Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation
Abstract
1. Introduction
2. Materials and Methods
2.1. Population of Study
2.2. Collection and Processing of Breast Milk Samples
2.3. Breast Milk Antioxidants and Oxidative Damage Biomarkers
2.3.1. Total Antioxidant Capacity
2.3.2. Thiol Groups
2.3.3. Catalase Activity
2.3.4. Superoxide Dismutase (SOD)
2.3.5. Melatonin
2.3.6. Lipid Peroxidation
2.3.7. Protein Oxidation
2.4. Statistical Analysis
3. Results
3.1. Differences in Breast Milk Antioxidants and Oxidative Damage Biomarkers at Days 7, 14 and 28 of Lactation
3.2. Association between Maternal Age, Breast Milk Antioxidants and Oxidative Damage Biomarkers
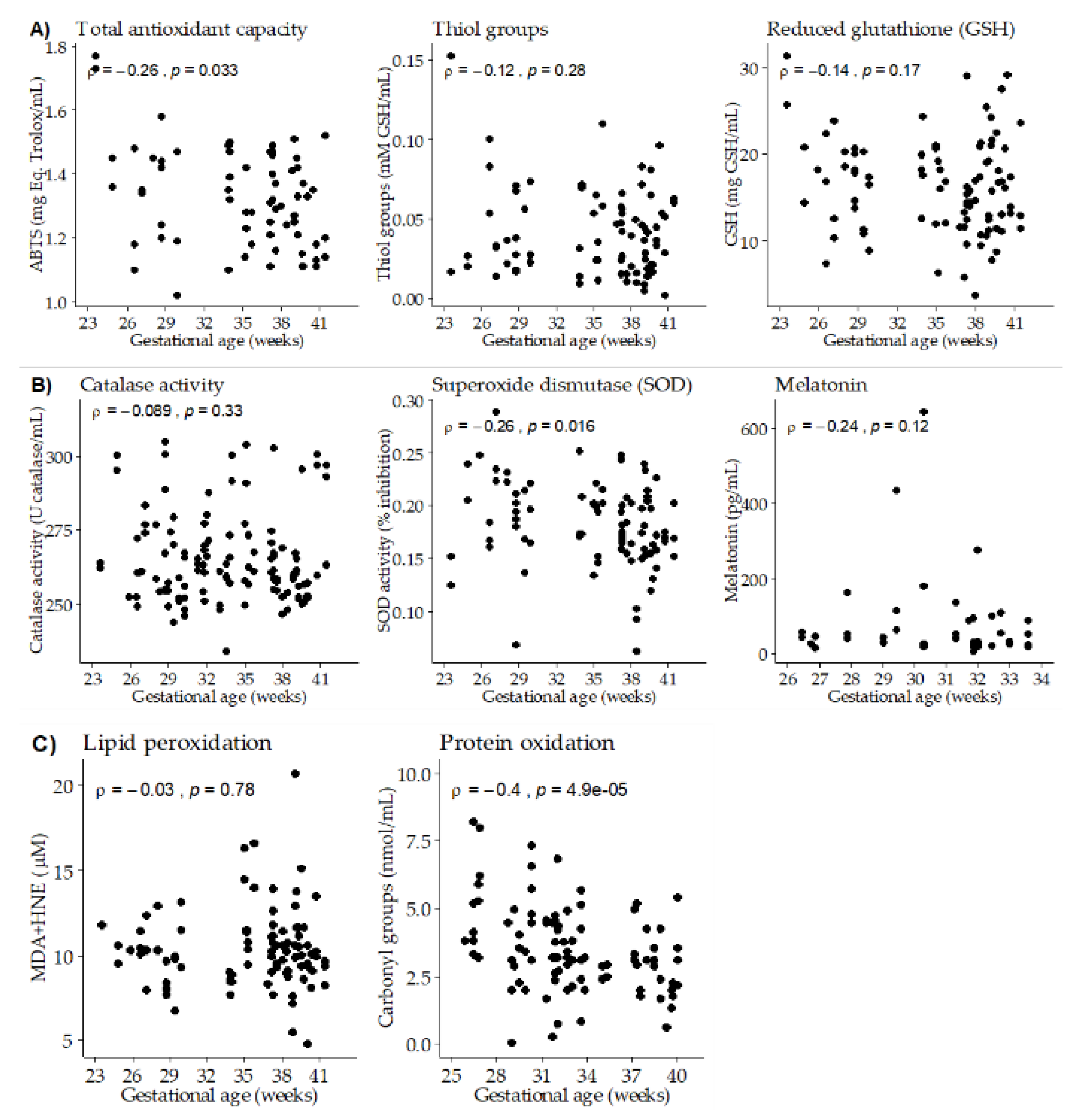
3.3. Association between Gestational Age, Breast Milk Antioxidants and Oxidative Damage Biomarkers
3.4. Linear Regression Models with Mixed Random Effects
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, J.; Hector, D. Benefits of breastfeeding. N. S. W. Public Health Bull. 2005, 16, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Benetti, S.; Liguori, S.A.; Sorrenti, M.; Cordero Di Montezemolo, L. Advances on human milk hormones and protection against obesity. Cell. Mol. Biol. 2013, 59, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martín-Cabrejas, M.A.; López de Pablo, Á.L.; Sáenz de Pipaón, M.; Ramiro-Cortijo, D. A review of bioactive factors in human breastmilk: A focus on prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Tudehope, D.I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 2013, 162, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Heal. 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Mitanchez, D.; Buffat, C.; Ligi, I.; Grandvuillemin, I.; Boubred, F.; Simeoni, U. Oxidative stress after preterm birth: Origins, biomarkers, and possible therapeutic approaches. Arch. Pediatr. 2015, 22, 1047–1055. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ozsurekci, Y.; Aykac, K. Oxidative Stress Related Diseases in Newborns. Oxid. Med. Cell. Longev. 2016, 2016, 2768365. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Bracciali, C.; Di Virgilio, N.; Buonocore, G. Oxygen Use in Neonatal Care: A Two-edged Sword. Front. Pediatr. 2017, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Shimizu, T. Effect of human breast milk on biological metabolism in infants. Pediatr. Int. 2019, 61, 6–15. [Google Scholar] [CrossRef]
- Turhan, A.H.; Atici, A.; Muslu, N. Antioxidant capacity of breast milk of mothers who delivered prematurely is higher than that of mothers who delivered at term. Int. J. Vitam. Nutr. Res. 2011, 81, 368–371. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, M.C.; Linde, J.; Bompadre, S.; Battino, M.; Narbona, E.; Maldonado, J.; Mataix, J. Coenzyme Q concentration and total antioxidant capacity of human milk at different stages of lactation in mothers of preterm and full-term infants. Free Radic. Res. 2006, 40, 199–206. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bagci, S. Melatonin Concentrations and Antioxidative Capacity of Human Breast Milk According to Gestational Age and the Time of Day. J. Hum. Lact. 2016, 32, 105–110. [Google Scholar] [CrossRef]
- Xavier, A.M.; Rai, K.; Hegde, A.M. Total antioxidant concentrations of breastmilk-an eye-opener to the negligent. J. Heal. Popul. Nutr. 2011, 29, 605–611. [Google Scholar] [CrossRef]
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), 5–8. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Tomeleri, C.M.; Fernandes, R.R.; Sugihara Junior, P.; Venturini, D.; Barbosa, D.S.; Deminice, R.; Sardinha, L.B.; Cyrino, E.S. Effects of pre- or post-exercise whey protein supplementation on oxidative stress and antioxidant enzymes in older women. Scand. J. Med. Sci. Sport. 2019, 29, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sinet, P.M. Metabolism of Oxygen Derivatives in Down’s Syndrome. Ann. N. Y. Acad. Sci. 1982, 396, 83–94. [Google Scholar] [CrossRef]
- Rodríguez-Sureda, V.; Vilches, Á.; Sánchez, O.; Audí, L.; Domínguez, C. Intracellular oxidant activity, antioxidant enzyme defense system, and cell senescence in fibroblasts with trisomy 21. Oxid. Med. Cell Longev. 2015, 2015, 509241. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Cortijo, D.; Herrera, T.; Rodríguez-Rodríguez, P.; De Pablo, Á.L.L.; De La Calle, M.; López-Giménez, M.R.; Mora-Urda, A.I.; Gutiérrez-Arzapalo, P.Y.; Gómez-Rioja, R.; Aguilera, Y.; et al. Maternal plasma antioxidant status in the first trimester of pregnancy and development of obstetric complications. Placenta 2016, 47, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, N.A.; Appiah-Opong, R.; Dzokoto, C. Human breastmilk storage and the glutathione content. J. Trop. Pediatr. 2000, 46, 111–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; de la Calle, M.; Rodríguez-Rodríguez, P.; López de Pablo, Á.L.; López-Giménez, M.R.; Aguilera, Y.; Martín-Cabrejas, M.A.; González, M.C.; Arribas, S.M. Maternal antioxidant status in early pregnancy and development of fetal complications in twin pregnancies: A pilot study. Antioxidants 2020, 9, 269. [Google Scholar] [CrossRef]
- Ledo, A.; Arduini, A.; Asensi, M.A.; Sastre, J.; Escrig, R.; Brugada, M.; Aguar, M.; Saenz, P.; Vento, M. Human milk enhances antioxidant defenses against hydroxyl radical aggression in preterm infants. Am. J. Clin. Nutr. 2009, 89, 210–215. [Google Scholar] [CrossRef]
- Chen, Y.; Fantuzzi, G.; Schoeny, M.; Meier, P.; Patel, A.L. High-Dose Human Milk Feedings Decrease Oxidative Stress in Premature Infant. J. Parenter. Enter. Nutr. 2019, 43, 126–132. [Google Scholar] [CrossRef]
- Koletzko, B.; Sauerwald, U.; Keicher, U.; Saule, H.; Wawatschek, S.; Böhles, H.; Bervoets, K.; Fleith, M.; Crozier-Willi, G. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diets without or with long-chain polyunsaturated fatty acids: A randomized clinical trial. Eur. J. Nutr. 2003, 42, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Ferreira, C.H.F.; Shifrin, Y.; Pan, J.; Belik, J. Human milk H2O2 content: Does it benefit preterm infants? Pediatr. Res. 2018, 83, 687–692. [Google Scholar] [CrossRef]
- Castillo-Castañeda, P.C.; García-González, A.; Bencomo-Alvarez, A.E.; Barros-Nuñez, P.; Gaxiola-Robles, R.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Micronutrient content and antioxidant enzyme activities in human breast milk. J. Trace Elem. Med. Biol. 2019, 51, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Sahin, S.; Ozdemir, T.; Katipoglu, N.; Akcan, A.B.; Kaynak Turkmen, M. Comparison of Changes in Breast Milk Macronutrient Content during the First Month in Preterm and Term Infants. Breastfeed. Med. 2020, 15, 56–62. [Google Scholar] [CrossRef]
- Zarban, A.; Taheri, F.; Chahkandi, T.; Sharifzadeh, G.; Khorashadizadeh, M. Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. J. Clin. Biochem. Nutr. 2009, 45, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.R.; Sadeghi, N.; Jannat, B.; Hajimahmoodi, M.; Behfar, A.O.A.; Jannat, F.; Nasab, F.M. Human breast milk provides better antioxidant capacity than infant formula. Iran. J. Pharm. Res. 2010, 9, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shi, W.; Zhuang, J.; Liu, Y.; Tang, L.; Bu, J.; Sun, J.; Bei, F. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. 2019, 9, 17984. [Google Scholar] [CrossRef]
- Hunter, L.P.; Rychnovsky, J.D.; Yount, S.M. A selective review of maternal sleep characteristics in the postpartum period. J Obs. Gynecol. Neonatal Nurs. 2009, 38, 60–68. [Google Scholar] [CrossRef]
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and antioxidant status of human breast milk during lactation period. Dairy Sci. Technol. 2015, 95, 295–302. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast milk lipids and fatty acids in regulating neonatal intestinal development and protecting against intestinal injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [PubMed]
- Al-Kerwi, E.A.A.; Al-Hashimi, A.H.M.; Salman, A.M. Mother’s milk and hydrogen peroxide. Asia Pac. J. Clin. Nutr. 2005, 14, 428–431. [Google Scholar] [PubMed]
- Mimouni, F.B.; Lubetzky, R.; Yochpaz, S.; Mandel, D. Preterm Human Milk Macronutrient and Energy Composition: A Systematic Review and Meta-Analysis. Clin. Perinatol. 2017, 44, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fleischer Michaelsen, K.; Skafte, L.; Badsberg, J.H.; Jørgensen, M. Variation in macronutrients in human bank milk: Influencing factors and implications for human milk banking. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 229–239. [Google Scholar] [CrossRef]
- L’Abbe, M.R.; Friel, J.K. Superoxide dismutase and glutathione peroxidase content of human milk from mothers of premature and full-term infants during the first 3 months of lactation. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 270–274. [Google Scholar] [CrossRef]
- Ellis, L.; Picciano, M.F.; Smith, A.M.; Hamosh, M.; Mehta, N.R. The impact of gestational length on human milk selenium concentration and glutathione peroxidase activity. Pediatr. Res. 1990, 27, 32–35. [Google Scholar] [CrossRef]
- Boyce, C.; Watson, M.; Lazidis, G.; Reeve, S.; Dods, K.; Simmer, K.; McLeod, G. Preterm human milk composition: A systematic literature review. Br. J. Nutr. 2016, 116, 1033–1045. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, Y.; Chen, Y.; Zhang, Y.; Xiong, B. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol. 2020, 28, 101327. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Huang, Z.; Han, L.; Wu, X.; Li, L.; Xin, Y.; Ge, J.; Sha, J.; Yin, Z.; et al. Melatonin ameliorates the advanced maternal age-associated meiotic defects in oocytes through the SIRT2-dependent H4K16 deacetylation pathway. Aging (Albany. NY). 2020, 12, 1610–1623. [Google Scholar] [CrossRef]
- Vural, E.M.S.; Van Munster, B.C.; De Rooij, S.E. Optimal dosages for melatonin supplementation therapy in older adults: A systematic review of current literature. Drugs Aging 2014, 31, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.L.; Areia, A.L.; Pinto, A.M.; Donato, H. Advanced maternal age: Adverse outcomes of pregnancy, a meta-analysis. Acta Med. Port. 2019, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.C.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Pediatr. 2012, 171, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Moran-Lev, H.; Mimouni, F.B.; Ovental, A.; Mangel, L.; Mandel, D.; Lubetzky, R. Circadian macronutrients variations over the first 7 weeks of human milk feeding of preterm infants. Breastfeed. Med. 2015, 10, 366–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Huang, N.; Xiao, L.; Lin, H.; Luo, J.; Zhang, Z.; Zou, Z. Changes in breast milk lutein concentrations and their associations with dietary lutein intake: A 12-week prospective analytical study. Br. J. Nutr. 2019, 122, 1033–1039. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Hernández-Aguilar, M.T.; Navarro-Ruiz, A.; López-Jaén, A.B.; Borja-Herrero, C.; Valls-Bellés, V. Diet supplementation during early lactation with non-alcoholic beer increases the antioxidant properties of breastmilk and decreases the oxidative damage in breastfeeding mothers. Breastfeed. Med. 2013, 8, 164–169. [Google Scholar] [CrossRef]
- Machado, M.R.; Kamp, F.; Nunes, J.C.; El-Bacha, T.; Torres, A.G. Breast milk content of vitamin a and e from early-to mid-lactation is affected by inadequate dietary intake in brazilian adult women. Nutrients 2019, 11, 2025. [Google Scholar] [CrossRef]
- Păduraru, L.; Dimitriu, D.C.; Avasiloaiei, A.L.; Moscalu, M.; Zonda, G.I.; Stamatin, M. Total antioxidant status in fresh and stored human milk from mothers of term and preterm neonates. Pediatr. Neonatol. 2018, 59, 600–605. [Google Scholar] [CrossRef]
- Silvestre, D.; Miranda, M.; Muriach, M.; Almansa, I.; Jareño, E.; Romero, F.J. Frozen Breast Milk at −20 Degrees C and −80 Degrees C: A Longitudinal Study of Glutathione Peroxidase Activity and Malondialdehyde Concentration. J. Hum. Lact. 2010, 26, 35–41. [Google Scholar] [CrossRef]

| Day 7 (n = 50) | Day 14 (n = 50) | Day 28 (n = 45) | p-Value | |
|---|---|---|---|---|
| ABTS (mg Eq. Trolox/mL) | 1.45 (1.35; 1.48) | 1.30 (1.18; 1.37) | 1.20 (1.11; 1.25) | < 0.001 |
| Thiol groups (mM GSH/mL) | 0.04 (0.02; 0.06) | 0.03 (0.02; 0.06) | 0.04 (0.02; 0.06) | 0.85 |
| GSH (mg GSH/mL) | 20.2 (15.8; 22.5) | 16.2 (12.1; 18.4) | 12.2 (10.5; 15.8) | < 0.001 |
| Catalase activity (U/mL) | 261.5 (256.6; 271.1) | 261.3 (253.6; 277.0) | 262.0 (253.5; 274.7) | 0.94 |
| SOD activity (% inhibition) | 0.17 (0.15; 0.20) | 0.17 (0.16; 0.22) | 0.19 (0.17; 0.20) | 0.95 |
| Melatonin (pg/mL) | 25.9 (17.2; 49.7) | 70.7 (26.3; 129.7) | 31.3 (23.2; 51.1) | 0.07 |
| MDA + HNE (µM) | 10.6 (9.7; 12.1) | 10.3 (9.2; 11.7) | 9.3 (8.3; 10.0) | < 0.001 |
| Carbonyl groups (nmol/mL) | 3.7 (2.3; 4.2) | 3.3 (2.0; 4.0) | 3.1 (2.0; 3.1) | 0.66 |
| Maternal Age | p-Value | RE | Gestational Age | p-Value | RE | |
|---|---|---|---|---|---|---|
| ABTS (mg Eq. Trolox/mL) | 0.001 ± 0.004 | 0.78 | 0.99 | −0.008 ± 0.003 | 0.006 | 0.99 |
| GSH (mg GSH/mL) | 0.19 ± 0.12 | 0.11 | 0.35 | −0.13 ± 0.10 | 0.22 | 0.94 |
| SOD (% inhibition) | - | - | −0.002 ± 0.001 | 0.043 | 0.97 | |
| Melatonin (pg/mL) | −7.41 ± 2.50 | 0.005 | 0.86 | - | - | - |
| Carbonyl groups (nmol/mL) | - | - | −0.22 ± 0.07 | 0.001 | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gila-Díaz, A.; Herranz Carrillo, G.; Cañas, S.; Saenz de Pipaón, M.; Martínez-Orgado, J.A.; Rodríguez-Rodríguez, P.; López de Pablo, Á.L.; Martin-Cabrejas, M.A.; Ramiro-Cortijo, D.; Arribas, S.M. Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation. Nutrients 2020, 12, 2569. https://doi.org/10.3390/nu12092569
Gila-Díaz A, Herranz Carrillo G, Cañas S, Saenz de Pipaón M, Martínez-Orgado JA, Rodríguez-Rodríguez P, López de Pablo ÁL, Martin-Cabrejas MA, Ramiro-Cortijo D, Arribas SM. Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation. Nutrients. 2020; 12(9):2569. https://doi.org/10.3390/nu12092569
Chicago/Turabian StyleGila-Díaz, Andrea, Gloria Herranz Carrillo, Silvia Cañas, Miguel Saenz de Pipaón, José Antonio Martínez-Orgado, Pilar Rodríguez-Rodríguez, Ángel Luis López de Pablo, María A. Martin-Cabrejas, David Ramiro-Cortijo, and Silvia M. Arribas. 2020. "Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation" Nutrients 12, no. 9: 2569. https://doi.org/10.3390/nu12092569
APA StyleGila-Díaz, A., Herranz Carrillo, G., Cañas, S., Saenz de Pipaón, M., Martínez-Orgado, J. A., Rodríguez-Rodríguez, P., López de Pablo, Á. L., Martin-Cabrejas, M. A., Ramiro-Cortijo, D., & Arribas, S. M. (2020). Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation. Nutrients, 12(9), 2569. https://doi.org/10.3390/nu12092569










