yeshak, M.Y.; Burman, R.; Asres, K.; Göransson, U. Cyclotides from an extreme habitat: Characterization of cyclic peptides from Viola abyssinica of the Ethiopian highlands. J. Nat. Prod. 2011, 74, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Youns, M.; Krauth-Siegel, R.L.; Wink, M. Biological activities of xanthatin from Xanthium strumarium leaves. Phytother. Res. 2011, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Gebrelibanos, M.; Asres, K.; Veeresham, C. In Vitro radical scavenging activity of the leaf and bark extracts of Senna singueana (Del). Lock. Ethiop. Pharm. J. 2007, 25, 77–84. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Youan, B.-B.C. Anticancer activity and nutritional value of extracts of the seed of Glinus lotoides. J. Nutr. Sci. Vitaminol. 2010, 56, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtemariam, S. Knipholone anthrone from Kniphofia foliosa induces a rapid onset of necrotic cell death in cancer cells. Fitoterapia 2010, 81, 1013–1019. [Google Scholar] [CrossRef]
- Tuasha, N.; Hailemeskel, E.; Erko, B.; Petros, B. Comorbidity of intestinal helminthiases among malaria outpatients of Wondo Genet health centers, southern Ethiopia: Implications for integrated control. BMC Infect. Dis. 2019, 19, 659. [Google Scholar] [CrossRef] [Green Version]
- Esubalew, S.T.; Belete, A.; Lulekal, E.; Gabriel, T.; Engidawork, E.; Asres, K. Review of ethnobotanical and ethnopharmacological evidences of some ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiop. J. Health Dev. 2017, 31, 161–187. [Google Scholar]
- Friis, I.; Demissew, S.; Van Breugel, P. Atlas of the potential vegetation of Ethiopia. In Det Kongelige Danske Videnskabernes Selskab; Nabu Press: Charleston, SC, USA, 2010. [Google Scholar]
- Makonnen, E.; Hagos, E. Antispasmodic effect of Bersama abyssinica aqueous extract on guinea-pig ileum. Phytother. Res. 1993, 7, 211–212. [Google Scholar] [CrossRef]
- Birhanu, Z. Traditional use of medicinal plants by the ethnic groups of Gondar Zuria District, North-Western Ethiopia. J. Nat. Rem. 2013, 13, 46–53. [Google Scholar]
- Tesfaye, S.; Belete, A.; Engidawork, E.; Gedif, T.; Asres, K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts, Ethiopia. Evid-Based Compl. Alt. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Agize, M.; Demissew, S.; Asfaw, Z. Ethnobotany of medicinal plants in Loma and Gena bosa districts (woredas) of dawro zone, southern Ethiopia. Topcls. J. Herb. Med. 2013, 2, 194–212. [Google Scholar]
- Giday, M.; Asfaw, Z.; Woldu, Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J. Ethnopharmacol. 2010, 132, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kidane, B.; van Andel, T.; van der Maesen, L.J.G.; Asfaw, Z. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giday, M.; Teklehaymanot, T.; Animut, A.; Mekonnen, Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J. Ethnopharmacol. 2007, 110, 516–525. [Google Scholar] [CrossRef]
- Abera, B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Abebe, D.; Debella, A.; Urga, K. Medicinal plants and other useful plants of Ethiopia. Ethiop. Health Nutr. Res. Inst. 2003, 156, 194–212. [Google Scholar]
- Regassa, R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J. Med. Plants Res. 2013, 7, 517–535. [Google Scholar]
- Kewessa, G.; Abebe, T.; Demessie, A. Indigenous knowledge on the use and management of medicinal trees and shrubs in Dale District, Sidama Zone, Southern Ethiopia. Ethnobot. Res. Appl. 2015, 14, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Abebe, W. A survey of prescriptions used in traditional medicine in Gondar region, northwestern Ethiopia: General pharmaceutical practice. J. Ethnopharmacol. 1986, 18, 147–165. [Google Scholar] [CrossRef]
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Birhanu, Z.; Endale, A.; Shewamene, Z. An ethnomedicinal investigation of plants used by traditional healers of Gondar town, North-Western Ethiopia. J. Med. Plants Stud. 2015, 3, 36–43. [Google Scholar]
- Chekole, G.; Asfaw, Z.; Kelbessa, E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J. Ethnobiol. Ethnomed. 2015, 11, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teklehaymanot, T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J. Ethnopharmacol. 2009, 124, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yineger, H.; Yewhalaw, D. Traditional medicinal plant knowledge and use by local healers in Sekoru District, Jimma Zone, Southwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kebede, A.; Ayalew, S.; Mesfin, A.; Mulualem, G. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Dire Dawa city, eastern Ethiopia. J. Med. Plants Stud. 2016, 4, 170–178. [Google Scholar]
- Ayele, T.T. A Review on traditionally used medicinal plants/herbs for cancer therapy in Ethiopia: Current status, challenge and future perspectives. Org. Chem. Curr. Res. 2018, 7. [Google Scholar] [CrossRef]
- Tolossa, K.; Debela, E.; Athanasiadou, S.; Tolera, A.; Ganga, G.; Houdijk, J.G. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2013, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bekele, G.; Reddy, P.R. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo tribes in Abaya District, Borana, Oromia, Ethiopia. Univers. J. Plant Sci. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Abera, B. Medicinal plants used in traditional medicine in Jimma Zone, Southwest Ethiopia. Ethiop. J. Health Sci. 2003, 13, 85–94. [Google Scholar]
- Wabe, N.; Mohammed, M.A.; Raju, N.J. An ethnobotanical survey of medicinal plants in the Southeast Ethiopia used in traditional medicine. Spatula DD 2011, 1, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Zenebe, G.; Zerihun, M.; Solomon, Z. An ethnobotanical study of medicinal plants in Asgede Tsimbila district, Northwestern Tigray, northern Ethiopia. Ethnobot. Res. Appl. 2012, 10, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Teklay, A.; Abera, B.; Giday, M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J. Ethnobiol. Ethnomed. 2013, 9, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.H. Medicinal plants in folk medicine system of Ethiopia. J. Poisonous Med. Plants Res. 2013, 1, 7–11. [Google Scholar]
- Yohannis, S.W.; Asfaw, Z.; Kelbessa, E. Ethnobotanical study of medicinal plants used by local people in menz gera midir district, north shewa zone, amhara regional state, Ethiopia. J. Med. Plants Res. 2018, 12, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Birhanu, A.; Ayalew, S. Indigenous knowledge on medicinal plants used in and around Robe Town, Bale Zone, Oromia Region, Southeast Ethiopia. J. Med. Plants Res. 2018, 12, 194–202. [Google Scholar] [CrossRef]
- Gebeyehu, G.; Asfaw, Z.; Enyew, A. An ethnobotanical study of traditional use of medicinal plants and their conservation status in Mecha Wereda, west Gojjam zone of Amhara region, Ethiopia. Int. J. Pharm. Health Care Res. 2014, 2, 137–154. [Google Scholar]
- Yineger, H.; Yewhalaw, D.; Teketay, D. Ethnomedicinal plant knowledge and practice of the Oromo ethnic group in southwestern Ethiopia. J. Ethnobiol. Ethnomed. 2008, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Demissew, S. A description of some essential oil bearing plants in Ethiopia and their indigenous uses. J. Essent. Oil Res. 1993, 5, 465–479. [Google Scholar] [CrossRef]
- Agisho, H.; Osie, M.; Lambore, T. Traditional medicinal plants utilization, management and threats in Hadiya zone, Ethiopia. J. Med. Plant 2014, 2, 94–108. [Google Scholar]
- Giday, M.; Asfaw, Z.; Woldu, Z. Medicinal plants of the Meinit ethnic group of Ethiopia: An ethnobotanical study. J. Ethnopharmacol. 2009, 124, 513–521. [Google Scholar] [CrossRef]
- Birhanu, T.; Abera, D.; Ejeta, E.; Nekemte, E. Ethnobotanical study of medicinal plants in selected Horro Gudurru Woredas, western Ethiopia. J. Biol. Agric. Healthc. 2015, 5, 83–93. [Google Scholar]
- Gidey, M.; Beyene, T.; Signorini, M.A.; Bruschi, P.; Yirga, G. Traditional medicinal plants used by Kunama ethnic group in Northern Ethiopia. J. Med. Plants Res. 2015, 9, 494–509. [Google Scholar]
- Giday, M.; Asfaw, Z.; Elmqvist, T.; Woldu, Z. An ethnobotanical study of medicinal plants used by the Zay people in Ethiopia. J. Med. Plants Res. 2015, 9, 494–509. [Google Scholar] [CrossRef]
- Ragunathan, M.; Abay, S.M. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, northwestern Ethiopia. Indian J. Tradit. Knowl. 2009, 8, 281–284. [Google Scholar]
- Mekuanent, T.; Zebene, A.; Solomon, Z. Ethnobotanical study of medicinal plants in Chilga district, northwestern Ethiopia. J. Nat. Remedies 2015, 15, 88–112. [Google Scholar] [CrossRef] [Green Version]
- Habtemariam, S. Cytotoxicity of diterpenes from Premna schimperi and Premna oligotricha. Planta Med. 1995, 61, 368–369. [Google Scholar] [CrossRef]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2010, 27, 79–132. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Addo, E.M.; Chai, H.-B.; Hymete, A.; Yeshak, M.Y.; Slebodnick, C.; Kingston, D.G.; Rakotondraibe, L.H. Antiproliferative constituents of the roots of Ethiopian Podocarpus falcatus and structure revision of 2α-hydroxynagilactone F and nagilactone I. J. Nat. Prod. 2015, 78, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Tesso, H.; Terfa, A.; Dekebo, A.; Dinku, W.; Lee, Y.H.; Shin, S.Y.; Lim, Y. Biological evaluation of the diterpenes from Croton macrostachyus. Appl. Biol. Chem. 2017, 60, 615–621. [Google Scholar] [CrossRef]
- Sathya, S.; Sudhagar, S.; Vidhya Priya, M.; Bharathi Raja, R.; Muthusamy, V.S.; Niranjali Devaraj, S.; Lakshmi, B.S. 3β-Hydroxylup-20(29)-ene-27,28-dioic acid dimethyl ester, a novel natural product from Plumbago zeylanica inhibits the proliferation and migration of MDA-MB-231 cells. Chem. Biol. Interact. 2010, 188, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kongue, M.D.; Talontsi, F.M.; Lamshöft, M.; Kenla, T.J.; Dittrich, B.; Kapche, G.D.; Spiteller, M. Sonhafouonic acid, a new cytotoxic and antifungal hopene-triterpenoid from Zehneria scabra camerunensis. Fitoterapia 2013, 85, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-W.; Lin, A.-S.; Wu, D.-C.; Wang, S.S.; Chang, F.-R.; Wu, Y.-C.; Huang, Y.-B. Euphol from Euphorbia tirucalli selectively inhibits human gastric cancer cell growth through the induction of ERK1/2-mediated apoptosis. Food Chem. Toxicol. 2012, 50, 4333–4339. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Tansini, A.; Oliveira, R.J.; Martinho, O.; Lima, J.P.; Pianowski, L.F.; Reis, R.M. In vitro screening of cytotoxic activity of euphol from Euphorbia tirucalli on a large panel of human cancer-derived cell lines. Exp. Ther. Med. 2018, 16, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef]
- Darmanin, S.; Wismayer, P.S.; Camilleri Podesta, M.T.; Micallef, M.J.; Buhagiar, J.A. An extract from Ricinus communis L. leaves possesses cytotoxic properties and induces apoptosis in SK-MEL-28 human melanoma cells. Nat. Prod. Res. 2009, 23, 561–571. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa. Families E–I.; Royal Botanic Gardens: Richmond, UK, 1994; Volume 2. [Google Scholar]
- Liu, J.-Q.; Yang, Y.-F.; Li, X.-Y.; Liu, E.-Q.; Li, Z.-R.; Zhou, L.; Li, Y.; Qiu, M.-H. Cytotoxicity of naturally occurring rhamnofolane diterpenes from Jatropha curcas. Phytochemistry 2013, 96, 265–272. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Li, F.; Zhao, Z.-G.; Liu, X.-L.; Tang, Y.-X.; Wang, M.-K. Diterpenoids from the root bark of Jatropha curcas and their cytotoxic activities. Phytochem. Lett. 2012, 5, 721–724. [Google Scholar] [CrossRef]
- Irungu, B.N.; Orwa, J.A.; Gruhonjic, A.; Fitzpatrick, P.A.; Landberg, G.; Kimani, F.; Midiwo, J.; Erdélyi, M.; Yenesew, A. Constituents of the roots and leaves of Ekebergia capensis and their potential antiplasmodial and cytotoxic activities. Molecules 2014, 19, 14235–14246. [Google Scholar] [CrossRef]
- Tang, X.-L.; Yang, X.-Y.; Jung, H.-J.; Kim, S.-Y.; Jung, S.-Y.; Choi, D.-Y.; Park, W.-C.; Park, H. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol. Pharm. Bull. 2009, 32, 1399–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsayari, A.; Kopel, L.; Ahmed, M.S.; Soliman, H.S.; Annadurai, S.; Halaweish, F.T. Isolation of anticancer constituents from Cucumis prophetarum var. prophetarum through bioassay-guided fractionation. BMC Complement. Altern. Med. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupchan, S.M.; Hemingway, R.J.; Karim, A.; Werner, D. Tumor inhibitors. XLVII. Vernodalin and vernomygdin, two new cytotoxic sesquiterpene lactones from Vernonia amygdalina Del. J. Org. Chem. 1969, 34, 3908–3911. [Google Scholar] [CrossRef] [PubMed]
- Selassie, C.D.; Kapur, S.; Verma, R.P.; Rosario, M. Cellular apoptosis and cytotoxicity of phenolic compounds: A quantitative structure- activity relationship study. J. Med. Chem. 2005, 48, 7234–7242. [Google Scholar] [CrossRef]
- Okoye, F.B.C.; Debbab, A.; Wray, V.; Esimone, C.O.; Osadebe, P.O.; Proksch, P. A phenyldilactone, bisnorsesquiterpene, and cytotoxic phenolics from Maytenus senegalensis leaves. Tetrahedron Lett. 2014, 55, 3756–3760. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Ding, Y.-Y.; Liu, F.; Li, N. Cytotoxic and anti-inflammatory activities of phenanthrenes from the medullae of Juncus effusus L. Arch. Pharm. Res. 2016, 39, 154–160. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Kosuge, Y.; Hamagami, H.; Ozaki, M.; Ishige, K.; Ito, Y.; Kitanaka, S. Chemical constituents isolated from Juncus effusus induce cytotoxicity in HT22 cells. J. Nat. Med. 2015, 69, 421–426. [Google Scholar] [CrossRef]
- Xu, T.-P.; Shen, H.; Liu, L.-X.; Shu, Y.-Q. Plumbagin from Plumbago Zeylanica L. Induces Apoptosis in Human Non-small Cell Lung Cancer Cell Lines through NF-κB Inactivation. Asian Pac. J. Cancer Prev. 2013, 14, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-A.; Chang, H.-H.; Kao, C.-Y.; Tsai, T.-H.; Chen, Y.-J. Plumbagin, isolated from Plumbago zeylanica, induces cell death through apoptosis in human pancreatic cancer cells. Pancreatology 2009, 9, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowska, J.; Chrobak, R.; Wróbel, J.T.; Czarnocki, Z. Novel bisindole derivatives of Catharanthus alkaloids with potential cytotoxic properties. In Developments in Tryptophan and Serotonin Metabolism; Springer: Berlin/Heidelberg, Germany, 2003; pp. 643–646. [Google Scholar]
- Jossang, A.; Fodor, P.; Bodo, B. A new structural class of bisindole alkaloids from the seeds of Catharanthus roseus: Vingramine and methylvingramine. J. Org. Chem. 1998, 63, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-K.; Xu, J.-K.; Tian, H.-Y.; Wang, L.; Zhang, X.-Q.; Xiao, X.-Z.; Li, P.; Ye, W.-C. Two new vinblastine-type N-oxide alkaloids from Catharanthus roseus. Nat. Prod. Res. 2013, 27, 1911–1916. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, G.-C.; Wang, Y.; Zhang, X.-Q.; Huang, X.-J.; Zhang, D.-M.; Chen, M.-F.; Ye, W.-C. Cytotoxic dimeric indole alkaloids from Catharanthus roseus. Fitoterapia 2012, 83, 765–769. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, C.-Y.; Jiang, M.-M.; Li, D.; Wen, P.; Song, X.; Chen, J.-D.; Guo, L.-X.; Hu, X.-P.; Li, G.-Q. Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine 2016, 23, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.J.; Ismail, Z.; Aisha, A.F.A.; Majid, A.M. Cytotoxic activity of Catharanthus roseus (Apocynaceae) crude extracts and pure compounds against human colorectal carcinoma cell line. Int. J. Pharmacol. 2010, 6, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Balkrishna, A.; Das, S.K.; Pokhrel, S.; Joshi, A.; Verma, S.; Sharma, V.K.; Sharma, V.; Sharma, N.; Joshi, C.S. Colchicine: Isolation, LC–MS QTof screening, and anticancer activity study of Gloriosa superba seeds. Molecules 2019, 24, 2772. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Cheng, S.; Zhang, S.; Sun, H.; Xu, Z. Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Med. 2013, 31, 113–119. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, F.; Yang, Y.; Li, M. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food Chem. 2013, 141, 1181–1186. [Google Scholar] [CrossRef]
- Maiyoa, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-oglucoside and β-amyrin from Prunus africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.I.; Hussain, S.; Yousuf, S.; Dar, A. Chlorinated and diepoxy withanolides from Withania somnifera and their cytotoxic effects against human lung cancer cell line. Phytochemistry 2010, 71, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Moniot, J.L.; Sigel, C.W.; Hemingway, R.J. Tumor inhibitors. LXV. Bersenogenin, berscillogenin, and 3-epiberscillogenin, three new cytotoxic bufadienolides from Bersama abyssinica. J. Org. Chem. 1971, 36, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Hemingway, R.J.; Hemingway, J.C. The isolation and characterization of hellebrigenin 3-acetate and hellebrigenin 3, 5-diacetate, bufadienolide tumor inhibitors from Bersama abyssinica. Tetrahedron Lett. 1968, 9, 149–152. [Google Scholar] [CrossRef]
- Abarzua, S.; Szewczyk, M.; Gailus, S.; Richter, D.-U.; Ruth, W.; Briese, V.; Piechulla, B. Effects of phytoestrogen extracts from Linum usitatissimum on the Jeg3 human trophoblast tumour cell line. Anticancer Res. 2007, 27, 2053–2058. [Google Scholar] [PubMed]
- Wangteeraprasert, R.; Lipipun, V.; Gunaratnam, M.; Neidle, S.; Gibbons, S.; Likhitwitayawuid, K. Bioactive compounds from Carissa spinarum. Phytother. Res. 2012, 26, 1496–1499. [Google Scholar] [CrossRef]
- Abarzua, S.; Serikawa, T.; Szewczyk, M.; Richter, D.-U.; Piechulla, B.; Briese, V. Antiproliferative activity of lignans against the breast carcinoma cell lines MCF 7 and BT 20. Arch. Gynecol. Obstet. 2012, 285, 1145–1151. [Google Scholar] [CrossRef]
- Ali, A.; Joshi, P.; Misra, L.; Sangwan, N.; Darokar, M. 5,6-De-epoxy-5-en-7-one-17-hydroxy withaferin A, a new cytotoxic steroid from Withania somnifera L. Dunal leaves. Nat. Prod. Res. 2014, 28, 392–398. [Google Scholar] [CrossRef]
- Aruna, S.R. In-vitro and in-vivo antitumor activity of Catharanthus roseus. Int. Res. J. Pharm. Appl. Sci. 2014, 4, 1–4. [Google Scholar]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. BioMed Res. Int. 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Valadares, M.C.; Carrucha, S.G.; Accorsi, W.; Queiroz, M.L. Euphorbia tirucalli L. modulates myelopoiesis and enhances the resistance of tumour-bearing mice. Int. Immunopharmacol. 2006, 6, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.G.; Appel, M.H.; Coutinho, D.S.; Soares, I.P.; Fischer, S.; de Oliveira, B.C.; Fachi, M.M.; Pontarolo, R.; Bonatto, S.J.; Iagher, F. Consumption of latex from Euphorbia tirucalli L. promotes a reduction of tumor growth and cachexia, and immunomodulation in Walker 256 tumor-bearing rats. J. Ethnopharmacol. 2020, 255, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Capistrano, I.R.; Vangestel, C.; Vanpachtenbeke, H.; Fransen, E.; Staelens, S.; Apers, S.; Pieters, L. Coadministration of a Gloriosa superba extract improves the in vivo antitumoural activity of gemcitabine in a murine pancreatic tumour model. Phytomedicine 2016, 23, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Drabick, J. Phase II Trial of Oral Colchicine in Men With Castrate-Resistant Prostate Cancer Who Have Failed Taxotere-Based Chemotherapy; Clinicaltrials Gov. National Library of Medicine: Bethesda, MD, USA, 2013.
- Balaji, R.; Rekha, N.; Deecaraman, M.; Manikandan, L. Antimetastatic and antiproliferative activity of methanolic fraction of Jatropha curcas against B16F10 melanoma induced lung metastasis in C57BL/6 mice. Afr. J. Pharm. Pharmacol. 2009, 3, 547–555. [Google Scholar]
- Fabian, C.J.; Khan, S.A.; Garber, J.E.; Dooley, W.C.; Yee, L.D.; Klemp, J.R.; Nydegger, J.L.; Powers, K.R.; Kreutzjans, A.L.; Zalles, C.M. Randomized phase IIB trial of the lignan secoisolariciresinol diglucoside in pre-menopausal women at increased risk for development of breast cancer. Cancer Prev. Res. 2020, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Shenouda, N.S.; Sakla, M.S.; Newton, L.G.; Besch-Williford, C.; Greenberg, N.M.; MacDonald, R.S.; Lubahn, D.B. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine 2007, 31, 72–81. [Google Scholar] [CrossRef] [Green Version]
- The University of Texas Health Science Center at San Antonio. Phase I Clinical Trial Testing the Synergism of Phytonutrients, Curcumin and Ursolic Acid, to Target Molecular Pathways in the Prostate; Clinicaltrials Gov, National Library of Medicine: Bethesda, MD, USA, 2020.
- Sand, J.M.; Hafeez, B.B.; Jamal, M.S.; Witkowsky, O.; Siebers, E.M.; Fischer, J.; Verma, A.K. Plumbagin (5-hydroxy-2-methyl-1, 4-naphthoquinone), isolated from Plumbago zeylanica, inhibits ultraviolet radiation-induced development of squamous cell carcinomas. Carcinogenesis 2012, 33, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Majumder, M.; Debnath, S.; Gajbhiye, R.L.; Saikia, R.; Gogoi, B.; Samanta, S.K.; Das, D.K.; Biswas, K.; Jaisankar, P.; Mukhopadhyay, R. Ricinus communis L. fruit extract inhibits migration/invasion, induces apoptosis in breast cancer cells and arrests tumor progression in vivo. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Q.; Feng, T.; Zhang, T.; Li, K.; Zhao, R.; Han, Z.; Gao, D. Antitumor activity of crude polysaccharides isolated from Solanum nigrum Linne on U14 cervical carcinoma bearing mice. Phytother. Res. 2007, 21, 832–840. [Google Scholar] [CrossRef]
- Hsu, J.-D.; Kao, S.-H.; Tu, C.-C.; Li, Y.-J.; Wang, C.-J. Solanum nigrum L. Extract inhibits 2-Acetylaminofluorene-induced hepatocarcinogenesis through overexpression of Glutathione S-Transferase and antioxidant enzymes. J. Agric. Food Chem. 2009, 57, 8628–8634. [Google Scholar] [CrossRef]
- Howard, C.B.; Johnson, W.K.; Pervin, S.; Izevbigie, E.B. Recent perspectives on the anticancer properties of aqueous extracts of Nigerian Vernonia amygdalina. Botanics 2015, 5, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupchan, S.M.; Hemingway, R.J.; Werner, D.; Karim, A. Tumor inhibitors. XLVI. Vernolepin, a novel sesquiterpene dilactone tumor inhibitor from Vernonia hymenolepis. J. Org. Chem. 1969, 34, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Kataria, H.; Kumar, S.; Chaudhary, H.; Kaur, G. Withania somnifera Suppresses Tumor Growth of Intracranial Allograft of Glioma Cells. Mol. Neurobiol. 2016, 53, 4143–4158. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, G.; Dinda, A.K.; Shakeel, F. Immunomodulatory effects of Withania Somnifera on Azoxymethane induced experimental colon cancer in mice. Immunol. Investig. 2010, 39, 688–698. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Nagalingam, A.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant activation of ETS-like transcription factor-1 and Death Receptor-5 via extracellular signal-regulated kinase in withaferin A-mediated inhibition of hepatocarcinogenesis in mice. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
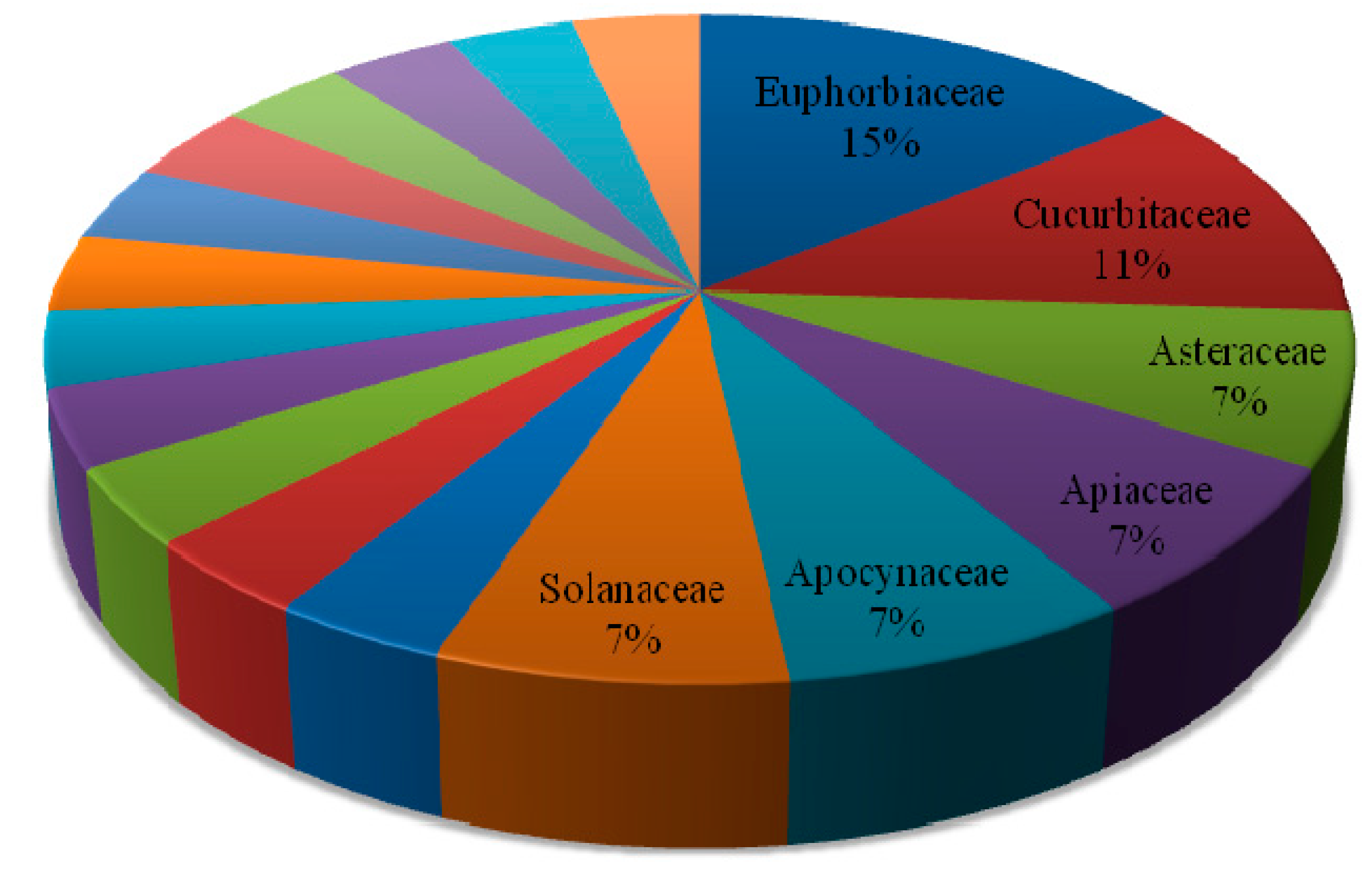



| Botanical Name (Family) | Illnesses/Symptoms Claimed to Be Treated Traditionally |
|---|---|
| Bersama abyssinica Fresen. (Melianthaceae) | Antispasmodic [19]; tumor [20] |
| Carissa spinarum (Apocynaceae) | Skin cancer [21] |
| Catharanthus roseus (L.) G. Don (Apocynaceae) | Cancer, liver infection, Wound, rheumatism [22] |
| Centella asiatica (L.) Urb. (Apiaceae) | Genital infection [23]; gastritis, evil eye, swelling [24]; Throat cancer [21] |
| Croton macrostachyus Hochst. Ex Delile (Euphorbiaceae) | Stomach ache, typhoid, worm expulsion, wounds, malaria [25]; wounds, malaria and gonorrhea [26]; tumor [27]; skin cancer, wound, ring worm [28]; cancer [29] |
| Cucumis prophetarum (Cucurbitaceae) | Skin cancer, cough, stomach-ache, diarrhoea [30]; wound, swollen body part [7] |
| Ekebergia capensis Sparrm. (Meliaceae) | Weight loss in children, stabbing pain, bovine tuberculosis [29]; cancer [6] |
| Euphorbia tirucalli L. (Euphorbiaceae) | Tumors [27]; wart, wounds [31] |
| Ferula communis L. (Apiaceae) | Gonorrhea [32]; Lung cancer [33] |
| Gloriosa superba (Colchicaceae) | Snake bite, impotence, stomach-ache [34]; tumors [35] |
| Jatropha curcas L. (Euphorbiaceae) | Abdominal pain [36]; rabies [25]; tumor [27,37] |
| Juncus effusus L. (Juncaceae) | Wound, stomach ache, bleeding after delivery, muscle cramps, tumors [27] |
| Kniphofia foliosa Hochst (Asphodelaceae) | Cervical cancer [21] |
| Lagenaria siceraria (Molina) Standl. (Cucurbitaceae) | Diarrhea, vomiting [38]; gonorrhea [39]; wound [25]; cough, cancer [28] |
| Linum usitatissimum (Linaceae) | Gastritis [40,41] |
| Maytenus senegalensis (Celastraceae) | Stomach-ache [42]; snake bite, tonsillitis, diarrhoea [43]; tumors [20] |
| Olea europaea subsp. Cuspidate (Wall. ex. G. Don) Cif. (Oleaceae) | Stomach problems, malaria, dysentery [44]; Eye disease [45]; wound [46]; brain tumor [47] |
| Plumbago zeylanica L. (Plumbaginaceae) | Cancer [26]; external body swelling, internal cancer, bone cancer [7]; cancer, cough, snake bite, swelling [31] |
| Podocarpus falcatus (Podocarpaceae) | Cancer [34]; amoeba, gastritis [6]; rabies [48] |
| Premna schimperi Engl. (Verbenaceae) | Antiseptic [49]; cancer [35] |
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | Breast cancer [21]; benign prostatic hyperplasia, prostate gland hypertrophy [26] |
| Ricinus communis L. (Euphorbiaceae) | Rabies [48]; dysentery [50]; stomach ache [34,51]; Liver disease [52]; tooth ache [31]; breast cancer [28] |
| Solanum nigrum (Solanaceae) | Painful and expanding swelling on finger [7]; cancer [27] |
| Vernonia amygdalina Delile (Asteraceae) | Tonsillitis [34]; cancer [6] |
| Vernonia hymenolepis A. Rich. (Asteraceae) | Tumor [6,40,41] |
| Withania somnifera (Solanaceae) | Snake bite [53]; chest pain [54]; cancer [27] |
| Zehneria scabra (L.F. Sond) (Cucurbitaceae) | Fever, head ache [55]; tumor [56]; eye disease, wart [45] |
| Plant | Class of Compounds | Cell Lines | IC50 | Pharmacology | Isolated Active Compounds | Reference |
|---|---|---|---|---|---|---|
| Maytenus senegalensis (Celastraceae) | Phenolic | L5178Y | 10 µg/mL (100% inhibition) | - | (−) Epigallocathechin (25) | [79] |
| Juncus effusus L. (Juncaceae) | Phenanthrenes | MCF-7 | 10.87 ± 0.82 µM | - | 5-(1-Methoxyethyl)-1-methyl-phenanthren-2,7-diol (26) | [80] |
| 26.68 ± 2.95 µM | Effususol A (27) | |||||
| HepG-2 | 23.90 ± 3.32 µM | Effusol | ||||
| SHSY-5Y | 22.83 ± 0.98 µM | Dehydroeffusol | ||||
| HepG-2 | 23.13 ± 1.79 µM | |||||
| SMMC-7721 | 25.35 ± 2.08 µM | Dehydroeffusal | ||||
| HepG-2 | 12.43 ± 0.41 µM | |||||
| Hela | 13.07 ± 2.56 µM | |||||
| HepG-2 | 26.04 ± 4.49 µM | 5-Hydroxymethyl-1-methylphenanthrene-2,7-diol | ||||
| Hela | 16.35 ± 6.04 µM | |||||
| 29.63 ± 0.67 µM | 2,7-Dihydroxy-1,8-dimethyl-5-vinyl-9,10-dihydrophenanthrene and juncusol | |||||
| HepG-2 | 16.45 ± 1.12 µM | Dehydrojuncusol | ||||
| Hela | 15.17 ± 2.47 µM | 1-Methylpyrene-2,7-diol | ||||
| MCF-7 | 27.10 ± 1.17 µM | |||||
| 9,10-Dihydrophenanthrene | HT22 | 100 µM | Caspase-3-mediated cytotoxicity | Effususol A (27) | [81] | |
| Plumbago zeylanica | Naphthoquinones | A549 | 10.3 µM | Apoptosis | Plumbagin (28) | [82] |
| H292 | 7.3 µM | |||||
| H460 | 6.1 µM | |||||
| Panc-1 | 2.1 µM | [83] | ||||
| Kniphofia foliosa Hochst* | Phenylanthraquinones | B16 | 3.3 ± 0.39 µM | Necrotic cell death | Knipholone (29) | [15] |
| RAW 264.7 | 1.6 ± 0.25 µM | |||||
| U937 | 0.5 ± 0.05 µM | |||||
| THP-1 | 0.9 ± 0.09 µM |
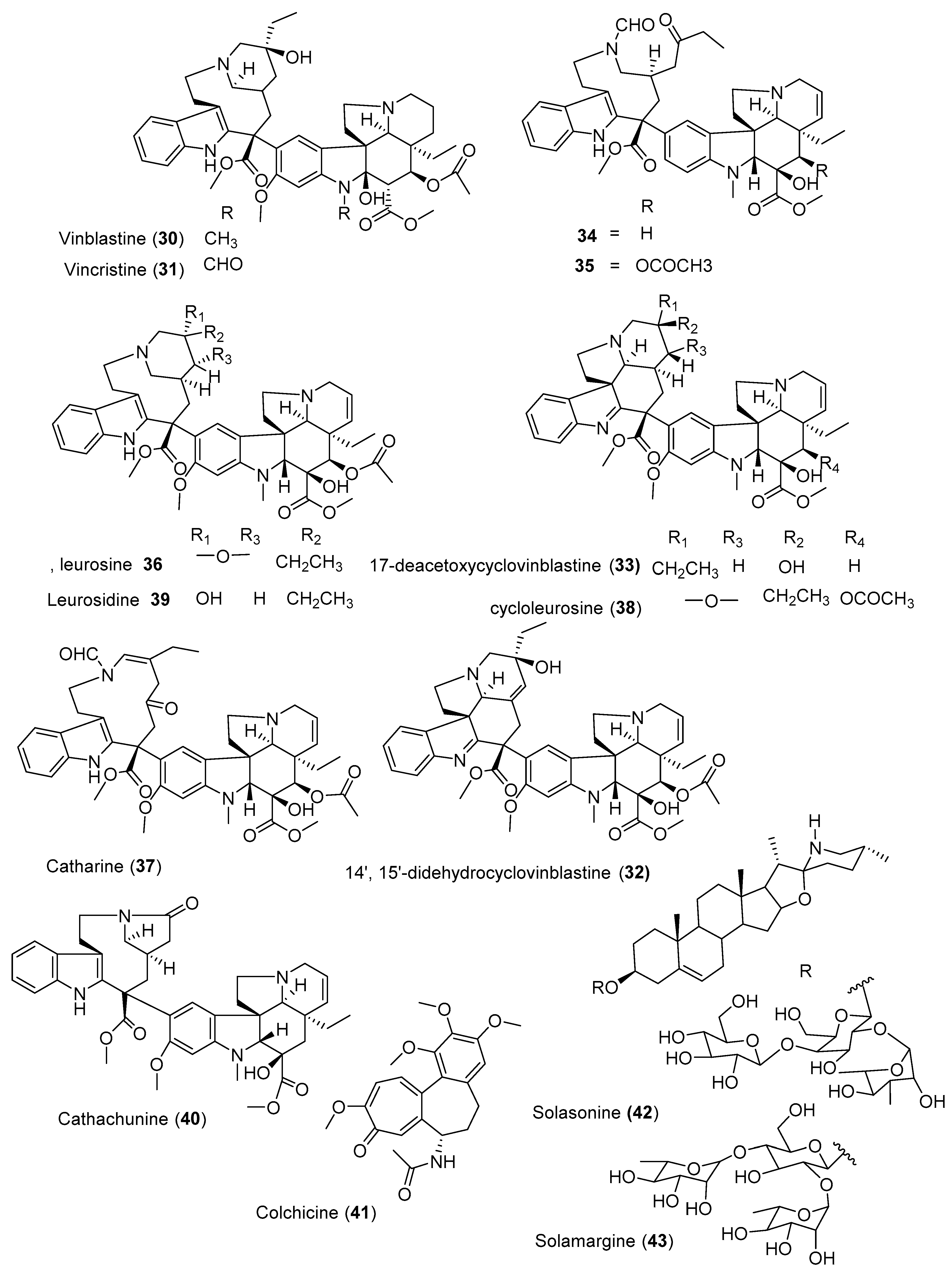
| Plants | Class of Compounds | Cell Lines | IC50 Values | Pharmacology | Isolated Active Compounds | Reference |
|---|---|---|---|---|---|---|
| Catharanthus roseus (L.) G.Don (Apocynaceae) | Bisindole alkaloid | SH-SY5Y | 0.1 µM | Mitotic arest and apoptosis | Vincristine (31) | [91] |
| MDA-MB-231 | 0.67 ± 0.03 nM | Vinblastine (30) | [87] | |||
| Indole alkaloids | 0.97 ± 0.07 µM | - | 14′,15′-Didehydrocyclovinblastine (32) | |||
| 7.93 ± 0.42 µM | 17-Deacetoxycyclovinblastine (33) | |||||
| 3.55 ± 0.19 µM | 17–Deacetoxyvinamidine (34) | |||||
| 10.67 ± 0.63 µM | Vinamidine (35) | |||||
| 0.73 ± 0.06 µM | Leurosine (36) | |||||
| 8.59 ± 0.51 µM | Catharine (37) | |||||
| 1.11 ± 0.07 µM | Cycloleurosine (38) | |||||
| 4.26 ± 0.23 µM | Leurosidine (39) | |||||
| HCT 116 | >200 µg/mL | Vindoline | [89] | |||
| 60 µg/mL | Catharanthine | |||||
| Bisindole alkaloid | HL-60 | 9.1 ± 0.7 µM | Induction of apoptosis via an intrinsic pathway | Cathachunine (40) | [88] | |
| Gloriosa superba (Colchicaceae) | Alkaloid | A-549 and MDA-MB-231 | 60 nM | G2/M phase arrest | Colchicine (41) | [90] |
| Solanum nigrum (Solanaceae) | Steroidal glycoalkaloids | MGC-803 | 5.2 µg/mL | Apoptosis | Solasonine (42) | [92] |
| 26.5 µg/mL | β1-Solasonine | |||||
| 8.77 µg/mL | Solamargine (43) | |||||
| 20.1 µg/mL | Solanigroside P |
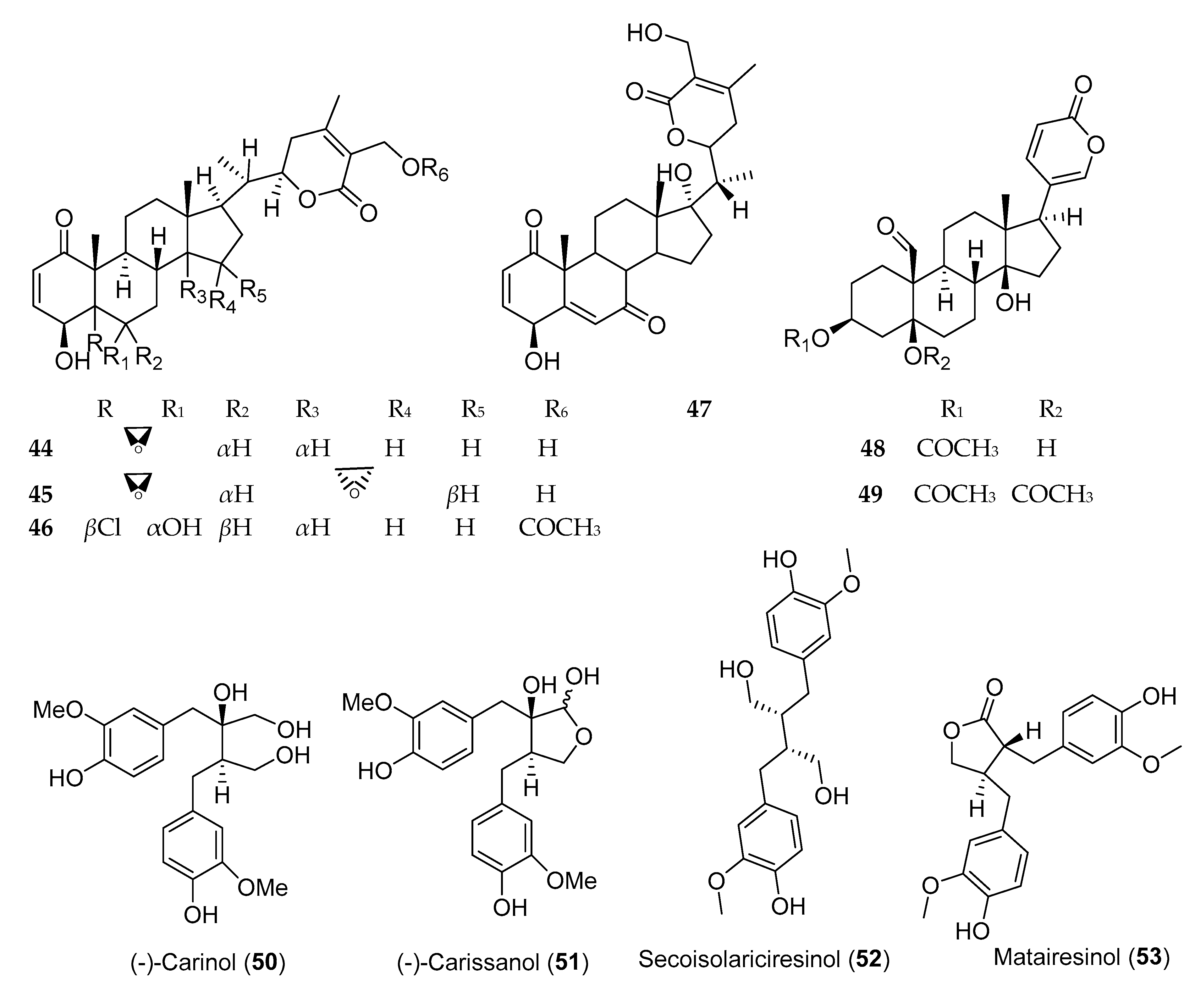
| Plant | Class of Compounds | Cell Lines | IC50 | Isolated Active Compounds | Reference |
|---|---|---|---|---|---|
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | Steroids | HEK293 | 937 µg/mL | β-Sitosterol-3-O-glucoside | [93] |
| HepG2 | 251 µg/mL | ||||
| Caco-2 | 54 µg/mL | ||||
| Withania somnifera (Solanaceae) | Steroidal lactone | NCI-H460 | 0.45 ± 0.00 µg/mL | Withaferin A (44) | [94] |
| 8.3 ± 0.12 µg/mL | 5β,6β,14α,15α-Diepoxy-4β,27-dihydroxy-1-oxowitha-2,24-dienolide (45) | ||||
| 95.6 ± 2.60 µg/mL | 27-Acetoxy-4β,6α-dihydroxy-5β-chloro-1-oxowitha-2,24-dienolide (46) | ||||
| Withasteroid | MCF-7 and WRL-68 | 1.0 µg/mL | 5,6-De-epoxy-5-en-7-one-17-hydroxy withaferin A (47) | [100] | |
| Caco-2 | 3.4 µg/mL | ||||
| PC-3 | 7.4 µg/mL | ||||
| Bersama abyssinica Fresen.* (Melianthaceae) | Steroids (bufadienolide) | KB | 0.028 µg/mL (ED50) | Berscillogenin | [95] |
| 0.62 µg/mL (ED50) | 3-Epiberscillogenin | ||||
| 0.0046 µg/mL (ED50) | Bersenogenin | ||||
| 10−7 µg/mL (ED50) | Hellebrigenin 3-acetate (48) | [96] | |||
| 10−3 µg/mL (ED50) | Hellebrigenin 3,5-diacetate (49) | ||||
| Carissa spinarum (Apocynaceae) | Lignans | A549 | <1 µg/mL | (−)-Carinol (50) | [98] |
| MCF-7 | |||||
| WI-38 | |||||
| A549 | 11.0 µg/mL | (−)-Carissanol (51) | |||
| MCF-7 | 17.4 µg/mL | ||||
| WI-38 | 6.2 µg/mL | ||||
| A549 | 29.0 µg/mL | (−)-Nortrachelogenin | |||
| MCF-7 | 88.3 µg/mL | ||||
| WI-38 | >100 µg/mL | ||||
| Linum usitatissimum (Linaceae) | Lignans | MCF-7 | 1 × 10−5 mol/L | Secoisolariciresinol (52) | [99] |
| 1 × 10−6 M | Matairesinol (53) |
| Plants | Crude Extract | Isolated Compounds | In Vivo Studies | Clinical Trials (Status) | Clinically Approved for |
|---|---|---|---|---|---|
| Bersama abyssinica | Hellebrigenin 3-acetate (48) | Significantly inhibits Walker intramuscular carcinosarcoma 256 in rats [96] | - | - | |
| Catharanthus roseus | Ethanolic extract | Significantly increased the life span and decreased the tumor volume in Ehrlich ascites carcinoma-bearing mice [101] | - | - | |
| Vincristine (31) | - | - | Childhood leukaemia, Hodgkin’s disease and acute panmyelosis [102] | ||
| Vinblastine (30) | - | - | Lymphosarcoma, choriocarcinoma, neuroblastoma and lymphocytic leukemia [103] | ||
| Euphorbia tirucalli | Hydroalcoholic extract | Significantly enhanced survival and reduced tumor growth in Ehrlich ascites tumor-bearing mice [104] | - | - | |
| Latex | Significantly reduced tumor growth and cachexia in Walker 256 tumor-bearing rats [105] | - | - | ||
| Gloriosa superba | Ethanolic crude extract | Significantly reduced tumor growth in combination with gemcitabine in a murine model of pancreatic adenocarcinoma [106] | - | - | |
| Colchicine (41) | - | Phase II for castrate resistant prostate cancer (Withdrawn due to funding) [107] | - | ||
| Jatropha curcas | Methanolic fractions | Showed significant anti-metastatic and antiprolifertaive activity in C57BL/6 mice [108] | - | - | |
| Linum usitatissimum | Secoisolariciresinol (52) | - | Phase II (Completed) [109] | - | |
| Prunus Africana | Ethanol extract | Showed significant reduction in prostate cancer incidence in mice [110] | - | ||
| Ursolic Acid | - | Early Phase I [111] | - | ||
| Plumbago zeylanica L. | Plumbagin | Significantly inhibits squamous cell carcinomas in FVB/N mice [112] | |||
| Ricinus communis | Fruit extract | Significantly reduced tumor volume in 4T1 syngeneic mouse model [113] | - | - | |
| Solanum nigrum | Crude polysaccharides | Significant growth inhibition in cervical cancer tumor-bearing mice [114] | - | - | |
| Aqueous extract | Significantly inhibits early hepatocarcinogenesis [115] | - | - | ||
| Vernonia amygdalina | Aqueous crude extract | Increase efficacies and optimizes treatment outcomes when given with paclitaxel in athymic mice [116] | _ | - | |
| Vernonia hymenolepis | Vernolepin | Significantly inhibited intramuscular carcinosarcoma in walker tumor bearing rats [117] | - | - | |
| Withania somnifera | Aqueous extract | Decreased tumor volume in orthotopic glioma allograft rat model [118] | - | - | |
| Ethanolic extract | Significantly improve colon cancer treatment in mice [119] | - | - | ||
| Withaferin A | Significantly inhibited HepG2-xenografts and diethylnitrosamine (DEN)-induced-hepatocellular carcinoma (HCC) in C57BL/6 mice [120] | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfaye, S.; Asres, K.; Lulekal, E.; Alebachew, Y.; Tewelde, E.; Kumarihamy, M.; Muhammad, I. Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer, Part 2: A Review on Cytotoxic, Antiproliferative, and Antitumor Phytochemicals, and Future Perspective. Molecules 2020, 25, 4032. https://doi.org/10.3390/molecules25174032
Tesfaye S, Asres K, Lulekal E, Alebachew Y, Tewelde E, Kumarihamy M, Muhammad I. Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer, Part 2: A Review on Cytotoxic, Antiproliferative, and Antitumor Phytochemicals, and Future Perspective. Molecules. 2020; 25(17):4032. https://doi.org/10.3390/molecules25174032
Chicago/Turabian StyleTesfaye, Solomon, Kaleab Asres, Ermias Lulekal, Yonatan Alebachew, Eyael Tewelde, Mallika Kumarihamy, and Ilias Muhammad. 2020. "Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer, Part 2: A Review on Cytotoxic, Antiproliferative, and Antitumor Phytochemicals, and Future Perspective" Molecules 25, no. 17: 4032. https://doi.org/10.3390/molecules25174032