Virus-Induced Asthma Exacerbations: SIRT1 Targeted Approach
Abstract
:1. Introduction
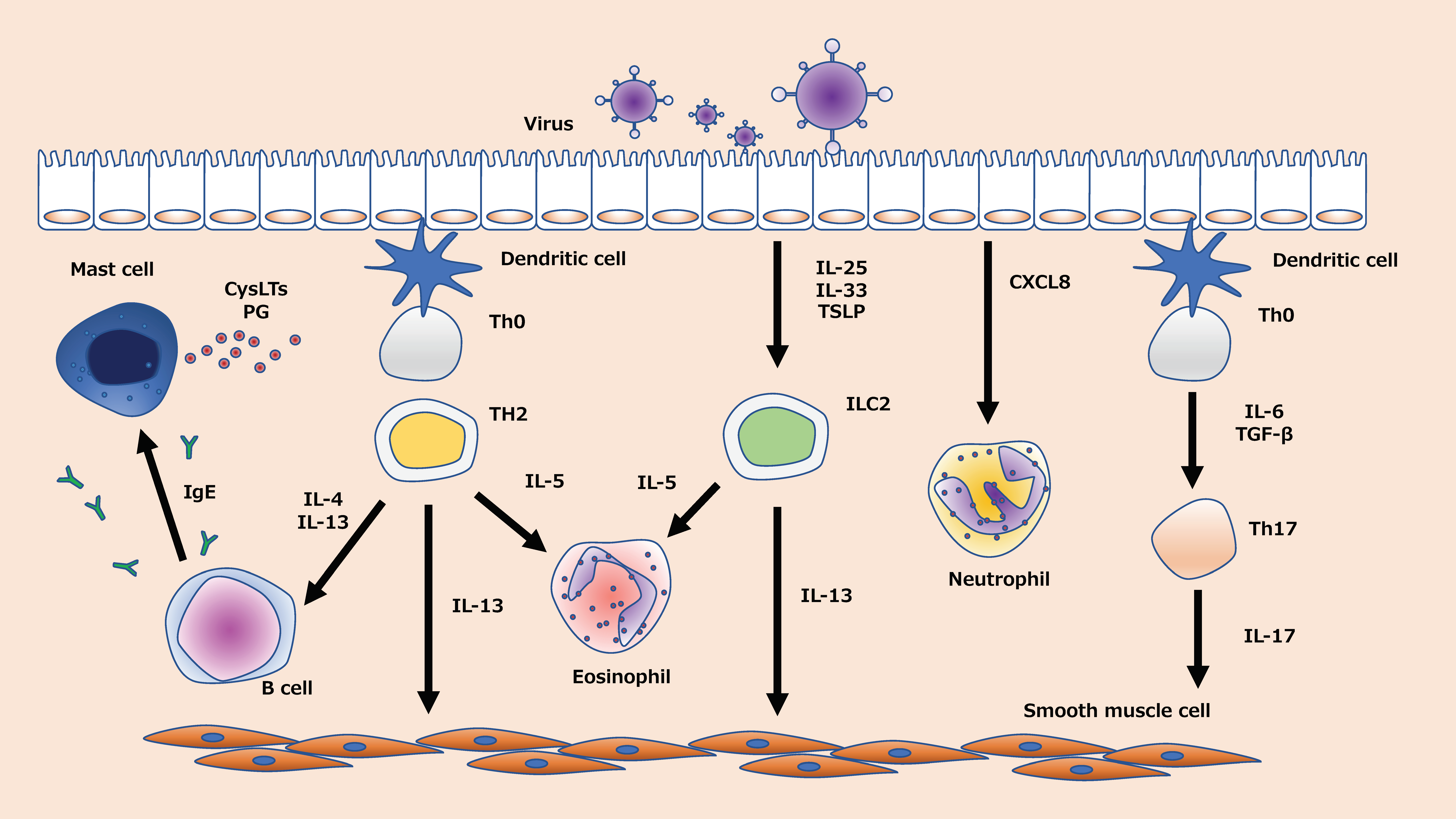
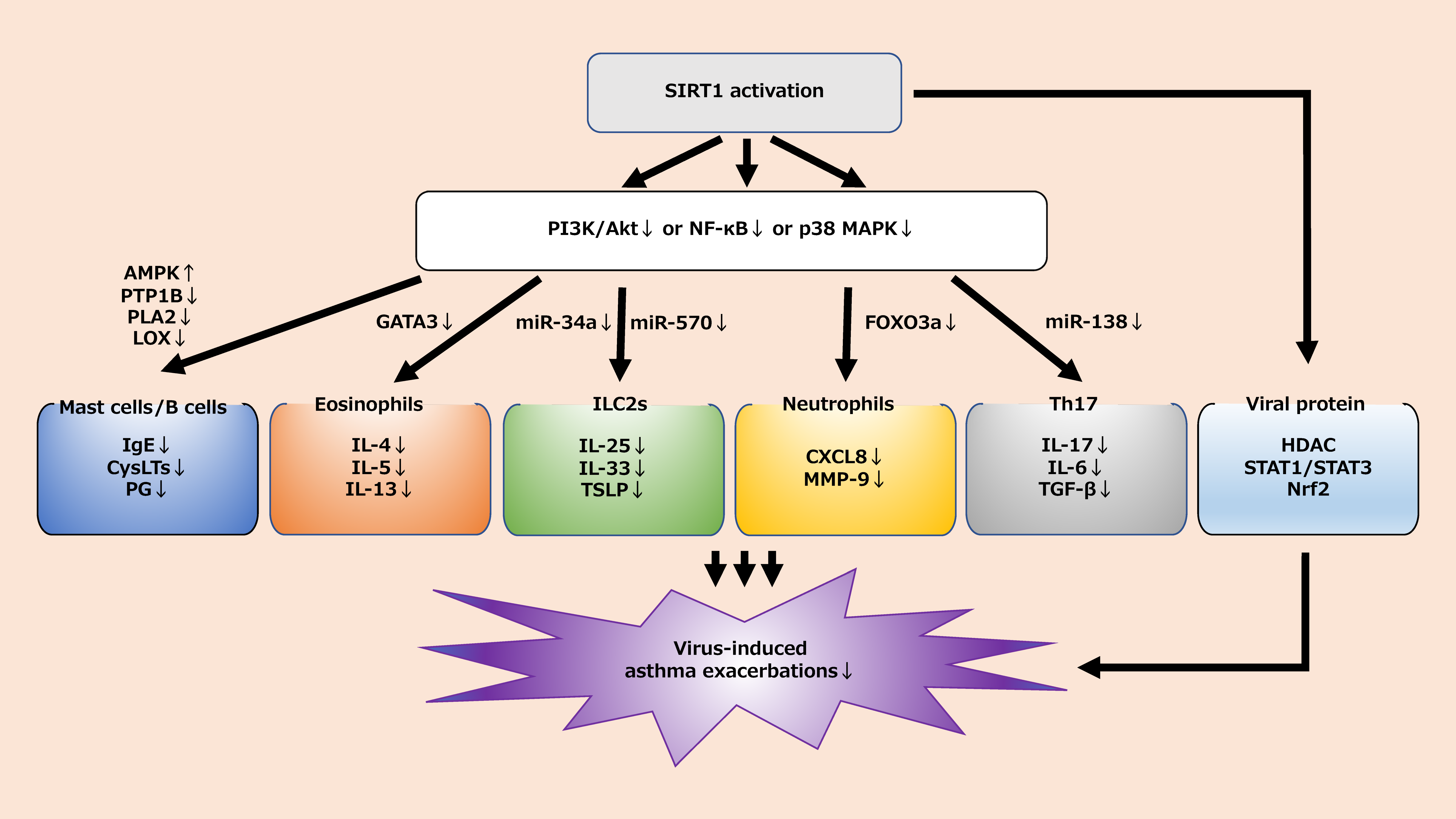
2. The Role of SIRT1 in Inflammatory Cells
2.1. Neutrophils
2.1.1. Neutrophils in Virus-Induced Asthma Exacerbations
2.1.2. The Relationship between SIRT1 and Neutrophils in Virus-Induced Asthma Exacerbations
2.2. Eosinophils
2.2.1. Eosinophils in Virus-Induced Asthma Exacerbations
2.2.2. The Relationship between SIRT1 and Eosinophils in Virus-Induced Asthma Exacerbations
2.3. Mast Cells and B Cells
2.3.1. Mast Cells and B Cells in Virus-Induced Asthma Exacerbations
2.3.2. The Relationship between SIRT1 and Mast Cells and B Cells in Virus-Induced Asthma Exacerbations
2.4. Type 2 Innate Lymphoid Cells (ILC2)
2.4.1. ILC2 in Virus-Induced Asthma Exacerbations
2.4.2. The Relationship between SIRT1 and ILC2 in Virus-Induced Asthma Exacerbations
2.5. Th17 Cells
2.5.1. Th17 Cells in Virus-Induced Asthma Exacerbations
2.5.2. The Relationship between SIRT1 and Th17 Cells in Virus-Induced Asthma Exacerbations
2.6. Viral Protein
2.6.1. Viral Protein in Virus-Induced Asthma Exacerbations
2.6.2. The Relationship between SIRT1 and Viral Proteins in Virus-Induced Asthma Exacerbations
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Eddine Aichour, M.T.; Alam, K.; et al. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez, A.; Cho, S.; Soriano, J.B.; Rosenwasser, L.J.; Rodrigo, G.J.; Rabe, K.F.; Peters, S.; Niimi, A.; Ledford, D.K.; Katial, R.; et al. Asthma in the Elderly: What We Know and What We Have yet to Know. World Allergy Organ. J. 2014, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Homma, T.; Kawahara, T.; Mikuni, H.; Uno, T.; Sato, H.; Fujiwara, A.; Uchida, Y.; Fukuda, Y.; Manabe, R.; Ida, H.; et al. Beneficial Effect of Early Intervention With Garenoxacin for Bacterial Infection-Induced Acute Exacerbation of Bronchial Asthma and Chronic Obstructive Pulmonary Disease. Int. Arch. Allergy Immunol. 2019, 178, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Homma, T.; Suzuki, S.; Takuma, T.; Tanaka, A.; Yokoe, T.; Ohnishi, T.; Niki, Y.; Sagara, H. High Burden of Aspergillus Fumigatus Infection among Chronic Respiratory Diseases. Chron. Respir. Dis. 2018, 15, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Message, S.D.; Laza-Stanca, V.; Mallia, P.; Parker, H.L.; Zhu, J.; Kebadze, T.; Contoli, M.; Sanderson, G.; Kon, O.M.; Papi, A.; et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. USA 2008, 105, 13562–13567. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Lemanske, R.F.; Evans, M.D.; Vang, F.; Pappas, T.; Gangnon, R.; Jackson, D.J.; Gern, J.E. Human Rhinovirus Species and Season of Infection Determine Illness Severity. Am. J. Respir. Crit. Care Med. 2012, 186, 886–891. [Google Scholar] [CrossRef] [Green Version]
- Hansel, T.T.; Tunstall, T.; Trujillo-torralbo, M.; Shamji, B.; Telcian, A.; Aniscenko, J.; Gogsadze, L.; Bakhsoliani, E.; Stanciu, L.; Bartlett, N.; et al. A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-γ and IFN-λ) and Type 2 in Fl Ammation (IL-5 and IL-13). EBioMedicine 2017, 19, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kloepfer, K.M.; Lee, W.M.; Pappas, T.E.; Kang, T.J.; Vrtis, R.F.; Evans, M.D.; Gangnon, R.E.; Bochkov, Y.A.; Jackson, D.J.; Lemanske, R.F., Jr.; et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014, 133, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Henderson, J.; Hilliard, T.N.; Sherriff, A.; Stalker, D.; Al Shammari, N.; Thomas, H.M. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: A longitudinal birth cohort study. Pediatr. Allergy Immunol. 2005, 16, 386–392. [Google Scholar] [CrossRef]
- Korppi, M. Respiratory Morbidity 20 Years after RSV Infection in Infancy. Pediatr. Pulmonol. 2004, 38, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Ramsahai, J.M.; Hansbro, P.M.; Wark, P.A.B. Mechanisms and Management of Asthma Exacerbations. Am. J. Respir. Crit. Care Med. 2019, 199, 423–432. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision Medicine and Phenotypes, Endotypes, Genotypes, Regiotypes, and Theratypes of Allergic Diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A. Tight Junction, Mucin, and Inflammasome—Related Molecules Are Differentially Expressed in Eosinophilic, Mixed, and Neutrophilic Experimental Asthma in Mice. Allergy 2019, 74, 294–307. [Google Scholar] [CrossRef]
- Agache, I.; Strasser, D.S.; Pierlot, G.M.; Farine, H.; Izuhara, K.; Akdis, C.A. Monitoring inflammatory heterogeneity with multiple biomarkers for multidimensional endotyping of asthma. J. Allergy Clin. Immunol. 2018, 141, 442–445. [Google Scholar] [CrossRef] [Green Version]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Homma, T.; Matsukura, S.; Hirose, T.; Ohnishi, T.; Kimura, T.; Kurokawa, M.; Ieki, K.; Odaka, M.; Suzuki, S.; Watanabe, S.; et al. Cooperative Activation of CCL5 Expression by TLR3 and Tumor Necrosis Factor-α or Interferon-γ through Nuclear Factor-ΚB or STAT-1 in Airway Epithelial Cells. Int. Arch. Allergy Immunol. 2010, 152, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Homma, T.; Fukuda, Y.; Uchida, Y.; Uno, T.; Jinno, M.; Kishino, Y.; Yamamoto, M.; Sato, H.; Akimoto, K.; Kaneko, K.; et al. Inhibition of Virus—Induced Cytokine Production from Airway Epithelial Cells by the Late Addition of Budesonide. Medicina (Kaunas) 2020, 56, 98. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Ocampo, C.J.; Stevens, W.W.; Price, C.P.E.; Thompson, C.F.; Homma, T.; Huang, J.H.; Norton, J.E.; Suh, L.A.; Kathryn, L.; et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: Possible role of the nongastric H,K-ATPase. J. Allergy Clin. Immunol. 2017, 139, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Homma, T.; Kato, A.; Bhushan, B.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Gupta, D.S.; Schleimer, R.P. Role of Aspergillus Fumigatus in Triggering Protease-Activated Receptor-2 in Airway Epithelial Cells and Skewing the Cells toward a T-Helper 2 Bias. Am. J. Respir. Cell Mol. Biol. 2016, 54, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Homma, T.; Norton, J.E.; Sha, Q.; Siebert, J.; Gupta, D.S.; Schroeder, J.W.; Schleimer, R.P. Suppression of Epithelial Signal Transducer and Activator of Transcription 1 Activation by Extracts of Aspergillus Fumigatus. Am. J. Respir. Cell Mol. Biol. 2015, 53, 87–95. [Google Scholar] [CrossRef]
- Matsukura, S.; Kurokawa, M.; Homma, T.; Watanabe, S.; Suzuki, S.; Ieki, K.; Takeuchi, H.; Notomi, K.; Schleimer, R.P.; Kawaguchi, M.; et al. Basic research on virus-induced asthma exacerbation: Inhibition of inflammatory chemokine expression by fluticasone propionate. Int. Arch. Allergy Immunol. 2013, 161, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. New Molecular Targets for the Treatment of Neutrophilic Diseases. J. Allergy Clin. Immunol. 2007, 119, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Saturni, S.; Contoli, M.; Spanevello, A.; Papi, A. Models of Respiratory Infections: Virus-Induced Asthma Exacerbations and Beyond. Allergy Asthma Immunol. Res. 2015, 7, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Skloot, G.S.; Busse, P.J.; Braman, S.S.; Kovacs, E.J.; Dixon, A.E.; Vaz Fragoso, C.A.; Scichilone, N.; Prakash, Y.S.; Pabelick, C.M.; Mathur, S.K.; et al. An Official American Thoracic Society Workshop Report: Evaluation and Management of Asthma in the Elderly. Ann. Am. Thorac. Soc. 2016, 13, 2064–2077. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Morton, J.P.; Athineos, D.; Kang, T.; Lasitschka, F.; Andrulis, M.; Pascual, G.; et al. Europe PMC Funders Group Europe PMC Funders Author Manuscripts A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Guarente, L.; Franklin, H. Epstein Lecture: Sirtuins, aging, and medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef]
- Baker, J.R.; Donnelly, L.E.; Barnes, P.J. Senotherapy. Chest 2020, 158, 562–570. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020. ahead of print. [Google Scholar] [CrossRef]
- Rahman, I.; Kinnula, V.L.; Gorbunova, V.; Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 2012, 54, S20–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Resp. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on Its Biological Functions. Cell Commun. Signal. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, M.Y.; Hull, P.A.; Fei, M.; Kwon, H.S.; Tsou, C.L.; Kasler, H.; Ng, C.P.; Gordon, D.E.; Johnson, J.; Krogan, N.; et al. Metabolic Reprogramming of Human CD8 + Memory T Cells through Loss of SIRT1. J. Exp. Med. 2018, 215, 51–62. [Google Scholar] [CrossRef]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4 + T Cell Activation by Enhancing the Expression and Activity of Sirt1. PLoS ONE 2013, 8, e75139. [Google Scholar] [CrossRef] [Green Version]
- Ghirotto, B.; Terra, F.F.; Olsen, N.; Câmara, S.; Basso, P.J. Sirtuins in B Lymphocytes Metabolism and Function. World J. Exp. Med. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Sands, B.E.; Joshi, S.; Haddad, J.; Freudenberg, J.M.; Oommen, D.E.; Hoffmann, E.; McCallum, S.W.; Jacobson, E. Assessing Colonic Exposure, Safety, and Clinical Activity of SRT2104, a Novel Oral SIRT1 Activator, in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel. Dis. 2016, 22, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Westerberg, G.; Chiesa, J.A.; Andersen, C.A.; Diamanti, D.; Magnoni, L.; Pollio, G.; Darpo, B.; Zhou, M. Safety, Pharmacokinetics, Pharmacogenomics and QT Concentration—Effect Modelling of the SirT1 Inhibitor Selisistat in Healthy Volunteers. Br. J. Clin. Pharmacol. 2015, 79, 477–491. [Google Scholar] [CrossRef]
- Süssmuth, S.D.; Haider, S.; Landwehrmeyer, G.B.; Farmer, R.; Frost, C.; Tripepi, G.; Andersen, C.A.; Di Bacco, M.; Lamanna, C.; Diodato, E. An exploratory double-blind, randomized clinical trial with selisistat, a SirT1 inhibitor, in patients with Huntington’s disease. Br. J. Clin. Pharmacol. 2015, 79, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Pizzichini, M.M.M.; Pizzichini, E.; Efthimiadis, A.N.N.; Chauhan, A.J.; Johnston, S.L.; Hussack, P.A.T.; Mahony, J.I.M.; Dolovich, J.; Hargreave, F.E. Asthma and Natural Colds Inflammatory Indices in Induced Sputum: A Feasibility Study. Am. J. Respir. Crit. Care Med. 1998, 158, 1178–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kluijver, J.; Grünberg, K.; Pons, D.; De Klerk, E.P.A.; Dick, C.R.; Sterk, P.J.; Hiemstra, P.S. Interleukin-1β and Interleukin-1ra Levels in Nasal Lavages during Experimental Rhinovirus Infection in Asthmatic and Non-Asthmatic Subjects. Clin. Exp. Allergy 2003, 33, 1415–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudy, M.H.; Traves, S.L.; Wiehler, S.; Proud, D. Cigarette Smoke Modulates Rhinovirus- Induced Airway Epithelial Cell Chemokine Production. Eur. Respir. J. 2010, 35, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Herbert, C.; Siegle, J.S.; Vuppusetty, C.; Hansbro, N.; Thomas, P.S.; Foster, P.S.; Barnes, P.J.; Kumar, R.K. Steroid-Resistant Neutrophilic Inflammation in a Mouse Model of an Acute Exacerbation of Asthma. Am. J. Respir. Cell Mol. Biol. 2008, 39, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Thomson, N.C. Novel Approaches to the Management of Noneosinophilic Asthma. Ther. Adv. Respir. Dis. 2016, 10, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Beigelman, A.; Isaacson-Schmid, M.; Sajol, G.; Baty, J.; Rodriguez, O.M.; Leege, E.; Lyons, K.; Schweiger, T.L.; Zheng, J.; Schechtman, K.B.; et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2015, 135, 1171–1178.e1. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.D.; Watson, J.; Roberts, L.R.; Gill, S.K.; Groves, H.; Dhariwal, J.; Almond, M.H.; Wong, E.; Walton, R.P.; Jones, L.H.; et al. Identification of novel macrolides with antibacterial, anti-inflammatory and type I and III IFN-augmenting activity in airway epithelium. J. Antimicrob. Chemother. 2016, 71, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.L.; Szigeti, M.; Cross, M.; Brightling, C.; Chaudhuri, R.; Harrison, T.; Mansur, A.; Robison, L.; Sattar, Z.; Jackson, D.; et al. Azithromycin for Acute Exacerbations of Asthma: The AZALEA Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1630–1637. [Google Scholar] [CrossRef]
- Tacon, C.E.; Wiehler, S.; Holden, N.S.; Newton, R.; Proud, D.; Leigh, R. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-{kappa}B. Am. J. Respir. Cell Mol. Biol. 2010, 43, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Ramezanpour, M.; Cooksley, C.; Li, J.; Nakamaru, Y.; Homma, A.; Psaltis, A.; Wormald, P.J.; Vreugde, S. Sirtuin-1 Controls Poly (I:C)-Dependent Matrix Metalloproteinase 9 Activation in Primary Human Nasal Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2018, 59, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Sagara, H.; Okada, T.; Okumura, K.; Ogawa, H.; Ra, C.; Fukuda, T.; Nakao, A. Activation of TGF-beta/Smad2 signaling is associated with airway remodeling in asthma. J. Allergy Clin. Immunol. 2002, 110, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Xia, Z.; Liu, Y.; Meng, F.; Wu, X.; Fang, Y.; Zhang, C.; Liu, D. Sirt1 Inhibits Rheumatoid Arthritis Fibroblast-like Synoviocyte Aggressiveness and Inflammatory Response via Suppressing Nf-Κb Pathway. Biosci. Rep. 2018, 38, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an Antiinflammatory and Antiaging Protein, Is Decreased in Lungs of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xue, Z.; He, H.N.; Liu, X.; Yin, S.Y.; Wu, D.Y.; Zhang, X.; Schatten, H.; Miao, Y.L. Resveratrol Delays Postovulatory Aging of Mouse Oocytes through Activating Mitophagy. Aging 2019, 11, 11504–11519. [Google Scholar] [CrossRef]
- Knobloch, J.; Wahl, C.; Feldmann, M.; Jungck, D.; Strauch, J.; Stoelben, E.; Koch, A. Resveratrol Attenuates the Release of Inflammatory Cytokines from Human Bronchial Smooth Muscle Cells Exposed to Lipoteichoic Acid in Chronic Obstructive Pulmonary Disease. Basic Clin. Pharmacol. Toxicol. 2014, 114, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Iwai, M.; Aoki, S.; Takimoto, K.; Maruyama, M.; Maruyama, W.; Motoyama, N. SIRT1 Suppresses the Senescence-Associated Secretory Phenotype through Epigenetic Gene Regulation. PLoS ONE 2015, 10, e0116480. [Google Scholar] [CrossRef]
- Knobloch, J.; Hag, H.; Jungck, D.; Urban, K.; Koch, A. Resveratrol impairs the release of steroid-resistant cytokines from bacterial endotoxin-exposed alveolar macrophages in chronic obstructive pulmonary disease. Basic Clin. Pharmacol. Toxicol. 2011, 109, 138–143. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, K.Y.; Min, H.G.; Lee, S.J.; Kim, J.J.; Choi, J.S.; Kim, W.S.; Cha, H.J. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp. Dermatol. 2010, 19, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, S.L.; Busse, W.W. The role of rhinovirus in asthma exacerbations. J. Allergy Clin. Immunol. 2005, 116, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hedman, J.; Moilanen, E.; Poussa, T.; Nieminen, M.M. Serum ECP and MPO, but not urinary LTE4, are associated with bronchial hyper-responsiveness. Respir. Med. 1999, 93, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Calhoun, W.J.; Dick, E.C.; Schwartz, L.B.; Busse, W.W. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J. Clin. Investig. 1994, 94, 2200–2208. [Google Scholar] [CrossRef] [Green Version]
- Tsukagoshi, H.; Ishioka, T.; Noda, M.; Kozawa, K.; Kimura, H. Molecular epidemiology of respiratory viruses in virus-induced asthma. Front. Microbiol. 2013, 4, 278. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Yamada, Y.; Maruyama, K.; Hayashi, Y. Differential effects of corticosteroids on serum eosinophil cationic protein and cytokine production in rhinovirus- and respiratory syncytial virus-induced acute exacerbation of childhood asthma. Int Arch Allergy Immunol. 2011, 155 (Suppl. 1), 77–84. [Google Scholar] [CrossRef]
- Kato, M.; Suzuki, K.; Yamada, Y.; Maruyama, K.; Hayashi, Y.; Mochizuki, H. Allergology International Virus Detection and Cytokine profile in Relation to Age among Acute Exacerbations of Childhood Asthma. Allergol. Int. 2015, 64, S64–S70. [Google Scholar] [CrossRef] [Green Version]
- Price, D.B.; Buhl, R.; Chan, A.; Freeman, D.; Gardener, E.; Godley, C.; Gruffydd-Jones, K.; McGarvey, L.; Ohta, K.; Ryan, D.; et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: A randomised controlled trial. Lancet Respir. Med. 2018, 6, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Bjerregaard, A.; Laing, I.A.; Backerm, V.; Sverrild, A.; Khoo, S.-K.; Chidlow, G.; Sikazwe, C.; Smith, D.W.; Le, Souëf, P.; Porsbjerg, C. High Fractional Exhaled Nitric Oxide and Sputum Eosinophils Are Associated with an Increased Risk of Future Virus-Induced Exacerbations: A Prospective Cohort Study. Clin. Exp. Allergy 2017, 47, 1007–1013. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Ma, G.; Li, W.; Wu, J.; Lai, T.; Huang, D.; Zhao, X.; Lv, Q.; Chen, M.; et al. Increases in Peripheral SIRT1: A New Biological Characteristic of Asthma. Respirology 2015, 20, 1066–1072. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hayashi, R.; Suzuki, K.; Imanishi, S.; Kambara, K.; Okazawa, S.; Inomata, M.; Yamada, T.; Yamazaki, Y.; Koshimizu, Y.; et al. Sirtuin 1 Activator SRT1720 Suppresses Inflammation in an Ovalbumin-Induced Mouse Model of Asthma. Respirology 2013, 18, 332–339. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Luo, Y.; Zhang, Q.; Bian, Y.; Wang, R. Resveratrol-mediated SIRT1 activation attenuates ovalbumin-induced allergic rhinitis in mice. Mol. Immunol. 2020, 122, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Colley, T.; Mercado, N.; Kunori, Y.; Brightling, C.; Bhavsar, P.K.; Barnes, P.J.; Ito, K. Defective Sirtuin-1 Increases IL-4 Expression through Acetylation of GATA-3 in Patients with Severe Asthma. J. Allergy Clin. Immunol. 2016, 137, 1595–1597. [Google Scholar] [CrossRef] [Green Version]
- Green, R.H.; Brightling, C.E.; Mckenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma Exacerbations and Sputum Eosinophil Counts: A Randomised Controlled Trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Ashton, V.L.; Burden, A.; Hillyer, E.V.; Pavord, I. Blood Eosinophil Count and Prospective Annual Asthma Disease Burden: A UK Cohort Study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar]
- Sabogal Piñeros, Y.S.; Bal, S.M.; van de Pol, M.A.; Dierdorp, B.S.; Dekker, T.; Dijkhuis, A.; Brinkman, P.; van der Sluijs, K.F.; Zwinderman, A.H.; Majoor, C.J.; et al. Anti–IL-5 in Mild Asthma Alters Rhinovirus-Induced Macrophage, B-Cell, and Neutrophil Responses (MATERIAL) A Placebo-Controlled, Double-Blind Study. Am. J. Respir. Crit. Care Med. 2019, 199, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ravanetti, L.; Dijkhuis, A.; Sabogal Pineros, Y.S.; Bal, S.M.; Dierdorp, B.S.; Dekker, T.; Logiantara, A.; Adcock, I.M.; Rao, N.L.; Boon, L.; et al. An Early Innate Response Underlies Severe Influenza-Induced Exacerbations of Asthma in a Novel Steroid-Insensitive and Anti-IL-5-Responsive Mouse Model. Allergy 2017, 72, 737–753. [Google Scholar] [CrossRef]
- Ortega, H.; Li, H.; Suruki, R.; Albers, F.; Gordon, D.; Yancey, S. Cluster Analysis and Characterization of Response to Mepolizumab: A Step Closer to Personalized Medicine for Patients with Severe Asthma. Ann. Am. Thorac. Soc. 2014, 11, 1011–1017. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Wechsler, M.E.; Mark FitzGerald, J.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline Patient Factor Impact on the Clinical Efficacy of Benralizumab for Severe Asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef] [Green Version]
- Gill, M.A.; Liu, A.H.; Calatroni, A.; Krouse, R.Z.; Shao, B.; Schiltz, A.; Gern, J.E.; Togias, A.; Busse, W.W.; Hill, C. Enhanced Plasmacytoid Dendritic Cell Antiviral Responses after Omalizumab. J. Allergy Clin. Immunol. 2018, 141, 1735–1743. [Google Scholar] [CrossRef] [Green Version]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Asthma and Lower Airway Disease Preseasonal Treatment with Either Omalizumab or an Inhaled Corticosteroid Boost to Prevent Fall Asthma Exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Hershey, G.K.; et al. Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992. [Google Scholar] [CrossRef]
- Busse, P.J.; Mathur, S.K. Age-Related Changes in Immune Function: Effect on Airway Inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, V.C.; Quehenberger, O.; Oshansky, C.M.; Suen, R.; Aaron, M.; Treuting, P.M.; Thomas, P.G.; Dennis, E.A.; Aderem, A. That Induce and Resolve Inflammation. Cell 2014, 154, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Sokolowska, M.; Chen, L.Y.; Liu, Y.; Martinez-Anton, A.; Logun, C.; Alsaaty, S.; Cuento, R.A.; Cai, R.; Sun, J.; Quehenberger, O.; et al. Dysregulation of lipidomic profile and antiviral immunity in response to hyaluronan in patients with severe asthma. J. Allergy Clin. Immunol. 2017, 139, 1379–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolowska, M.; Rovati, G.E.; Diamant, Z.; Untersmayr, E.; Schwarze, J.; Lukasik, Z.; Sava, F.; Angelina, A.; Palomares, O.; Akdis, C.; et al. Current perspective on eicosanoids in asthma and allergic diseases—EAACI Task Force consensus report, part I. Allergy 2020. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, H.; Fukahori, S.; Tsuchida, T.; Kawano, T.; Tomari, S.; Matsuo, N.; Nishino, T.; Fukushima, C.; Kohno, S. Effects of a short course of pranlukast combined with systemic corticosteroid on acute asthma exacerbation induced by upper respiratory tract infection. J. Asthma 2012, 49, 637–641. [Google Scholar] [CrossRef] [Green Version]
- Bisgaard, H.; Zielen, S.; Garcia-Garcia, M.L.; Johnston, S.L.; Gilles, L.; Menten, J.; Tozzi, C.A.; Polos, P. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Peters-Golden, M.; Swern, A.; Bird, S.S.; Hustad, C.M.; Grant, E.; Edelman, J.M. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006, 27, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L.; Luo, X.F.; Li, M.T.; Xu, D.; Zhou, S.; Chen, H.Z.; Gao, N.; Chen, Z.; Zhang, L.L.; Zeng, X.F. Resveratrol Possesses Protective Effects in a Pristane-Induced Lupus Mouse Model. PLoS ONE 2014, 9, e114792. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Li, X.; Lee, Y.J.; Jin, F.; Park, Y.N.; Deng, Y.; Kang, Y.; Yang, J.H.; Chang, J.H.; Kim, D.Y.; Kim, J.A.; et al. Sirt1 Negatively Regulates FcϵRI-Mediated Mast Cell Activation through AMPK-and PTP1B-Dependent Processes. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Yuan, Y.; Liu, Q.; Zhao, J.; Tang, H.; Sun, J. SIRT1 attenuates murine allergic rhinitis by downregulated HMGB 1/TLR4 pathway. Scand. J. Immunol. 2018, 87, e12667. [Google Scholar] [CrossRef] [Green Version]
- Han, S.Y.; Bae, J.Y.; Park, S.H.; Kim, Y.H.; Park, J.H.; Kang, Y.H. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J. Nutr. 2013, 143, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Lim, L.H. Trans-Resveratrol, an extract of red wine, inhibits human eosinophil activation and degranulation. Br. J. Pharmacol. 2008, 155, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, D.W.; Khalmuratova, R.; Kim, J.H.; Jung, M.H.; Chang, D.; Shin, E.; Lee, H.; Shin, H.; Rhee, C.; et al. Resveratrol Prevents Development of Eosinophilic Rhinosinusitis with Nasal Polyps in a Mouse Model. Br. J. Pharmacol. 2013, 68, 862–869. [Google Scholar] [CrossRef]
- Liou, C.J.; Wei, C.H.; Chen, Y.L.; Cheng, C.Y.; Wang, C.L.; Huang, W.C. Fisetin Protects Against Hepatic Steatosis Through Regulation of the Sirt1/AMPK and Fatty Acid β-Oxidation Signaling Pathway in High-Fat Diet-Induced Obese Mice. Cell. Physiol. Biochem. 2018, 49, 1870–1884. [Google Scholar] [CrossRef]
- Liou, C.J.; Lee, Y.K.; Ting, N.C.; Chen, Y.L.; Shen, S.C.; Wu, S.J.; Huang, W.C. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells 2019, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between dietary scores with asthma symptoms and asthma control in adults. Eur. Respir. J. 2018, 52, 1702572. [Google Scholar] [CrossRef] [Green Version]
- Starkey, M.R.; McKenzie, A.N.; Belz, G.T.; Hansbro, P.M. Pulmonary Group 2 Innate Lymphoid Cells: Surprises and Challenges. Mucosal. Immunol. 2019, 12, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.K.; Foster, P.S.; Rosenberg, H.F. Respiratory Viral Infection, Epithelial Cytokines, and Innate Lymphoid Cells in Asthma Exacerbations. J. Leukoc. Biol. 2014, 96, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; de Bruijn, M.; Lukkes, M.; van Nimwegen, M.; Bergen, I.M.; KleinJan, A.; GeurtsvanKessel, C.H.; Andeweg, A.; Rimmelzwaan, G.F.; Hendriks, R.W. T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. Eur. J. Immunol. 2019, 49, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.; Rajput, C.; Hong, J.Y.; Lei, J.; Hinde, J.L.; Wu, Q.; Bentley, J.K.; Hershenson, M. The Innate Cytokines Il-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Exansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J. Immunol. 2017, 199, 1308–1318. [Google Scholar] [CrossRef]
- Jackson, D.J.; Makrinioti, H.; Rana, B.M.J.; Shamji, B.W.H.; Trujillo-Torralbo, M.B.; Footitt, J.; Del-Rosario, J.; Telcian, A.G.; Nikonova, A.; Zhu, J.; et al. IL-33-Dependent Type 2 Inflammation during Rhinovirus-Induced Asthma Exacerbations in Vivo. Am. J. Respir. Crit. Care Med. 2014, 190, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Gavala, M.L.; Bashir, H.; Gern, J.E. Virus/Allergen Interactions in Asthma. Curr. Allergy Asthma Rep. 2013, 13, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; Headley, M.B.; Loo, Y.M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic Stromal Lymphopoietin Is Induced by Respiratory Syncytial Virus-Infected Airway Epithelial Cells and Promotes a Type 2 Response to Infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol Ameliorates 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Lesions through Effects on the Epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.U.N.; Guo, L.; Yuen, B.; Law, K.; Liang, X.; Ma, N.; Xu, G.; Wang, X.; Yuan, X.; Tang, H.; et al. Resveratrol Decreases Cell Apoptosis through Inhibiting DNA Damage in Bronchial Epithelial Cells. Int. J. Mol. Med. 2020, 45, 1673–1684. [Google Scholar] [CrossRef]
- Sasaki, T. Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity. Front. Endocrinol. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Luo, X.; Tang, Y.; Liu, W.; Luo, R. Leptin Regulated ILC2 Cell through the PI3K/AKT Pathway in Allergic Rhinitis. Mediat. Inflamm. 2020, 2020, 4176082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, X.; Castillo, E.F.; Luo, Y.; Liu, M.; Yang, X.O. Leptin Enhances TH2 and ILC2 Responses in Allergic Airway Disease. J. Biol. Chem. 2016, 291, 22043–22052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.R.; Vuppusetty, C.; Colley, T.; Hassibi, S.; Fenwick, P.S.; Donnelly, L.E.; Ito, K.; Barnes, P.J. MicroRNA-570 is a novel regulator of cellular senescence and inflammaging. FASEB J. 2019, 33, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Ishimaru, K.; Kobayashi, A.; Yu, G.; Nakamura, Y.; Oh-oka, K.; Suzuki-Inoue, K.; Kono, K.; Nakao, A. Resveratrol Inhibits IL-33–Mediated Mast Cell Activation by Targeting the MK2/3–PI3K/Akt Axis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, D.C.; Boswell, M.G.; Reiss, S.; Goleniewska, K.; Toki, S.; Harintho, M.T.; Lukacs, N.W.; Kolls, J.K.; Peebles, R.S., Jr. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax 2013, 68, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Sikazwe, C.T.; Laing, I.A.; Imrie, A.; Smith, D.W. Nasal Cytokine Profiles of Patients Hospitalised with Respiratory Wheeze Associated with Rhinovirus, C. Viruses 2019, 11, 1038. [Google Scholar] [CrossRef] [Green Version]
- Niwa, M.; Fujisawa, T.; Mori, K.; Yamanaka, K.; Yasui, H.; Suzuki, Y.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; et al. IL-17A Attenuates IFN-λ Expression by Inducing Suppressor of Cytokine Signaling Expression in Airway Epithelium. J. Immunol. 2018, 201, 2392–2402. [Google Scholar] [CrossRef] [Green Version]
- Deschildre, A.; Pichavant, M.; Engelmann, I.; Langlois, C.; Drumez, E.; Pouessel, G.; Boileau, S.; Romero-cubero, D.; Decleyre-badiu, I.; Dewilde, A. Virus-Triggered Exacerbation in Allergic Asthmatic Children: Neutrophilic Airway Inflammation and Alteration of Virus Sensors Characterize a Subgroup of Patients. Respir. Res. 2017, 18, 191. [Google Scholar] [CrossRef] [Green Version]
- Saraya, T.; Kurai, D.; Ishii, H.; Ito, A.; Sasaki, Y.; Niwa, S.; Kiyota, N.; Tsukagoshi, H.; Kozawa, K.; Goto, H.; et al. Epidemiology of Virus-Induced Asthma Exacerbations: With Special Reference to the Role of Human Rhinovirus. Front. Microbiol. 2014, 5, 226. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, K.A.; Kedda, M.A.; Thompson, P.J.; Knight, D.A. Oncostatin M: An Interleukin-6-like Cytokine Relevant to Airway Remodelling and the Pathogenesis of Asthma. Clin. Exp. Allergy 2003, 33, 1026–1032. [Google Scholar] [CrossRef]
- Chu, D.K.; Al-Garawi, A.; Llop-Guevara, A.; Pillai, R.A.; Radford, K.; Shen, P.; Walker, T.D.; Goncharova, S.; Calhoun, W.J.; Nair, P.; et al. Therapeutic Potential of Anti-IL-6 Therapies for Granulocytic Airway Inflammation in Asthma. Allergy Asthma Clin. Immunol. 2015, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.A.; Revez, J.A.; Loh, Z.; Simpson, J.; Zhang, V.; Bain, L.; Varelias, A.; Rose-John, S.; Blumenthal, A.; Smyth, M.J. Allergen-induced IL-6 trans-signaling activates γδ T cells to promote type 2 and type 17 airway inflammation. J. Allergy Clin. Immunol. 2015, 136, 1065–1073. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chen, S.H.; Wang, J.Y. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J. Mol. Med. 2016, 94, 51–59. [Google Scholar] [CrossRef]
- Mehta, A.K.; Doherty, T.; Broide, D.; Croft, M. Tumor necrosis factor family member LIGHT acts with IL-1β and TGF-β to promote airway remodeling during rhinovirus infection. Allergy 2018, 73, 1415–1424. [Google Scholar] [CrossRef]
- Denney, L.; Branchett, W.; Gregory, L.G.; Oliver, R.A.; Lloyd, C.M. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunol. 2018, 11, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Elesela, S.; Morris, S.B.; Narayanan, S.; Kumar, S.; Lombard, D.B.; Lukacs, N.W. Sirtuin 1 Regulates Mitochondrial Function and Immune Homeostasis in Respiratory Syncytial Virus Infected Dendritic Cells. PLoS Pathog. 2020, 16, e1008319. [Google Scholar] [CrossRef]
- Krueger, J.G.; Suarez-Fariñas, M.; Cueto, I.; Khacherian, A.; Matheson, R.; Parish, L.C.; Leonardi, C.; Shortino, D.; Gupta, A.; Haddad, J.; et al. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS ONE 2015, 10, e0142081. [Google Scholar] [CrossRef] [Green Version]
- Kjær, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol Ameliorates Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Yang, H.; Tartar, D.M.; Gao, B.; Luo, X.; Ye, S.Q.; Zaghouani, H.; Fang, D. Prevention and Treatment of Diabetes with Resveratrol in a Non-Obese Mouse Model of Type 1 Diabetes. Diabetologia 2011, 54, 1136–1146. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lin, Y.; Liu, X. Protective Effects of SIRT1 in Patients with Proliferative Diabetic Retinopathy via the Inhibition of IL-17 Expression. Exp. Ther. Med. 2016, 11, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wu, Q.J.; Yang, X.; Xing, X.X.; Chen, Y.Y.; Wang, H. Effects of SIRT1/Akt Pathway on Chronic Inflammatory Response and Lung Function in Patients with Asthma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4948–4953. [Google Scholar] [PubMed]
- Tang, L.; Chen, Q.; Meng, Z.; Sun, L.; Zhu, L.; Liu, J.; Hu, J.; Ni, Z.; Wang, X. Suppression of Sirtuin-1 Increases IL-6 Expression by Activation of the Akt Pathway during Allergic Asthma. Cell. Physiol. Biochem. 2018, 43, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Zhu, M.; Huang, Y.; Zhao, Y.M.; Wen, J.J.; Yang, X.J.; Wu, P. Metformin Relieves Acute Respiratory Distress Syndrome by Reducing MiR-138 Expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5355–5363. [Google Scholar]
- Li, X.; Li, J.; Wang, L.; Li, A.; Qiu, Z.; Qi, L.W.; Kou, J.; Liu, K.; Liu, B.; Huang, F. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue. Br. J. Pharmacol. 2016, 173, 2001–2015. [Google Scholar] [CrossRef]
- Li, C.Y.; Erickson, S.R.; Wu, C.H. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology 2016, 21, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Cheng, S.; Chen, H.; Li, Q.; Hu, Y.; Wang, Q.; Zhu, X.; Wang, J. Activation and overexpression of Sirt1 attenuates lung fibrosis via P300. Biochem. Biophys. Res. Commun. 2017, 486, 1021–1026. [Google Scholar] [CrossRef]
- Liu, F.; Shang, Y.X. Sirtuin 6 attenuates epithelial-mesenchymal transition by suppressing the TGF-β1/Smad3 pathway and c-Jun in asthma models. Int. Immunopharmacol. 2020, 82, 106333. [Google Scholar] [CrossRef]
- Minagawa, S.; Araya, J.; Numata, T.; Nojiri, S.; Hara, H.; Yumino, Y.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Nishimura, S.L. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L391–L401. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, I.K.; Yoon, H.K.; Kwon, S.S.; Rhee, C.K.; Lee, S.Y. Inhibitory Effects of Resveratrol on Airway Remodeling by Transforming Growth Factor-β/Smad Signaling Pathway in Chronic Asthma Model. Allergy Asthma Immunol. Res. 2017, 9, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Leus, N.G.; van der Wouden, P.E.; van den Bosch, T.; Hooghiemstra, W.; Ourailidou, M.E.; Kistemaker, L.E.; Bischoff, R.; Gosens, R.; Haisma, H.J.; Dekker, F.J. HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-κB p65 transcriptional activity. Biochem. Pharmacol. 2016, 108, 58–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Caramori, G.; Lim, S.; Oates, T.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Expression and Activity of Histone Deacetylases in Human Asthmatic Airways. Am. J. Respir. Crit. Care Med. 2002, 166, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Komaravelli, N.; Tian, B.; Ivanciuc, T.; Mautemps, N.; Brasier, A.R.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 2015, 88, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Cosío, B.G.; Mann, B.; Ito, K.; Jazrawi, E.; Barnes, P.J.; Chung, K.F.; Adcock, I.M. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 141–147. [Google Scholar] [CrossRef]
- Ito, K.; Lim, S.; Caramori, G.; Cosio, B.; Chung, K.F.; Adcock, I.M.; Barnes, P.J. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc. Natl. Acad. Sci. USA 2002, 99, 8921–8926. [Google Scholar] [CrossRef] [Green Version]
- Spears, M.; Donnelly, I.; Jolly, L.; Brannigan, M.; Ito, K.; McSharry, C.; Lafferty, J.; Chaudhuri, R.; Braganza, G.; Adcock, I.M. Effect of low-dose theophylline plus beclometasone on lung function in smokers with asthma: A pilot study. Eur. Respir. J. 2009, 33, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Cosio, B.G.; Iglesias, A.; Rios, A.; Noguera, A.; Sala, E.; Ito, K.; Barnes, P.J.; Agusti, A. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax 2009, 64, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Hodge, G.; Tran, H.B.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther. Adv. Respir. Dis. 2020, 14, 1753466620905280. [Google Scholar] [CrossRef]
- Jiao, P.; Li, W.; Shen, L.; Li, Y.; Yu, L.; Liu, Z. The protective effect of doxofylline against lipopolysaccharides (LPS)-induced activation of NLRP3 inflammasome is mediated by SIRT1 in human pulmonary bronchial epithelial cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-Dependent SIRT1 Deacetylase Participates in Epigenetic Reprogramming during Endotoxin Tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Erion, D.M.; Yuan, Z.; Dietrich, M.; Shulman, G.I.; Horvath, T.L.; Gao, Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell Biol. 2009, 11, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Limagne, E.; Thibaudin, M.; Euvrard, R.; Berger, H.; Chalons, P.; Végan, F.; Humblin, E.; Boidot, R.; Rébé, C.; Derangère, V.; et al. Sirtuin-1 Activation Controls Tumor Growth by Impeding Th17 Differentiation via STAT3 Deacetylation. Cell Rep. 2017, 19, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Xander, N.; Reddy Vari, H.; Eskandar, R.; Li, W.; Bolla, S. Rhinovirus-Induced SIRT-1 via TLR2 Regulates Subsequent Type I and Type III IFN Responses in Airway Epithelial Cells. J. Immunol. 2019, 203, 2508–2519. [Google Scholar] [CrossRef]
- Li, X.N.; Ma, L.Y.; Ji, H.; Qin, Y.H.; Jin, S.S.; Xu, L.X. Resveratrol protects against oxidative stress by activating the Keap-1/Nrf2 antioxidant defense system in obese-asthmatic rats. Exp. Ther. Med. 2018, 16, 4339–4348. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhao, G.; Chen, L.; Ding, Y.; Lian, J.; Hong, G.; Lu, Z. Resveratrol protects mice from paraquat-induced lung injury: The important role of SIRT1 and NRF2 antioxidant pathways. Mol. Med. Rep. 2016, 13, 1833–1838. [Google Scholar] [CrossRef]
- Chu, Q.; Yu, X.; Jia, R.; Wang, Y.; Zhang, Y.; Zhang, S.; Liu, Y.; Li, Y.; Chen, W.; Ye, X.; et al. Flavonoids from Apios americana Medikus Leaves Protect RAW264.7 Cells against Inflammation via Inhibition of MAPKs, Akt-mTOR Pathways, and Nfr2 Activation. Oxid. Med. Cell. Longev. 2019, 2019, 1563024. [Google Scholar] [CrossRef]
- Martinez, F.J.; Calverley, P.M.; Goehring, U.M.; Brose, M.; Fabbri, L.M.; Rabe, K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicentre randomised controlled trial. Lancet 2015, 385, 857–866. [Google Scholar] [CrossRef]
- Barreiro, E.; Puig-Vilanova, E.; Salazar-Degracia, A.; Pascual-Guardia, S.; Casadevall, C.; Gea, J. The phosphodiesterase-4 inhibitor roflumilast reverts proteolysis in skeletal muscle cells of patients with COPD cachexia. J. Appl. Physiol. 2018, 125, 287–303. [Google Scholar] [CrossRef]
- Mata, M.; Martinez, I.; Melero, J.A.; Tenor, H.; Cortijo, J. Roflumilast inhibits respiratory syncytial virus infection in human differentiated bronchial epithelial cells. PLoS ONE 2013, 8, e69670. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Fung, S.Y.; Xie, G.; Wong, L.R.; Jin, D.Y.; Cai, Z. Identification of lysine acetylation sites on MERS-CoV replicase pp1ab. Mol. Cell. Proteom. 2020, 19, 1303–1309. [Google Scholar] [CrossRef]
- Koyuncu, E.; Budayeva, H.G.; Miteva, Y.V.; Ricci, D.P.; Silhavy, T.J.; Shenk, T.; Cristea, I.M. Sirtuins are evolutionarily conserved viral restriction factors. mBio 2014, 5, e02249-14. [Google Scholar] [CrossRef] [Green Version]
- Mastromarino, P.; Capobianco, D.; Cannata, F.; Nardis, C.; Mattia, E.; De Leo, A.; Restignoli, R.; Francioso, A.; Mosca, L. Resveratrol inhibits rhinovirus replication and expression of inflammatory mediators in nasal epithelia. Antiviral Res. 2015, 123, 15–21. [Google Scholar] [CrossRef]
- Chu, H.; Zhou, J.; Wong, B.H.; Li, C.; Chan, J.F.; Cheng, Z.S.; Yang, D.; Wang, D.; Lee, A.C.; Li, C. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Seong, R.K.; Shin, O.S. Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw. 2016, 16, 286–295. [Google Scholar] [CrossRef] [Green Version]
| Target Cells | Effect of SIRT1 Activation | References (Model) |
|---|---|---|
| Neutrophils | CXCL8 ↓ | [54,55,56,57,58,59,60] (in vitro) |
| MMP-9 ↓ | [53,61] (animal) | |
| Eosinophils | IL-4, IL-5, IL-13 ↓ | [70,71,72] (in vitro) |
| GATA3 ↓ | [73] (animal) | |
| Mast cells and B cells | IgE ↓ | [92,94] (animal) |
| [93] (in vitro) | ||
| LTC4, PG ↓ | [96,97,98,99] (animal) | |
| Degranulation ↓ | [95] (animal) | |
| ILC2 | Il-25, IL-33, TSLP ↓ | [109] (in vitro) |
| epithelial damage ↓ | [110,111] (animal) | |
| miRNA34a, miRNA570 ↓ | [114,115] (in vitro) | |
| Th17 cells | [128] (animal) | |
| IL-17 ↓ | [129,130,131,132] (clinical trial) | |
| [17,133,134,135,136] (animal) | ||
| IL-6 ↓ | [139,140] (in vitro) | |
| TGF-β↓ | [138,141] (animal) | |
| Viral protein | HDAC activity ↑ | [149] (clinical trial) |
| steroid resistance ↓ | [150] (in vitro) | |
| NF-κB↓ | [152] (in vitro) | |
| STAT1, STAT3 ↓ | [154,155] (in vitro) | |
| [153] (animal) | ||
| Nrf2 ↓ | [161] (in vitro) | |
| [156,157,158] (animal) | ||
| [159,160] (clinical trial) | ||
| Viral replication ↓ | [162,163,164,165,166,167] (in vitro) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, Y.; Akimoto, K.; Homma, T.; Baker, J.R.; Ito, K.; Barnes, P.J.; Sagara, H. Virus-Induced Asthma Exacerbations: SIRT1 Targeted Approach. J. Clin. Med. 2020, 9, 2623. https://doi.org/10.3390/jcm9082623
Fukuda Y, Akimoto K, Homma T, Baker JR, Ito K, Barnes PJ, Sagara H. Virus-Induced Asthma Exacerbations: SIRT1 Targeted Approach. Journal of Clinical Medicine. 2020; 9(8):2623. https://doi.org/10.3390/jcm9082623
Chicago/Turabian StyleFukuda, Yosuke, Kaho Akimoto, Tetsuya Homma, Jonathan R Baker, Kazuhiro Ito, Peter J Barnes, and Hironori Sagara. 2020. "Virus-Induced Asthma Exacerbations: SIRT1 Targeted Approach" Journal of Clinical Medicine 9, no. 8: 2623. https://doi.org/10.3390/jcm9082623







