Second-Generation Antiandrogen Therapy Radiosensitizes Prostate Cancer Regardless of Castration State through Inhibition of DNA Double Strand Break Repair
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
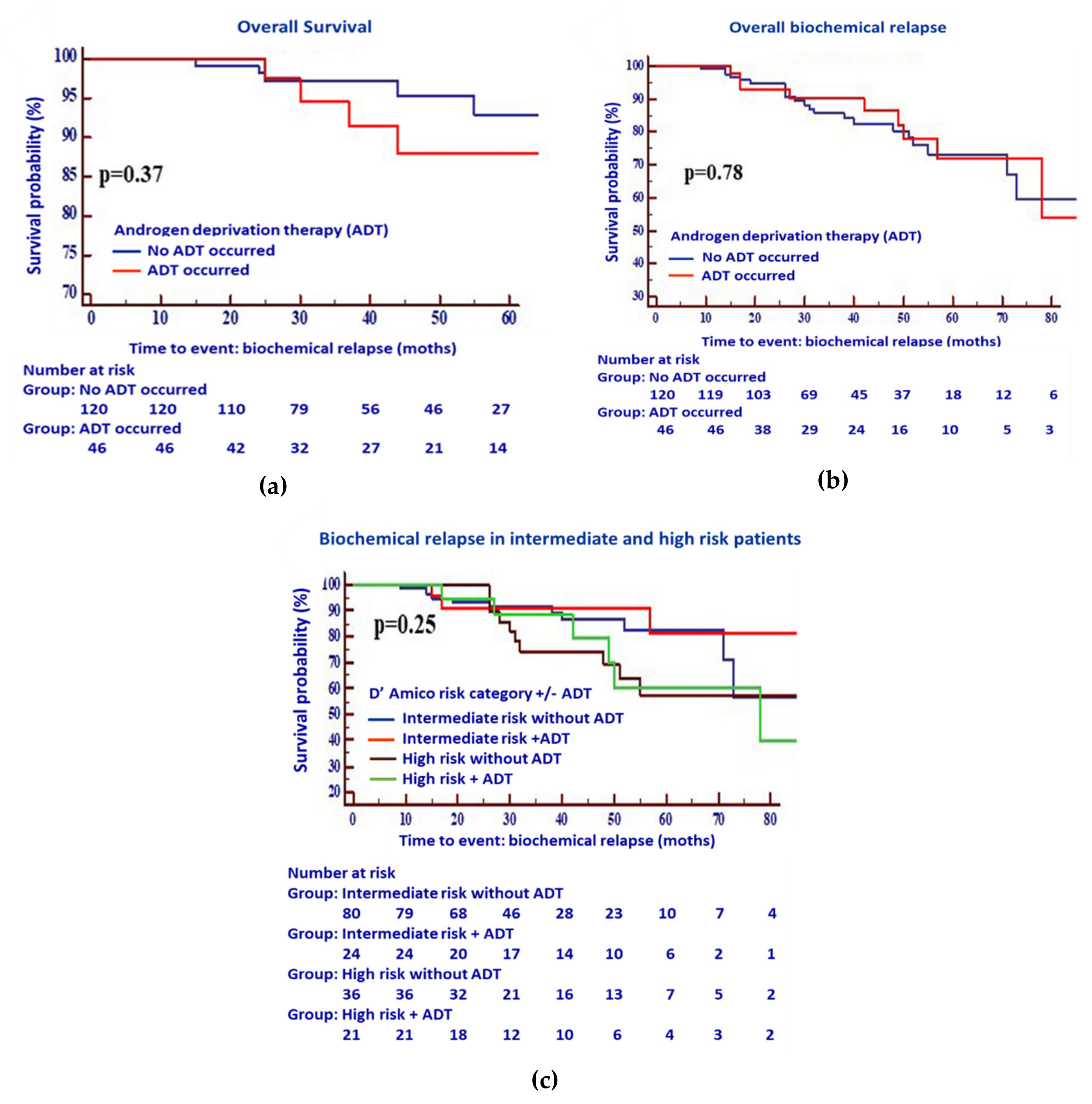
2.1. ADT Plus RT Confers a Slight but Not Significant Increase in the Biochemical Relapse-Free Survival of Patients with Intermediate- and High-Risk PCa
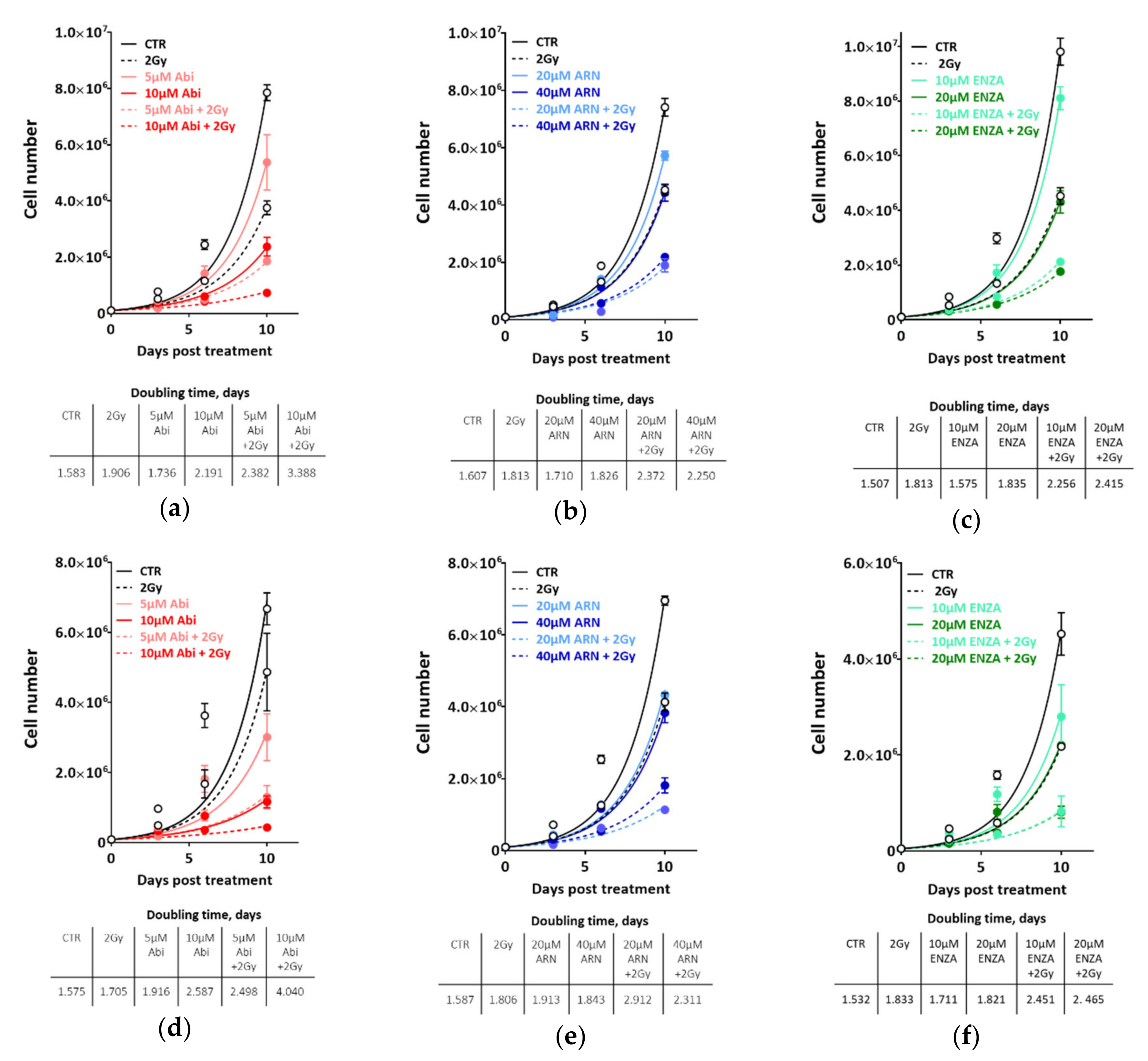
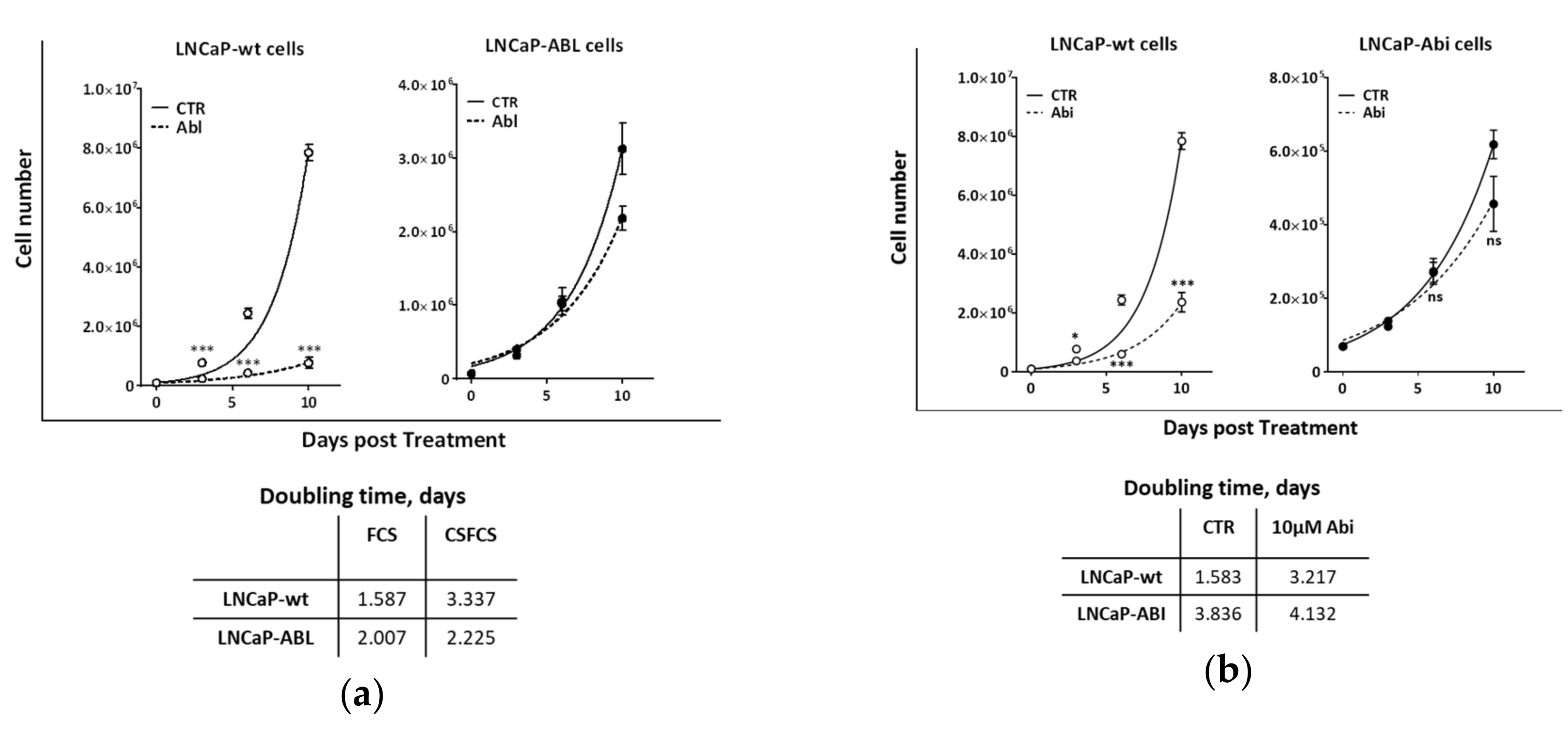
2.2. Second-Generation Antiandrogens Enhance the Response of Prostate Cancer Cells to IR
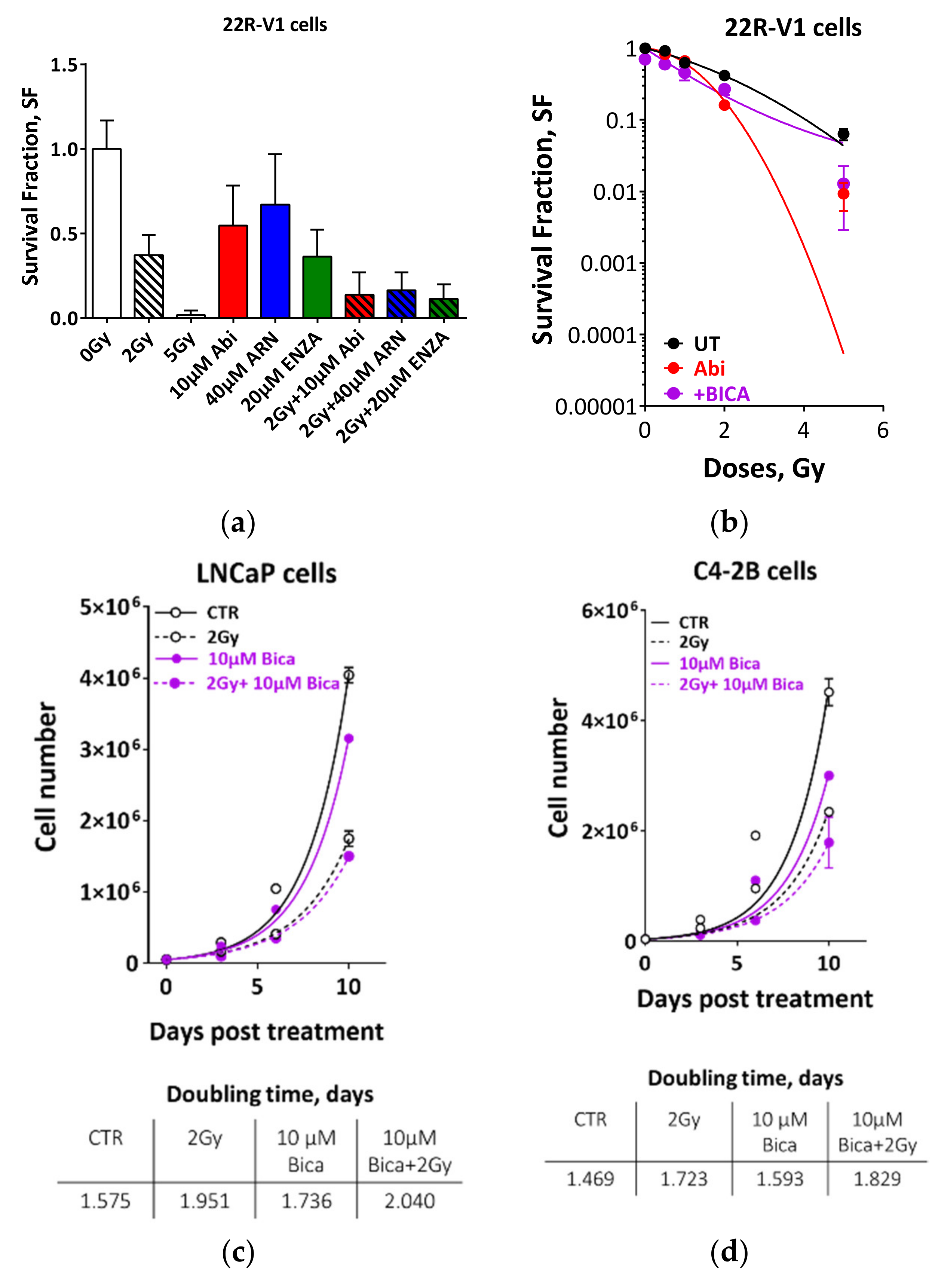
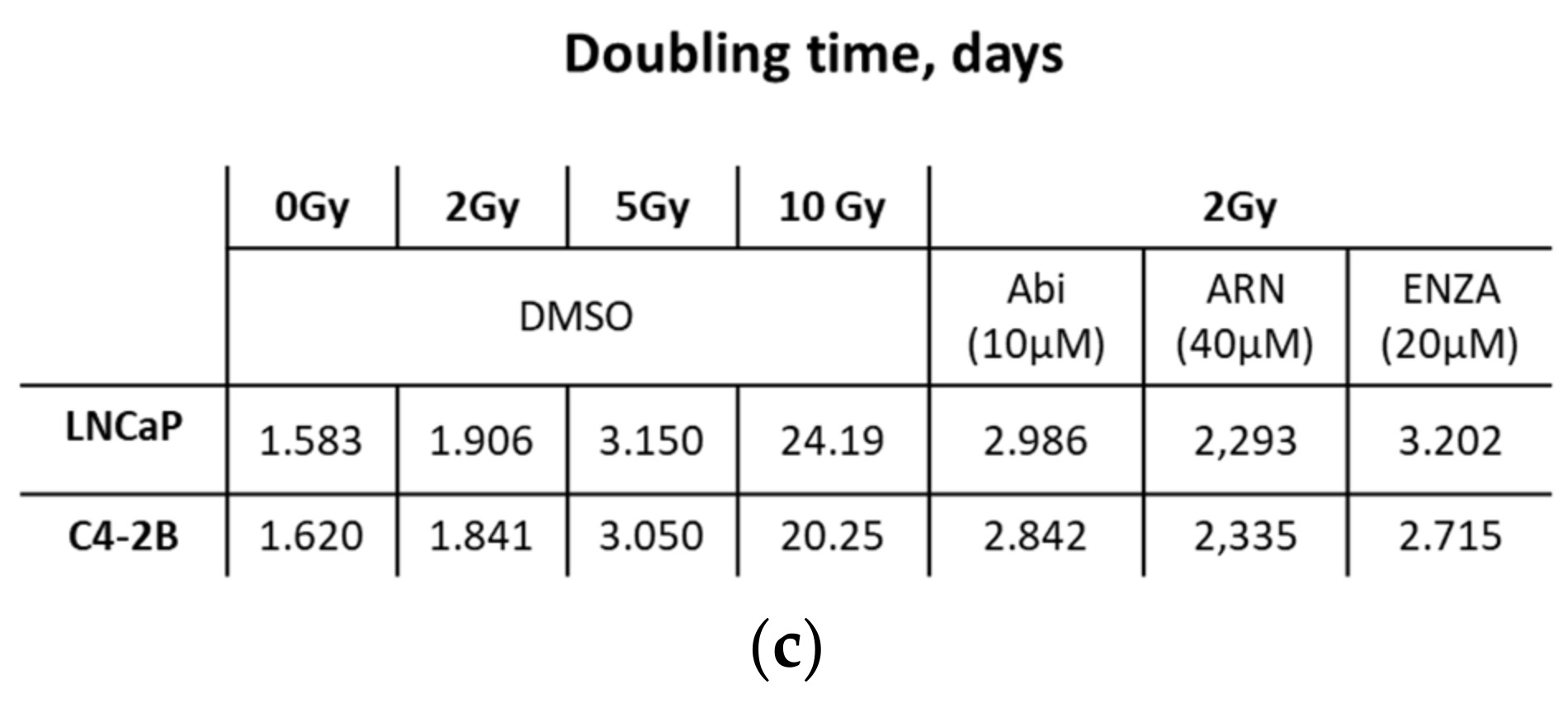
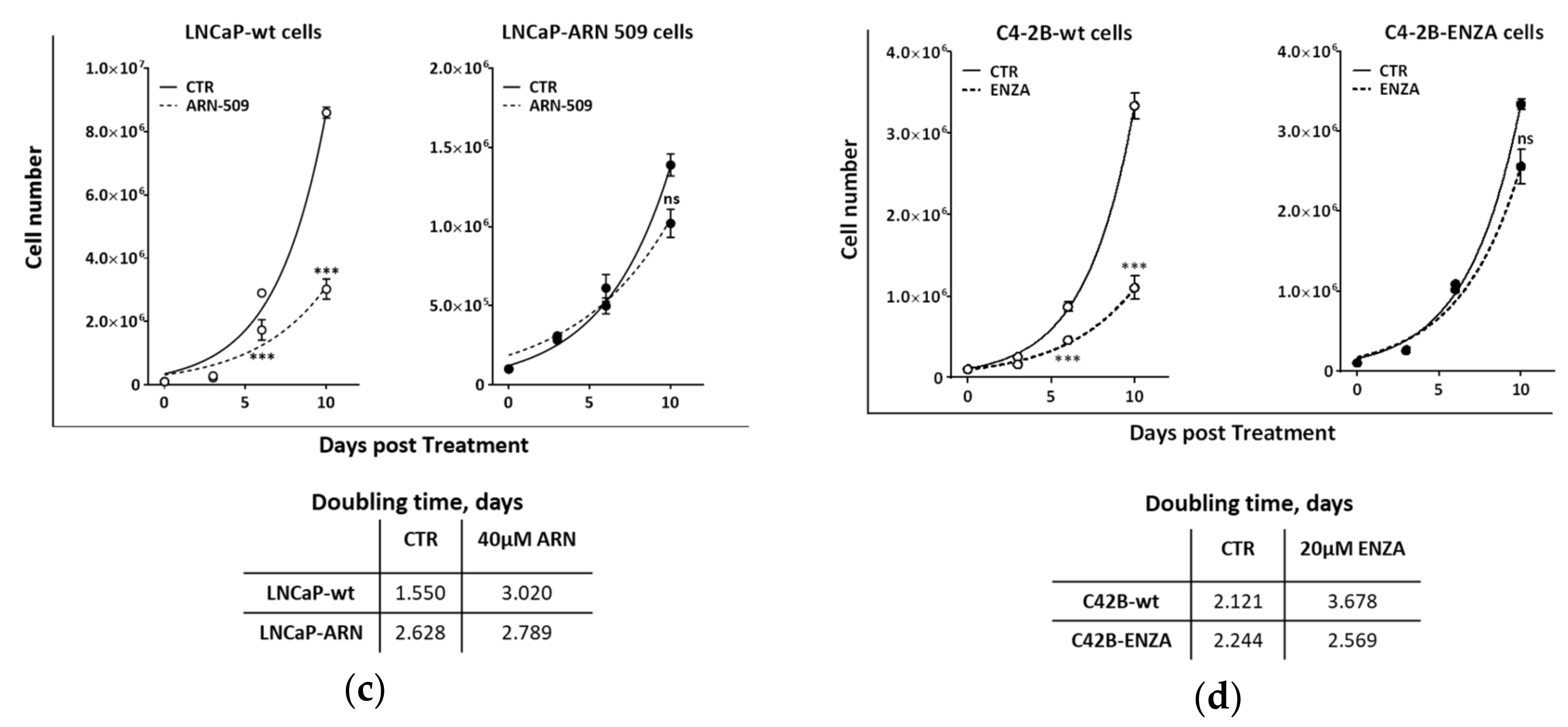
2.3. Second Generation Antiandrogens Escalate the IR Effect About 2 Times
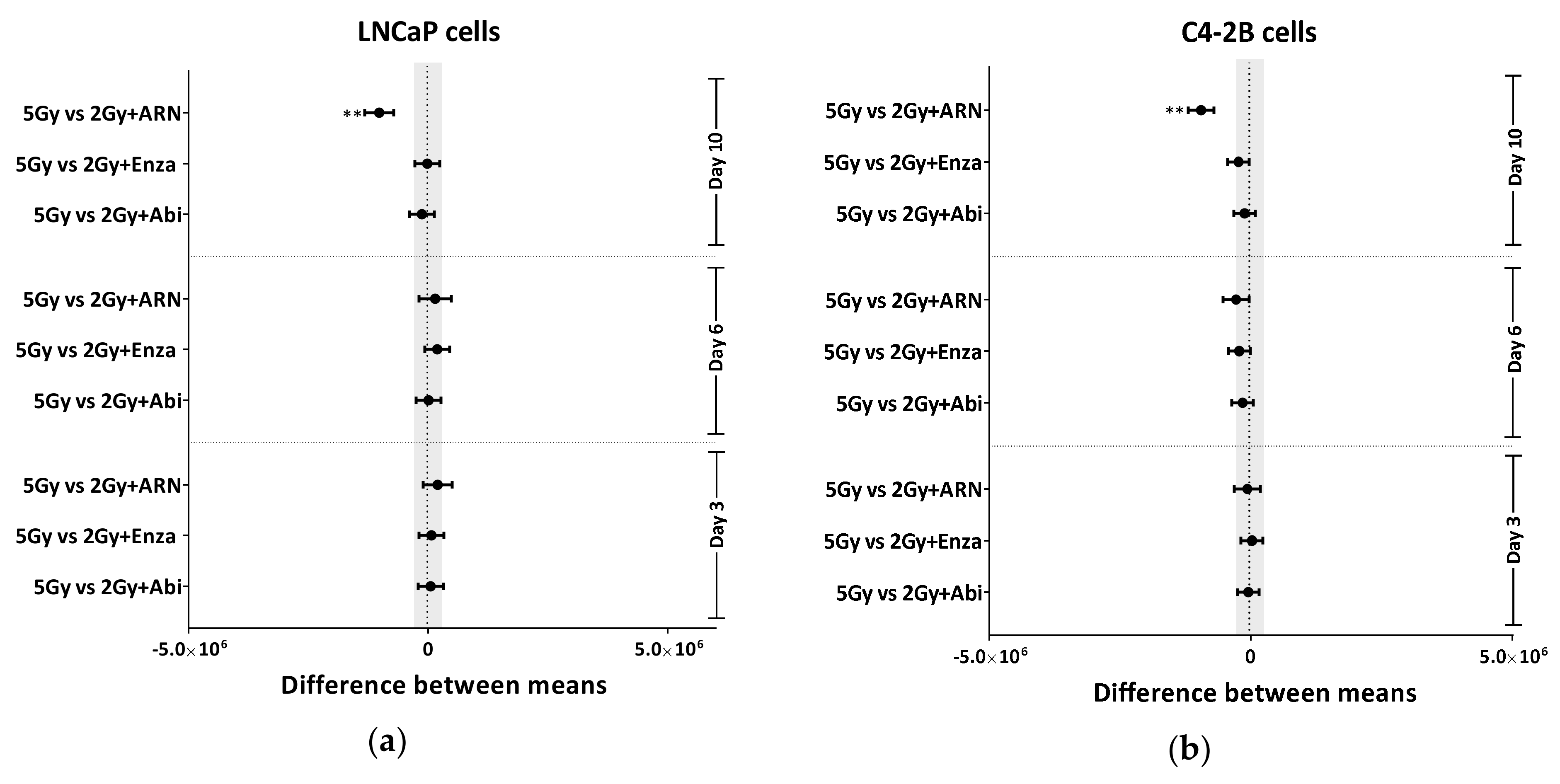
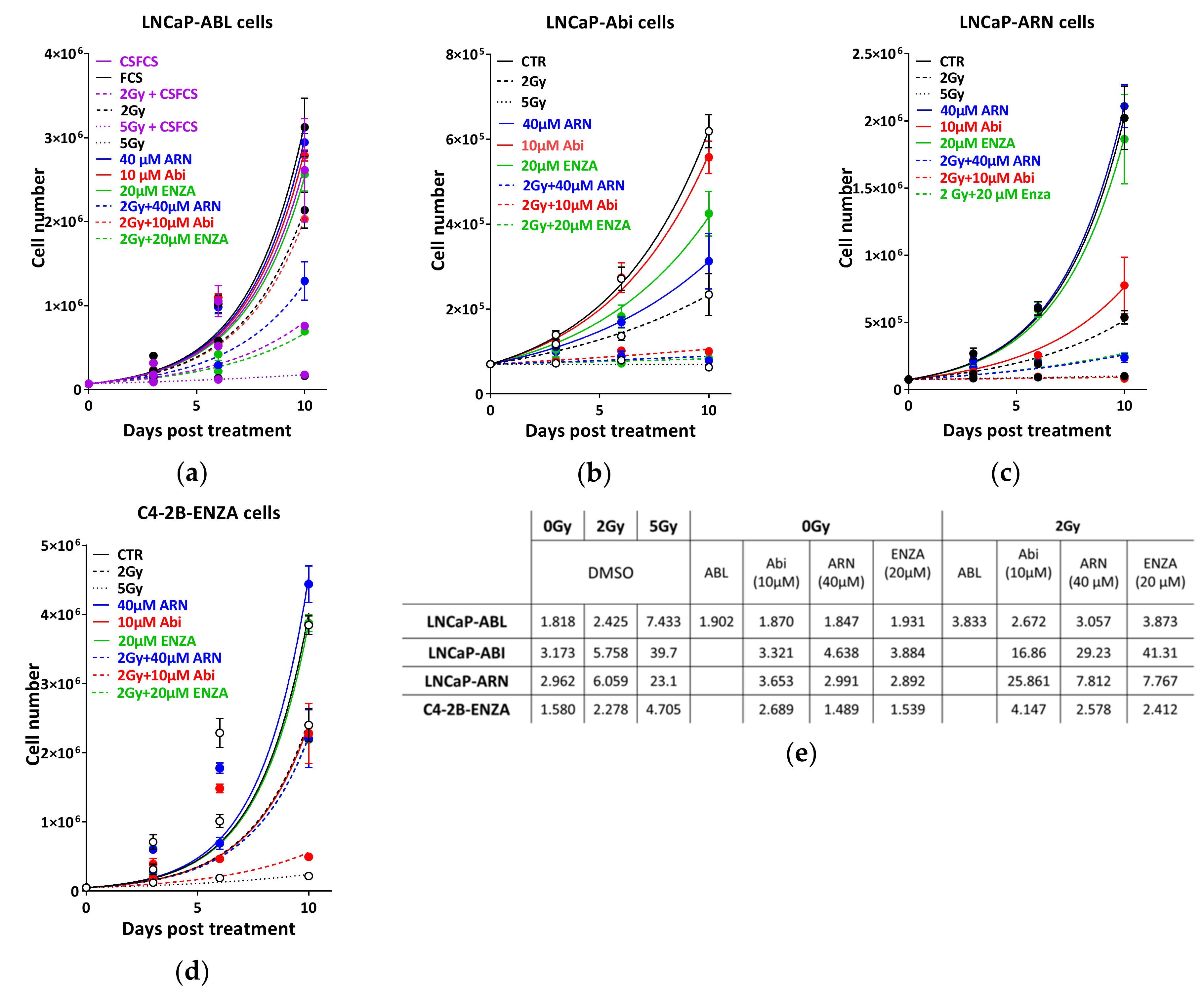
2.4. Appropriate Choice of Antiandrogen to Maximize the Therapeutic Effect of IR in Acquired AHT-Resistant PCa Cells
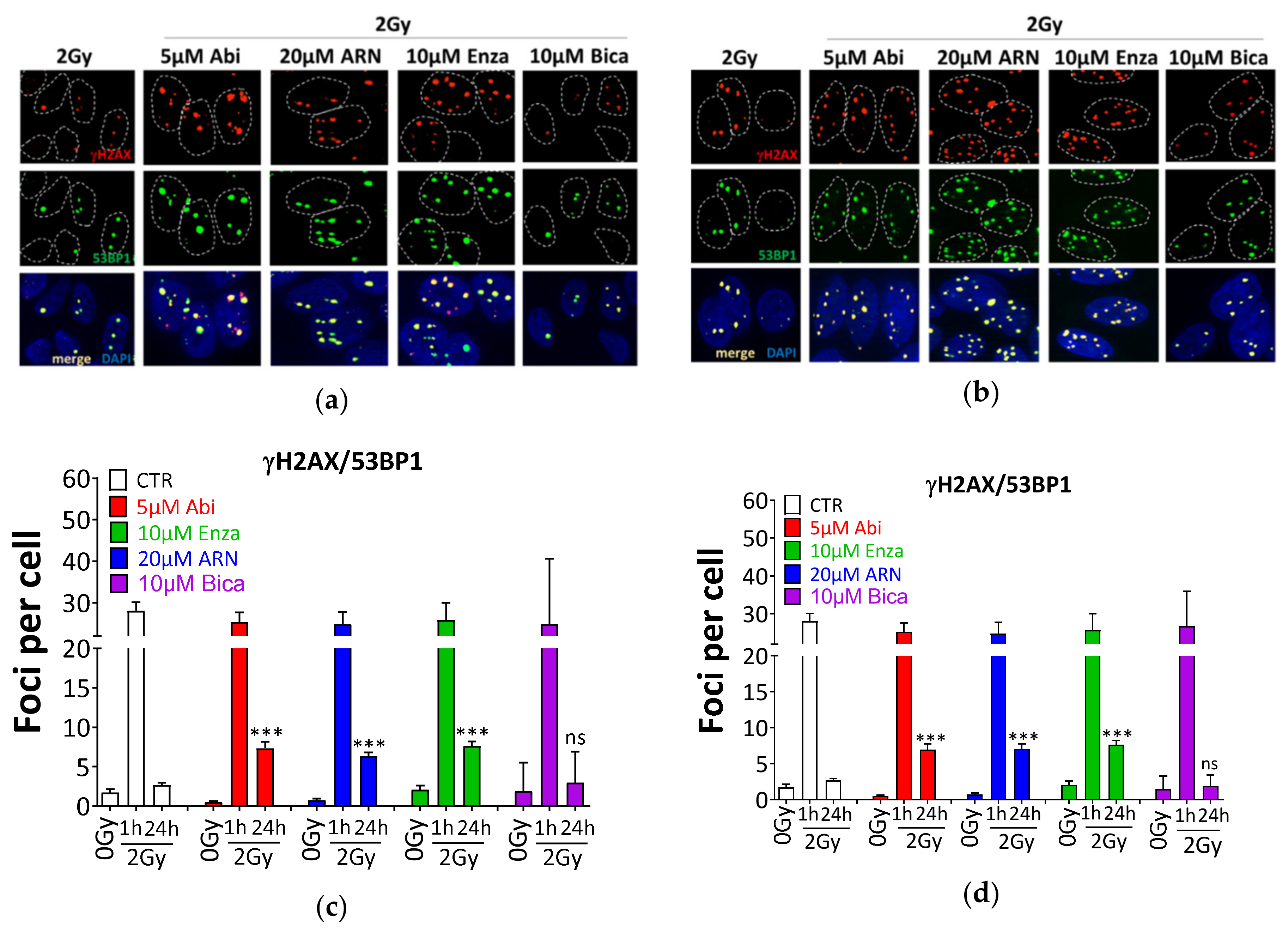
2.5. Second-Generation Antiandrogens Radiosensitize PCa Cells Through the Inhibition of DSB Repair
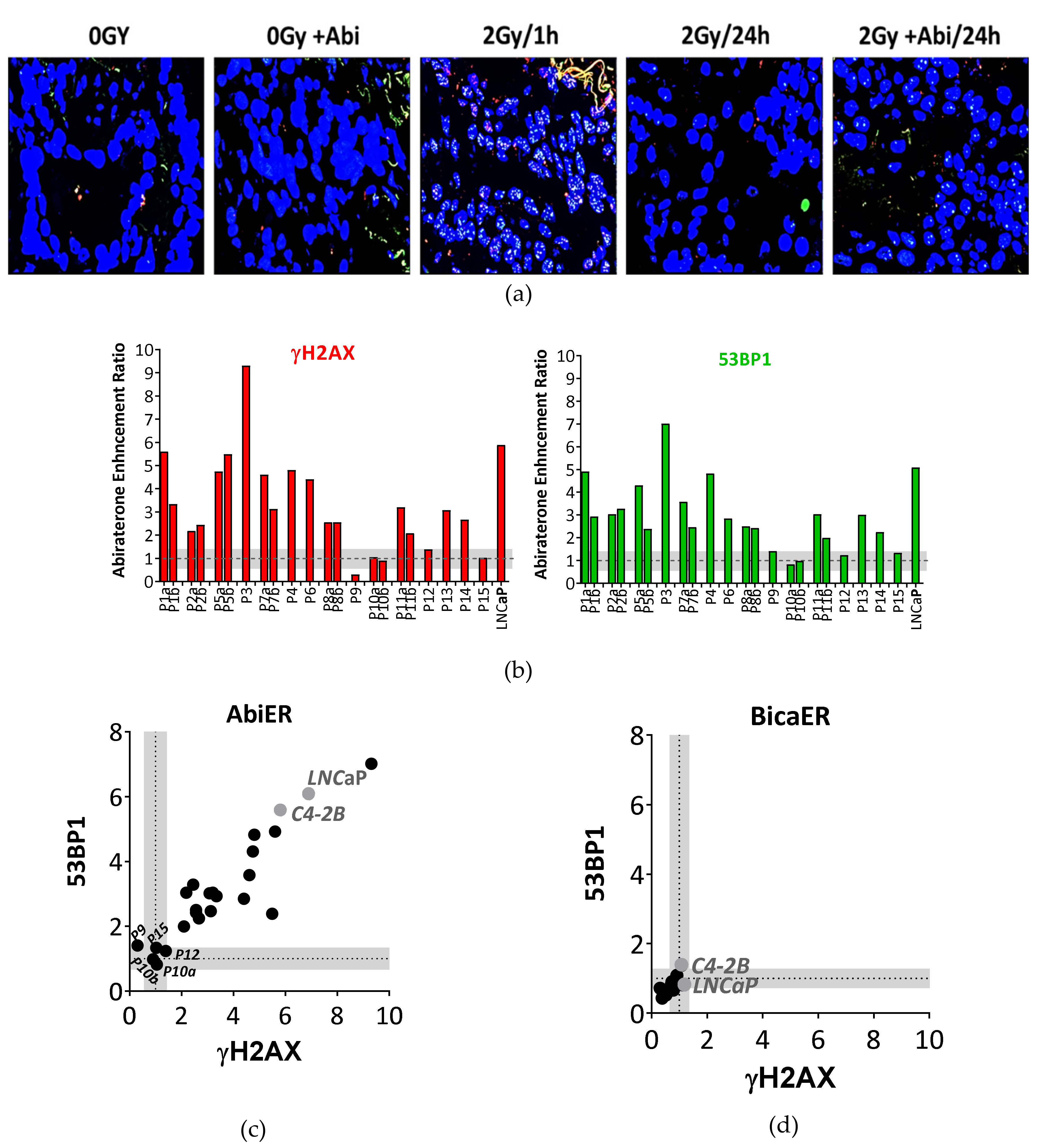
2.6. Abiraterone But Not Bicalutamide Reduces Repair Capacity of IR-Induced DSBs in Fresh Prostate Cancer Tissues
3. Discussion
4. Materials and Methods
4.1. Patients and Retrospective Study Design
4.2. Cell Culture, Drugs, and X-Irradiation
4.3. Proliferation Assay
4.4. Colony Formation Assay
4.5. Immunofluorescence
4.6. Cell Cycle Analysis
4.7. Apoptosis Quantification
4.8. Patient Sample Collection
4.9. Tissue Slice Cultures
4.10. Graphs and Statistics
4.11. Ethical Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, L.J.; Griffiths, K. Endocrine treatment in prostate cancer. Semin. Surg. Oncol. 2000, 18, 52–74. [Google Scholar] [CrossRef]
- Dal Pra, A.; Cury, F.L.; Souhami, L. Combining radiation therapy and androgen deprivation for localized prostate cancer-a critical review. Curr. Oncol. 2010, 17, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen deprivation therapy for prostate cancer. Jama 2005, 294, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Stevens, R.A. The Effect of Castration on Benign Hypertrophy of the Prostate in Man11This investigation was supported by a grant from the Committee on Research in Problems of Sex of the National Research Council. J. Urol. 1940, 43, 705–714. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Kelly, W.K. Outsmarting androgen receptor: Creative approaches for targeting aberrant androgen signaling in advanced prostate cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P. Current clinical trials in castrate-resistant prostate cancer. Curr. Urol. Rep. 2011, 12, 173–179. [Google Scholar] [CrossRef]
- Soifer, H.S.; Souleimanian, N.; Wu, S.; Voskresenskiy, A.M.; Collak, F.K.; Cinar, B.; Stein, C.A. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J. Biol. Chem. 2012, 287, 3777–3787. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.; Lim, A.C.; Hay, C.W.; Taylor, A.E.; Wingate, A.; Nowakowska, K.; Pezaro, C.; Carreira, S.; Goodall, J.; Arlt, W.; et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: A rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012, 72, 2176–2182. [Google Scholar] [CrossRef] [Green Version]
- Helleday, T.; Lo, J.; van Gent, D.C.; Engelward, B.P. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair 2007, 6, 923–935. [Google Scholar] [CrossRef]
- Michalski, J.M.; Pisansky, T.M.; Lawton, C.A.F.; Potters, L. Chapter 53—Prostate Cancer. In Clinical Radiation Oncology, 4th ed.; Gunderson, L.L., Tepper, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 1038–1095.e1018. [Google Scholar] [CrossRef]
- Tarish, F.L.; Schultz, N.; Tanoglidi, A.; Hamberg, H.; Letocha, H.; Karaszi, K.; Hamdy, F.C.; Granfors, T.; Helleday, T. Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci. Transl. Med. 2015, 7, 312re311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagars, G.K.; Pollack, A.; von Eschenbach, A.C. Prognostic factors for clinically localized prostate carcinoma: Analysis of 938 patients irradiated in the prostate specific antigen era. Cancer 1997, 79, 1370–1380. [Google Scholar] [CrossRef]
- D‘Amico, A.V.; Matelski, H.; O‘Leary, M.; Sussman, B. Prostate-specific antigen-producing cells in the bone marrow of a patient with early-stage prostate cancer. Urology 1997, 49, 279–282. [Google Scholar] [CrossRef]
- Zietman, A.L. Overview. In Clinical Radiation Oncology, 4th ed.; Gunderson, L.L., Tepper, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 1035–1037. [Google Scholar] [CrossRef]
- Joseph, D.J.; Lamb, D.S.; Denham, J.W.; Oldmeadow, C.; Attia, J.; Steigler, A. Ten year final results of the TROG 03.04 (RADAR) randomised phase 3 trial evaluating duration of androgen suppression ± zoledronate for locally advanced prostate cancer. J. Clin. Oncol. 2018, 36, 1. [Google Scholar] [CrossRef]
- Nabid, A.; Garant, M.-P.; Martin, A.-G.; Bahary, J.-P.; Lemaire, C.; Vass, S.; Bahoric, B.; Archambault, R.; Vincent, F.; Bettahar, R.; et al. Duration of androgen deprivation therapy in high risk prostate cancer: Final results of a randomized phase III trial. J. Clin. Oncol. 2017, 35, 5008. [Google Scholar] [CrossRef]
- Zietman, A.L.; Prince, E.A.; Nakfoor, B.M.; Park, J.J. Androgen deprivation and radiation therapy: Sequencing studies using the Shionogi in vivo tumor system. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 1067–1070. [Google Scholar] [CrossRef]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Yang, J.C.; Kung, H.J.; Shi, X.B.; Tilki, D.; Lara, P.N., Jr.; DeVere White, R.W.; Gao, A.C.; Evans, C.P. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene 2014, 33, 4521–4530. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, K.E.; Arden, K.C.; Cavenee, W.K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998, 273, 20213–20222. [Google Scholar] [CrossRef] [Green Version]
- Kocher, S.; Beyer, B.; Lange, T.; Nordquist, L.; Volquardsen, J.; Burdak-Rothkamm, S.; Schlomm, T.; Petersen, C.; Rothkamm, K.; Mansour, W.Y. A functional ex vivo assay to detect PARP1-EJ repair and radiosensitization by PARP-inhibitor in prostate cancer. Int. J. Cancer 2019, 144, 1685–1696. [Google Scholar] [CrossRef]
- McGowan, D.G.; Hunt, D.; Jones, C.U.; Amin, M.; Leibenhaut, M.H.; Husian, S.M.; Rotman, M.; Souhami, L.; Sandler, H.; Shipley, W.U. Short-term Endocrine Therapy Prior to and during Radiation Therapy Improves Overall Survival in Patients with T1b-T2b Adenocarcinoma of the Prostate and PSA ≤ 20: Initial Results of RTOG 94-08. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Chen, M.H.; Renshaw, A.A.; Loffredo, M.; Kantoff, P.W. Androgen suppression and radiation vs. radiation alone for prostate cancer: A randomized trial. Jama 2008, 299, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, A.V.; Chen, M.H.; de Castro, M.; Loffredo, M.; Lamb, D.S.; Steigler, A.; Kantoff, P.W.; Denham, J.W. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: An analysis of two randomised trials. Lancet Oncol. 2012, 13, 189–195. [Google Scholar] [CrossRef]
- Bolla, M.; Gonzalez, D.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Gil, T.; et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 1997, 337, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Hanks, G.E.; Pajak, T.F.; Porter, A.; Grignon, D.; Brereton, H.; Venkatesan, V.; Horwitz, E.M.; Lawton, C.; Rosenthal, S.A.; Sandler, H.M.; et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J. Clin. Oncol. 2003, 21, 3972–3978. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Bae, K.; Hanks, G.E.; Porter, A.; Grignon, D.J.; Brereton, H.D.; Venkatesan, V.; Lawton, C.A.; Rosenthal, S.A.; Sandler, H.M.; et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J. Clin. Oncol. 2008, 26, 2497–2504. [Google Scholar] [CrossRef]
- Laverdiere, J.; Nabid, A.; de Bedoya, L.D.; Ebacher, A.; Fortin, A.; Wang, C.S.; Harel, F. The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2-T3 prostate cancer. J. Urol. 2004, 171, 1137–1140. [Google Scholar] [CrossRef]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [Green Version]
- Al-Ubaidi, F.L.; Schultz, N.; Loseva, O.; Egevad, L.; Granfors, T.; Helleday, T. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin. Cancer Res. 2013, 19, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Asagoshi, K.; Tano, K.; Chastain, P.D., 2nd; Adachi, N.; Sonoda, E.; Kikuchi, K.; Koyama, H.; Nagata, K.; Kaufman, D.G.; Takeda, S.; et al. FEN1 functions in long patch base excision repair under conditions of oxidative stress in vertebrate cells. Mol. Cancer Res. 2010, 8, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Horikoshi, N.; Singh, M.; Gupta, A.; Misra, H.S.; Albuquerque, K.; Hunt, C.R.; Pandita, T.K. Chromatin modifications and the DNA damage response to ionizing radiation. Front. Oncol. 2013, 2, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanucci, A.; Sahu, B.; Seppala, J.; Larjo, A.; Latonen, L.M.; Waltering, K.K.; Tammela, T.L.; Vessella, R.L.; Lahdesmaki, H.; Janne, O.A.; et al. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene 2012, 31, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, J.F.; Schiewer, M.J.; Dean, J.L.; Schrecengost, R.S.; de Leeuw, R.; Han, S.; Ma, T.; Den, R.B.; Dicker, A.P.; Feng, F.Y.; et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013, 3, 1254–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Mansour, W.Y.; Bogdanova, N.V.; Kasten-Pisula, U.; Rieckmann, T.; Kocher, S.; Borgmann, K.; Baumann, M.; Krause, M.; Petersen, C.; Hu, H.; et al. Aberrant overexpression of miR-421 downregulates ATM and leads to a pronounced DSB repair defect and clinical hypersensitivity in SKX squamous cell carcinoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 106, 147–154. [Google Scholar] [CrossRef]
- Oing, C.; Tennstedt, P.; Simon, R.; Volquardsen, J.; Borgmann, K.; Bokemeyer, C.; Petersen, C.; Dikomey, E.; Rothkamm, K.; Mansour, W.Y. BCL2-overexpressing prostate cancer cells rely on PARP1-dependent end-joining and are sensitive to combined PARP inhibitor and radiation therapy. Cancer Lett. 2018, 423, 60–70. [Google Scholar] [CrossRef]
| Characteristics | n | % | Mean (±SD)/Median (Range) |
|---|---|---|---|
| Age (years) | 166 | 100 | 73 (53–80) |
| Baseline PSA-value (ng/mL) | 166 | 100 | 8 (2.1–165) |
| <10 | 100 | 60.2 | 6 (2.1–9.85) |
| 10–20 | 47 | 28.3 | 12.58 (10–20) |
| >20 | 19 | 11.4 | 39 (21.5–165) |
| Post-therapeutic PSA-nadir (ng/mL) | 164 | 100 | 0.1 (0–13.5) |
| Gleason-Score | 166 | 100 | 7 (6–10) |
| <7 | 19 | 11.4 | 6 |
| 7 | 112 | 67,5 | 7 |
| >7 | 35 | 21.1 | 8 (8–10) |
| T stage * | 165 * | 100 | - |
| T1c | 39 | 23.6 | - |
| T2a-b | 53 | 32.1 | - |
| T2c-T3a/b | 71 | 43 | - |
| Tx | 2 | 1.2 | - |
| Risk categories * | 166 | 100 | - |
| Low risk | 5 | 3 | - |
| Intermediate risk | 104 | 62.7 | - |
| High risk | 57 | 34.3 | - |
| Androgen deprivation therapy | 166 | 100 | - |
| Yes | 46 | 27.7 | - |
| No | 120 | 72.2 | - |
| Target volume (EBRT) | 166 | 100 | - |
| Prostate and seminal vesicles | 125 | 75.3 | - |
| Additional irradiation of pelvic lymph nodes | 41 | 24.7 | - |
| Charlson Comorbidity Index | 165 | 100 | 4 (1–9) |
| Side Effect | ADT | Total | ||
|---|---|---|---|---|
| No | Yes | |||
| Acute GIT Toxicity | No GIT | 48 | 15 | 63 |
| Grade 1 or 2 GIT | 65 | 29 | 94 | |
| Total | 113 | 44 | 157 | |
| Side Effect | ADT | Total | ||
|---|---|---|---|---|
| No | Yes | |||
| Acute GUT Toxicity | No GUT | 17 | 2 | 19 |
| Grade 1 or 2 GUT | 94 | 42 | 136 | |
| Total | 111 | 44 | 155 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsesy, M.E.; Oh-Hohenhorst, S.J.; Löser, A.; Oing, C.; Mutiara, S.; Köcher, S.; Meien, S.; Zielinski, A.; Burdak-Rothkamm, S.; Tilki, D.; et al. Second-Generation Antiandrogen Therapy Radiosensitizes Prostate Cancer Regardless of Castration State through Inhibition of DNA Double Strand Break Repair. Cancers 2020, 12, 2467. https://doi.org/10.3390/cancers12092467
Elsesy ME, Oh-Hohenhorst SJ, Löser A, Oing C, Mutiara S, Köcher S, Meien S, Zielinski A, Burdak-Rothkamm S, Tilki D, et al. Second-Generation Antiandrogen Therapy Radiosensitizes Prostate Cancer Regardless of Castration State through Inhibition of DNA Double Strand Break Repair. Cancers. 2020; 12(9):2467. https://doi.org/10.3390/cancers12092467
Chicago/Turabian StyleElsesy, Mohamed E., Su Jung Oh-Hohenhorst, Anastassia Löser, Christoph Oing, Sally Mutiara, Sabrina Köcher, Stefanie Meien, Alexandra Zielinski, Susanne Burdak-Rothkamm, Derya Tilki, and et al. 2020. "Second-Generation Antiandrogen Therapy Radiosensitizes Prostate Cancer Regardless of Castration State through Inhibition of DNA Double Strand Break Repair" Cancers 12, no. 9: 2467. https://doi.org/10.3390/cancers12092467
APA StyleElsesy, M. E., Oh-Hohenhorst, S. J., Löser, A., Oing, C., Mutiara, S., Köcher, S., Meien, S., Zielinski, A., Burdak-Rothkamm, S., Tilki, D., Huland, H., Schwarz, R., Petersen, C., Bokemeyer, C., Rothkamm, K., & Mansour, W. Y. (2020). Second-Generation Antiandrogen Therapy Radiosensitizes Prostate Cancer Regardless of Castration State through Inhibition of DNA Double Strand Break Repair. Cancers, 12(9), 2467. https://doi.org/10.3390/cancers12092467






