Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Winter Cultivation Period: Experimental Set up and Fertigation Scheme
2.2. Summer Cultivation Period: Experimental Set up and Fertigation Scheme
2.3. Sampling, Measurements, and Methods
2.3.1. Inoculation with Rhizobia
2.3.2. Growth and Yield Parameters
2.3.3. Soil Analyses
2.3.4. Biological N Fixation and Tissue Nitrogen Concentrations
2.4. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Yield Characteristics
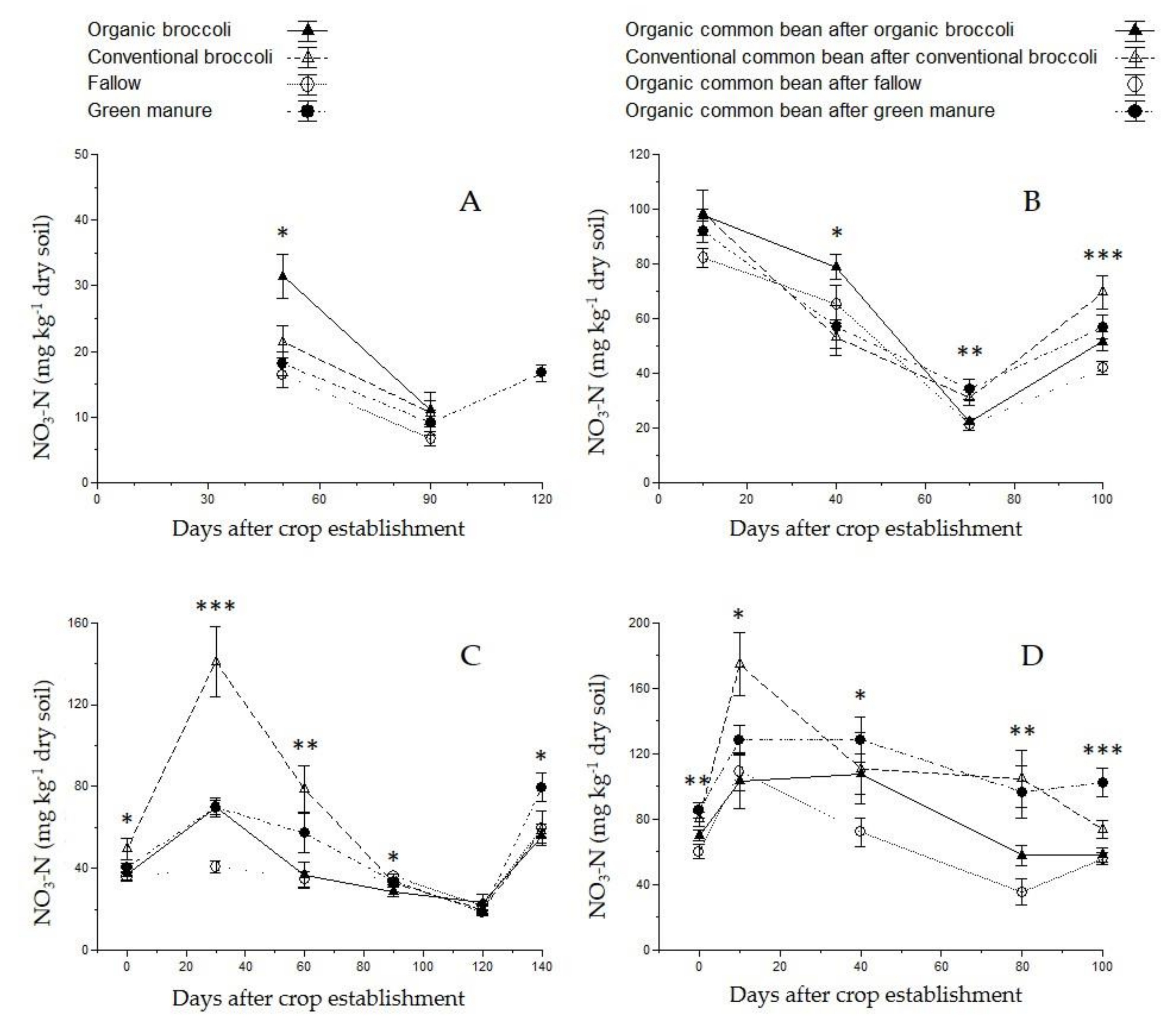
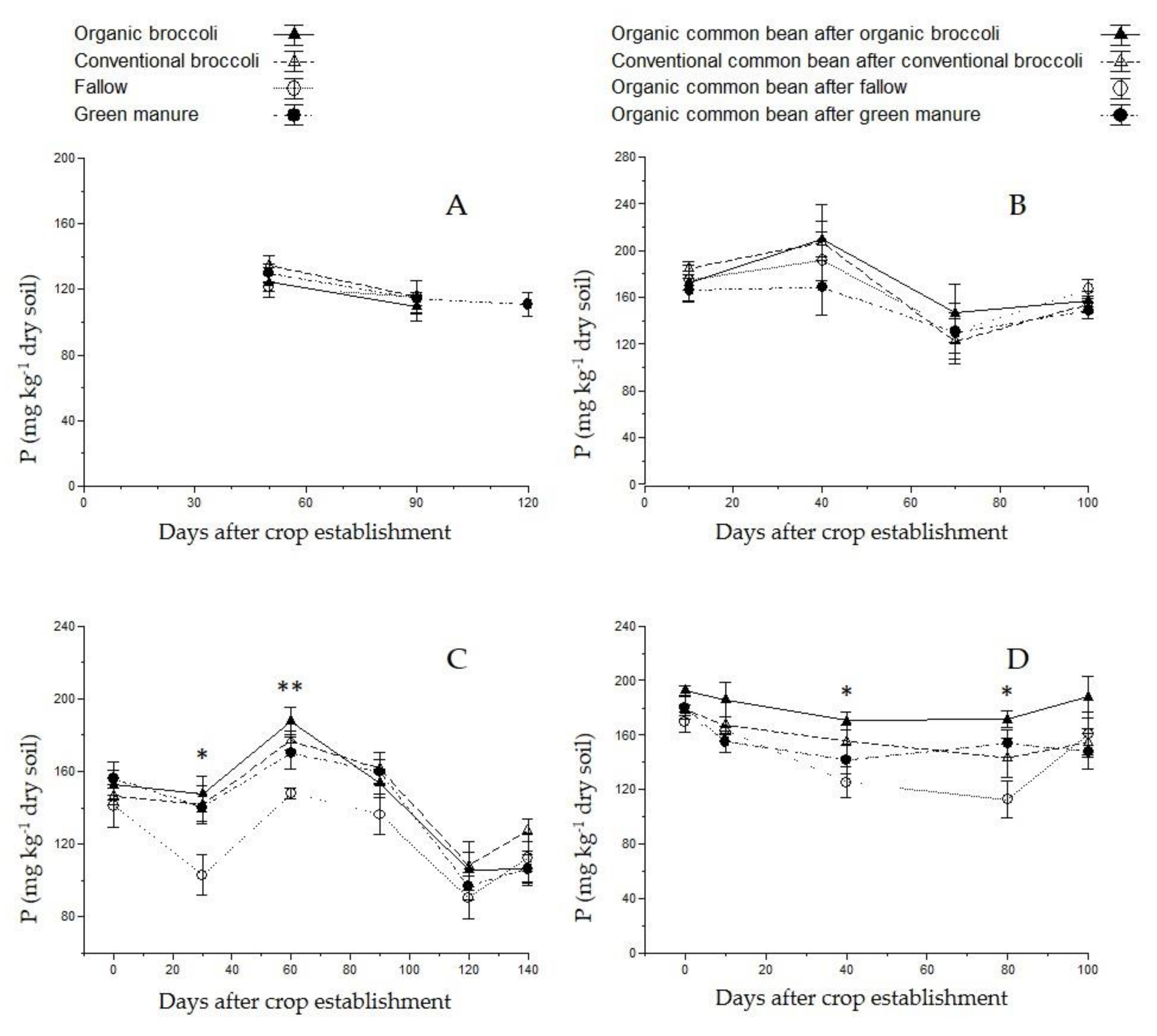
3.3. Soil Nutrient Content
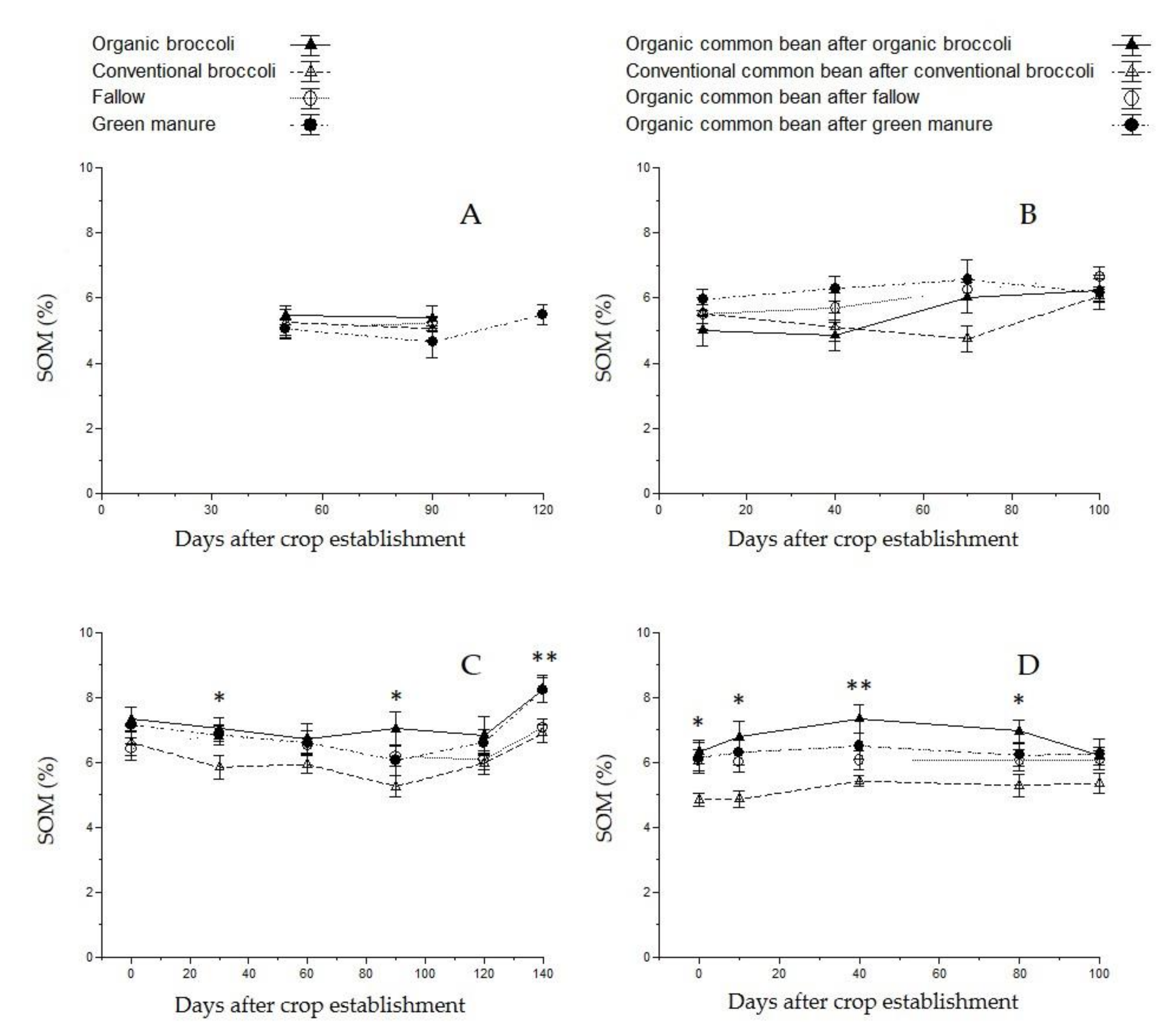
3.4. Soil Organic Matter
3.5. Nitrogen Content and BNF Activity of Legumes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lynch, D.H.; MacRae, R.; Martin, R.C. The Carbon and Global Warming Potential Impacts of Organic Farming: Does It Have a Significant Role in an Energy Constrained World? Sustainability 2011, 3, 322–362. [Google Scholar] [CrossRef]
- Lockeretz, W.; Shearer, G.; Kohl, D.H. Organic Farming in the Corn Belt. Science 1981, 211, 540–547. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Report and Recommendations on Organic Farming; U.S. Government Publishing Office: Washington, DC, USA, 1980; p. 94. [Google Scholar]
- CAST. Organic and conventional farming compared. Council Agric. Sci. Tech. 1980, 84, 32. [Google Scholar]
- Boeringa, R. Alternative methods of agriculture. Agric. Environ. 1980, 5, 1–199. [Google Scholar]
- Bezdicek, D.F.; Power, J.F.; Keeney, D.R.; Wright, M.J. Organic Farming: Current Technology and its Role in a Sustainable Agriculture. Am. Soc. Agron. 1984, 46, 192. [Google Scholar] [CrossRef]
- Connor, D.J.; Mínguez, M.I. Evolution not revolution of farming systems will best feed and green the world. Glob. Food Secur. 2012, 1, 106–113. [Google Scholar] [CrossRef]
- Emsley, J. Going one better than nature? Nature. 2001, 410, 633–634. [Google Scholar] [CrossRef]
- Trewavas, A. Urban myths of organic farming. Nature 2001, 410, 409–410. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Berentsen, P.B.M.; Giesen, G.W.J.; Schneiders, M.M.F.H. Conversion from Conventional to Biological Dairy Farming: Economic and Environmental Consequences at Farm Level. Biol. Agric. Hortic. 1998, 16, 311–328. [Google Scholar] [CrossRef]
- Torstensson, G. Nitrogen Delivery and Utilization by Subsequent Crops after Incorporation of Leys with Different Plant Composition. Biol. Agric. Hortic. 1998, 16, 129–143. [Google Scholar] [CrossRef]
- Berry, P.M.; Stockdale, E.A.; Sylvester-Bradley, R.; Philipps, L.; Smith, K.A.; Lord, E.I.; Watson, C.A.; Fortune, S. N, P and K budgets for crop rotations on nine organic farms in the UK. Soil Use Manag. 2006, 19, 112–118. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2006, 88, 97–185. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Denton, M.D.; Pearce, D.J.; Peoples, M.B. Nitrogen contributions from faba bean (Vicia faba L.) reliant on soil rhizobia or inoculation. Plant Soil 2012, 365, 363–374. [Google Scholar] [CrossRef]
- Herridge, D.F.; Robertson, M.J.; Cocks, B.; Peoples, M.B.; Holland, J.F.; Heuke, L. Low nodulation and nitrogen fixation of mungbean reduce biomass and grain yields. Aust. J. Exp. Agric. 2005, 45, 269. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant Activity in Common Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Sgarbieri, V.C.; Whitaker, J.R. Physical, Chemical, and Nutritional Properties of Common Bean (Phaseolus) Proteins. Adv. Food Res. 1982, 93–166. [Google Scholar] [CrossRef]
- Ntatsi, G.; Gutiérrez-Cortines, M.E.; Karapanos, I.; Barros, A.; Weiss, J.; Balliu, A.; Rosa, E.A. dos S.; Savvas, D. The quality of leguminous vegetables as influenced by preharvest factors. Sci. Hortic. 2018, 232, 191–205. [Google Scholar] [CrossRef]
- Graham, P.H. Some problems of nodulation and symbiotic nitrogen fixation in Phaseolus vulgaris L.: A review. Field Crops Res. 1981, 4, 93–112. [Google Scholar] [CrossRef]
- Martínez-Romero, E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: Overview and perspectives. Plant Soil 2003, 252, 11–23. [Google Scholar] [CrossRef]
- Oberson, A.; Frossard, E.; Bühlmann, C.; Mayer, J.; Mäder, P.; Lüscher, A. Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant Soil 2013, 371, 237–255. [Google Scholar] [CrossRef]
- Khan, M.K.; Yoshida, T. Nitrogen fixation in peanut at various concentrations of15N-Urea and slow release 15N-fertilizer. Soil Sci. Plant Nutr. 1995, 41, 55–63. [Google Scholar] [CrossRef]
- Leidi, E.O.; Rodriguez-Navaro, D.N. Nitrogen and phosphorus availability limit N2 fixation in bean. New Phytol. 2000, 147, 337–346. [Google Scholar] [CrossRef]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Olle, M.; Travlos, E.; Thanopoulos, R.; Bilalis, D.; Bebeli, P.; Savvas, D. Impact of variety and farming practices on growth, yield, weed flora and symbiotic nitrogen fixation in faba bean cultivated for fresh seed production. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 619–630. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic farms. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Junk, G.; Svec, H.J. The absolute abundance of the nitrogen isotopes in the atmosphere and compressed gas from various sources. Geochim. Cosmochim. Acta 1958, 14, 234–243. [Google Scholar] [CrossRef]
- Bedard-Haughn, A.; van Groenigen, J.W.; van Kessel, C. Tracing 15N through landscapes: Potential uses and precautions. J. Hydrol. 2003, 272, 175–190. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Herridge, D.F.; Peoples, M.B.; Cadish, G.; Boddey, R.; Giller, K.; Alves, B.J.R.; Chalk, P.M. Measuring plant associated nitrogen fixation in agricultural systems. ACIAR 2008, 136, 258. [Google Scholar]
- Collino, D.J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.; Racca, R.W. Biological nitrogen fixation in soybean in Argentina: Relationships with crop, soil, and meteorological factors. Plant Soil 2015, 392, 239–252. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2006, 18, 248–255. [Google Scholar] [CrossRef]
- De Ponti, T.; Rijk, B.; van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Bullock, D.G. Crop rotation. Crit. Rev. Plant Sci. 1992, 11, 309–326. [Google Scholar] [CrossRef]
- Martini, E.A.; Buyer, J.S.; Bryant, D.C.; Hartz, T.K.; Denison, R.F. Yield increases during the organic transition: Improving soil quality or increasing experience? Field Crops Res. 2004, 86, 255–266. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Turan, M.; Dursun, A.; Goktepe, F. Growth, Nutrient Uptake, and Yield Promotion of Broccoli by Plant Growth Promoting Rhizobacteria with Manure. HortScience 2011, 46, 932–936. [Google Scholar] [CrossRef]
- Øvsthus, I.; Breland, T.A.; Hagen, S.F.; Brandt, K.; Wold, A.-B.; Bengtsson, G.B.; Seljåsen, R. Effects of Organic and Waste-Derived Fertilizers on Yield, Nitrogen and Glucosinolate Contents, and Sensory Quality of Broccoli (Brassica oleraceaL. var.italica). J. Agric. Food Chem. 2015, 63, 10757–10767. [Google Scholar] [CrossRef]
- Kontopoulou, C.K.; Bilalis, D.; Pappa, V.A.; Rees, R.M.; Savvas, D. Effects of organic farming practices and salinity on yield and greenhouse gas emissions from a common bean crop. Sci. Hortic. 2015, 183, 48–57. [Google Scholar] [CrossRef]
- Rochester, I.J.; Peoples, M.B.; Hulugalle, N.R.; Gault, R.R.; Constable, G.A. Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crops Res. 2001, 70, 27–41. [Google Scholar] [CrossRef]
- Chalk, P.M. Dynamics of biologically fixed N in legume-cereal rotations: A review. Aust. J. Agric. Res. 1998, 49, 303–316. [Google Scholar] [CrossRef]
- Wani, S.P.; McGill, W.B.; Haugen-Kozyra, K.L.; Robertson, J.A.; Thurston, J.J. Improved soil quality and barley yields with fababeans, manure, forages and crop rotation on a Gray Luvisol. Can. J. Soil Sci. 1994, 74, 75–84. [Google Scholar] [CrossRef]
- Badr El-Din, S.M.S.; Moawad, H. Enhancement of nitrogen fixation in lentil, faba bean, and soybean by dual inoculation with Rhizobia and mycorrhizae. Plant Soil 1988, 108, 117–123. [Google Scholar] [CrossRef]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Giannakou, I.; Savvas, D. Nitrogen Nutrition Optimization in Organic Greenhouse Tomato Through the Use of Legume Plants as Green Manure or Intercrops. Agronomy 2019, 9, 766. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Crispino, C.C.; Moraes, J.Z.; Sibaldelli, R.N.R.; Mendes, I.C.; Arihara, J. Nitrogen nutrition of soybean in Brazil: Contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 2006, 86, 927–939. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop Residues and Management Practices: Effects on Soil Quality, Soil Nitrogen Dynamics, Crop Yield, and Nitrogen Recovery. Adv. Agron. 1999, 68, 197–319. [Google Scholar] [CrossRef]
- Parr, J.F.; Hornick, S.B.; Papendick, R.I. Soil Quality: The Foundation of a Sustainable Agriculture; U.S. Department of Agriculture: Washington, DC, USA, 1994; pp. 73–79. [Google Scholar]
- Bremer, E.; van Kessel, C. Seasonal Microbial Biomass Dynamics after Addition of Lentil and Wheat Residues. Soil Sci. Soc. Am. J. 1992, 56, 1141–1146. [Google Scholar] [CrossRef]
- Patriquin, D.G. Biological husbandry and thenitrogen problem. Biol. Agric. Horticulture 1986, 3, 167–189. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol. Fertil. Soils 2003, 39, 88–93. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Agtunong, T.; Redden, R.; Mangge-Nang, M.; Searle, C.; Fukai, S. Genotypic variation in response to high temperature at flowering in common bean (Phaseolus vulgaris L.). Aust. J. Exp. Agric. 1992, 32, 1135–1140. [Google Scholar] [CrossRef]
- DiPaola, J.M.; Beard, J.B. Physiological Effects of Temperature Stress. Am. Soc. Agron. 1992, 32, 231–267. [Google Scholar] [CrossRef]
- Bassirirad, H.; Caldwel, M.M.; Bilbrough, C. Effects of soil temperature and nitrogen status on kinetics of 15NO3- uptake by roots of field-grown Agropyron desertorum (Fisch. ex Link) Schult. New Phytol. 1993, 123, 485–489. [Google Scholar] [CrossRef]
- Sims, J.T.; Edwards, A.C.; Schoumans, O.F.; Simard, R.R. Integrating Soil Phosphorus Testing into Environmentally Based Agricultural Management Practices. J. Environ. Qual. 2000, 29, 60–71. [Google Scholar] [CrossRef]
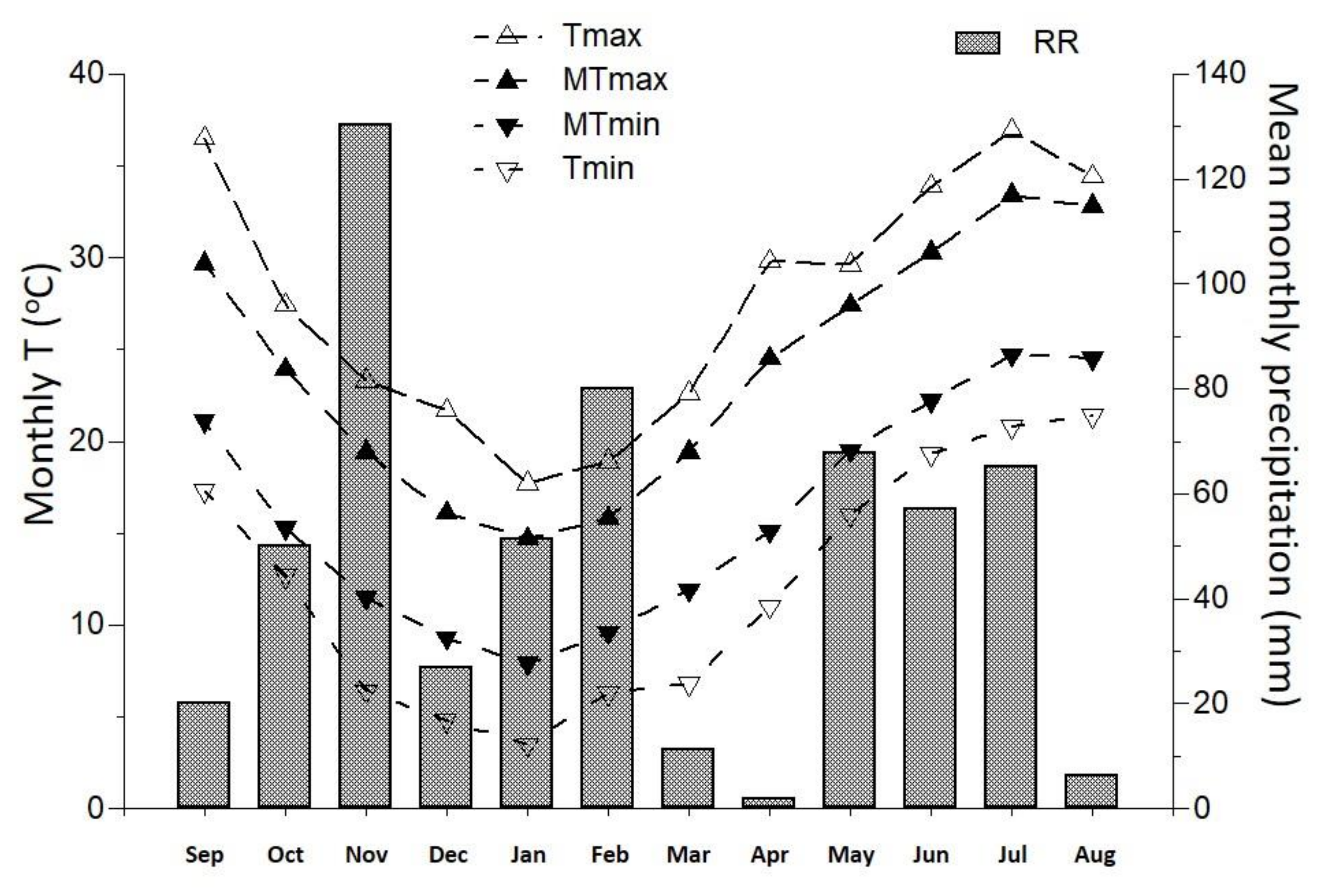



| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Clay | 20% | Total N | 0.2% |
| Silt | 14% | Available P | 153.5 mg kg−1 |
| Sand | 66% | Exchangeable K | 478 mg kg−1 |
| pH | 7.7 | Organic matter | 5% |
| Electrical conductivity | 710 μS cm−1 | Total CaCO3 | 15.98% |
| Winter Cultivation Period | Summer Cultivation Period | |||
|---|---|---|---|---|
| Treatments | Replicates | Treatments | Replicates | |
| Organic broccoli (1) | 8 | Inoculated | Organic common bean | 4 |
| Non-inoculated | 4 | |||
| Conventional broccoli (2) | 8 | Inoculated | Conventional common bean | 4 |
| Non-inoculated | 4 | |||
| Faba bean Inoculated (3a) | 4 | Inoculated | Organic common bean | 4 |
| Faba bean non-inoculated (3b) | 4 | Non-inoculated | 4 | |
| Fallow (4) | 8 | Inoculated | Organic common bean | 4 |
| Non-inoculated | 4 | |||
| 1st Year | 2nd Year | |
|---|---|---|
| Pre-crop treatment | g plant−1 | g plant−1 |
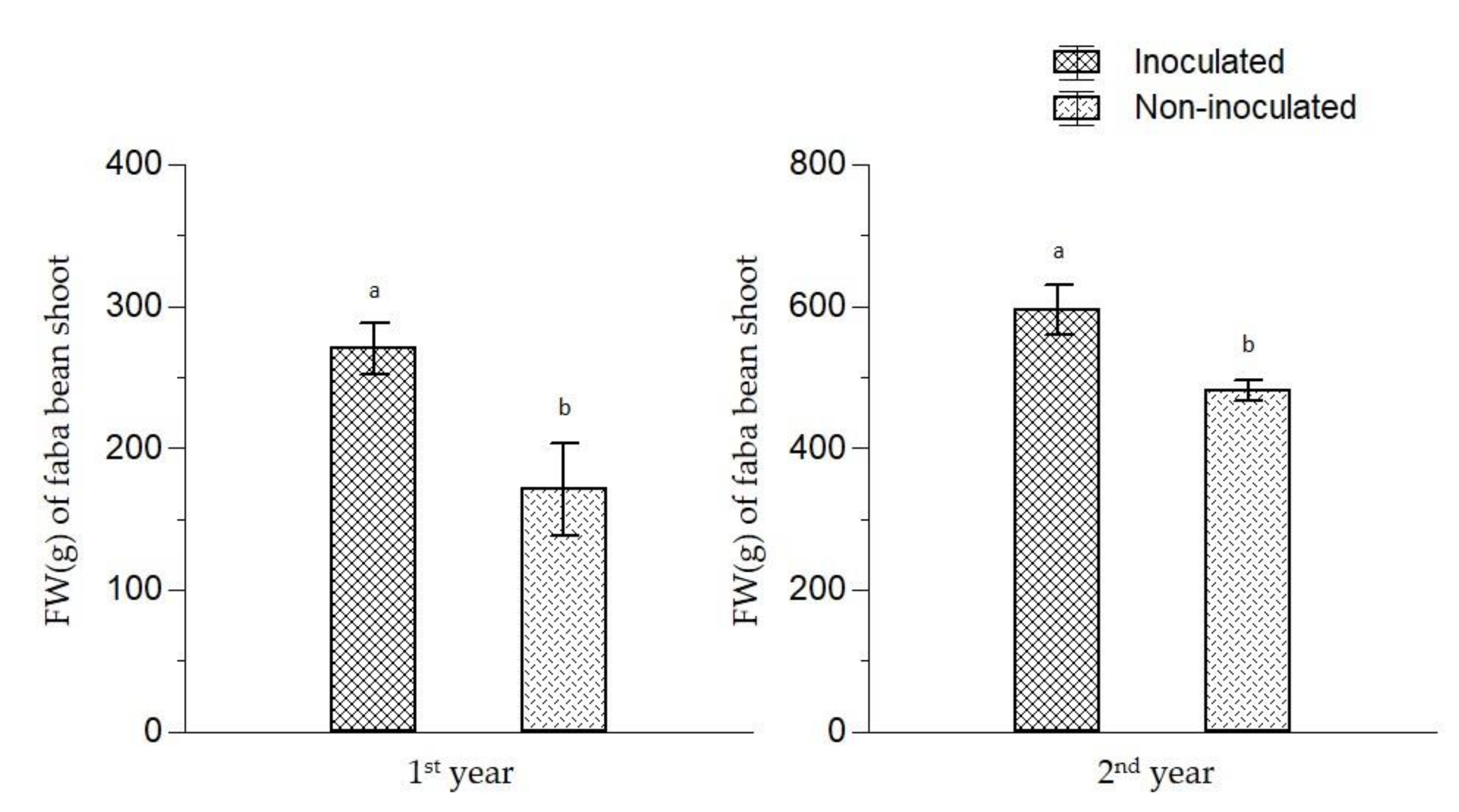
| Organic broccoli | 268 b | 142 bc |
| Conventional broccoli | 408 a | 167 ab |
| Fallow | 224 b | 117 c |
| Green manure | 296 b | 185 a |
| Inoculation with rhizobia | ||
| Inoculated | 344 | 151 |
| Non-inoculated | 253 | 154 |
| Statistical significance | ||
| Pre-crop | F = 9.036, p = 0.0003 | F = 4.43, p = 0.007 |
| Inoculation | F = 12.152, p = 0.002 | ns |
| Pre-crop×inoculation | ns | ns |
| Yield Characteristics of Common Bean | ||||
|---|---|---|---|---|
| Main Effects | ||||
| 1st Year | 2nd Year | |||
| TFW of pods (t ha−1) | Pods (No 105 ha−1) | TFW of pods (t ha−1) | Pods (No 105 ha) | |
| Pre-crop | ||||
| Organic broccoli | 42.36 b | 45.95 | 26.70 b | 27.74 b |
| Conventional broccoli | 52.84 a | 51.96 | 33.70 a | 34.06 a |
| Fallow | 47.40 ab | 47.15 | 29.74 ab | 30.44 ab |
| Green manure | 49.67 a | 50.57 | 32.07 a | 32.27 a |
| Inoculation with rhizobia | ||||
| Inoculated | 48.11 | 48.73 | 31.58 | 32.20 |
| Non-inoculated | 48.03 | 49.08 | 29.53 | 30.13 |
| Statistical significance | ||||
| Pre-crop | F = 5.274 p = 0.006 | ns | F = 4.173 p = 0.016 | F = 3.359 p = 0.035 |
| Inoculation | ns | ns | ns | ns |
| Pre-crop × Inoculation | ns | ns | ns | ns |
| Treatment | C/N | Ν (%) | Ndfa (%) | Dry Biomass (t ha−1) | BNF (kg ha−1) |
|---|---|---|---|---|---|
| 1st Experimental year (2017–2018) | |||||
| Inoculated | 11.43 | 3.66 | 86.00 | 8.08 | 255 |
| Non-inoculated | 10.36 | 3.93 | 75.9 | 5.09 | 152 |
| Statistical significance | ns | ns | F = 23.474 p = 0.002 | F = 9.279 p = 0.022 | F = 7.7454 p = 0.032 |
| 2nd Experimental year (2018–2019) | |||||
| Inoculated | 9.58 | 4.7 | 76.3 | 21.79 | 780 |
| Non-inoculated | 8.65 | 4.2 | 69.7 | 17.51 | 514 |
| Statistical significance | ns | ns | F = 21.43 p = 0.004 | F = 7.7481 p = 0.032 | F = 27.549 p = 0.002 |
| C/N | N (%) | Ndfa (%) | Dry biomass (t ha−1) | BNF (kg ha−1) | |
|---|---|---|---|---|---|
| Pre-crop | |||||
| Organic broccoli | 10.99 | 3.62 | 12.61 ab | 2.49 b | 11.52 bc |
| Conventional broccoli | 10.61 | 3.77 | 18.53 a | 3.46 a | 24.13 a |
| Fallow | 11.86 | 3.37 | 9.61 b | 2.08 b | 7.18 c |
| Green Manure | 10.63 | 3.77 | 18.47 a | 2.40 b | 17.89 ab |
| Inoculation with rhizobia | |||||
| Inoculated | 10.64 | 3.73 | 16.99 | 2.80 | 18.52 |
| Non-inoculated | 11.41 | 3.54 | 12.62 | 2.42 | 11.83 |
| Statistical significance | |||||
| Pre-crop | ns | ns | F = 4.419 p = 0.014 | F = 6.558 p = 0.002 | F = 7.931 p = 0.0007 |
| Inoculation | ns | ns | F = 4.264 p = 0.049 | ns | F = 6.470 p = 0.019 |
| Pre-crop × Inoculation | ns | ns | ns | ns | ns |
| C/N | N (%) | Ndfa (%) | Dry Biomass (t ha−1) | BNF (kg ha−1) | |
|---|---|---|---|---|---|
| Pre-crop | |||||
| Organic broccoli | 14.41 | 2.78 | 34.94 a | 1.41 bc | 12.62 |
| Conventional broccoli | 13.99 | 2.90 | 28.63 ab | 1.65 ab | 13.85 |
| Fallow | 14.36 | 2.81 | 40.69 a | 1.20 c | 13.80 |
| Green manure | 13.83 | 2.90 | 21.18 b | 1.82 a | 11.23 |
| Inoculation with rhizobia | |||||
| Inoculated | 14.25 | 2.82 | 32.28 | 1.56 | 14.00 |
| Non-inoculated | 14.05 | 2.87 | 30.44 | 1.48 | 11.78 |
| Statistical significance | |||||
| Pre-crop | ns | ns | F = 4.176 p = 0.009 | F = 4.462 p = 0.007 | ns |
| Inoculation | ns | ns | ns | ns | ns |
| Pre-crop × Inoculation | ns | ns | ns | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karavidas, I.; Ntatsi, G.; Ntanasi, T.; Vlachos, I.; Tampakaki, A.; Iannetta, P.P.M.; Savvas, D. Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production. Agronomy 2020, 10, 1269. https://doi.org/10.3390/agronomy10091269
Karavidas I, Ntatsi G, Ntanasi T, Vlachos I, Tampakaki A, Iannetta PPM, Savvas D. Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production. Agronomy. 2020; 10(9):1269. https://doi.org/10.3390/agronomy10091269
Chicago/Turabian StyleKaravidas, Ioannis, Georgia Ntatsi, Theodora Ntanasi, Ioannis Vlachos, Anastasia Tampakaki, Pietro P. M. Iannetta, and Dimitrios Savvas. 2020. "Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production" Agronomy 10, no. 9: 1269. https://doi.org/10.3390/agronomy10091269
APA StyleKaravidas, I., Ntatsi, G., Ntanasi, T., Vlachos, I., Tampakaki, A., Iannetta, P. P. M., & Savvas, D. (2020). Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production. Agronomy, 10(9), 1269. https://doi.org/10.3390/agronomy10091269









