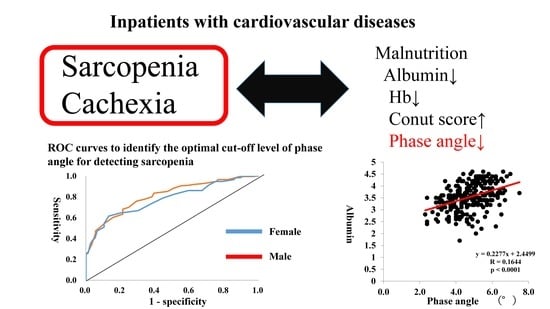
Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Short Physical Performance Battery
2.3. Measurement of Gait Speed, Grip Strength, and Voluntary Isometric Contraction
2.4. Measurements with the Bioelectrical Impedance Analyzer (BIA)
2.5. Controlling Nutritional Status (CONUT) Score
2.6. Measurement of Muscle Thickness by Ultrasound
2.7. Statistical Analysis
3. Results
3.1. Physical Characteristics and Clinical Data
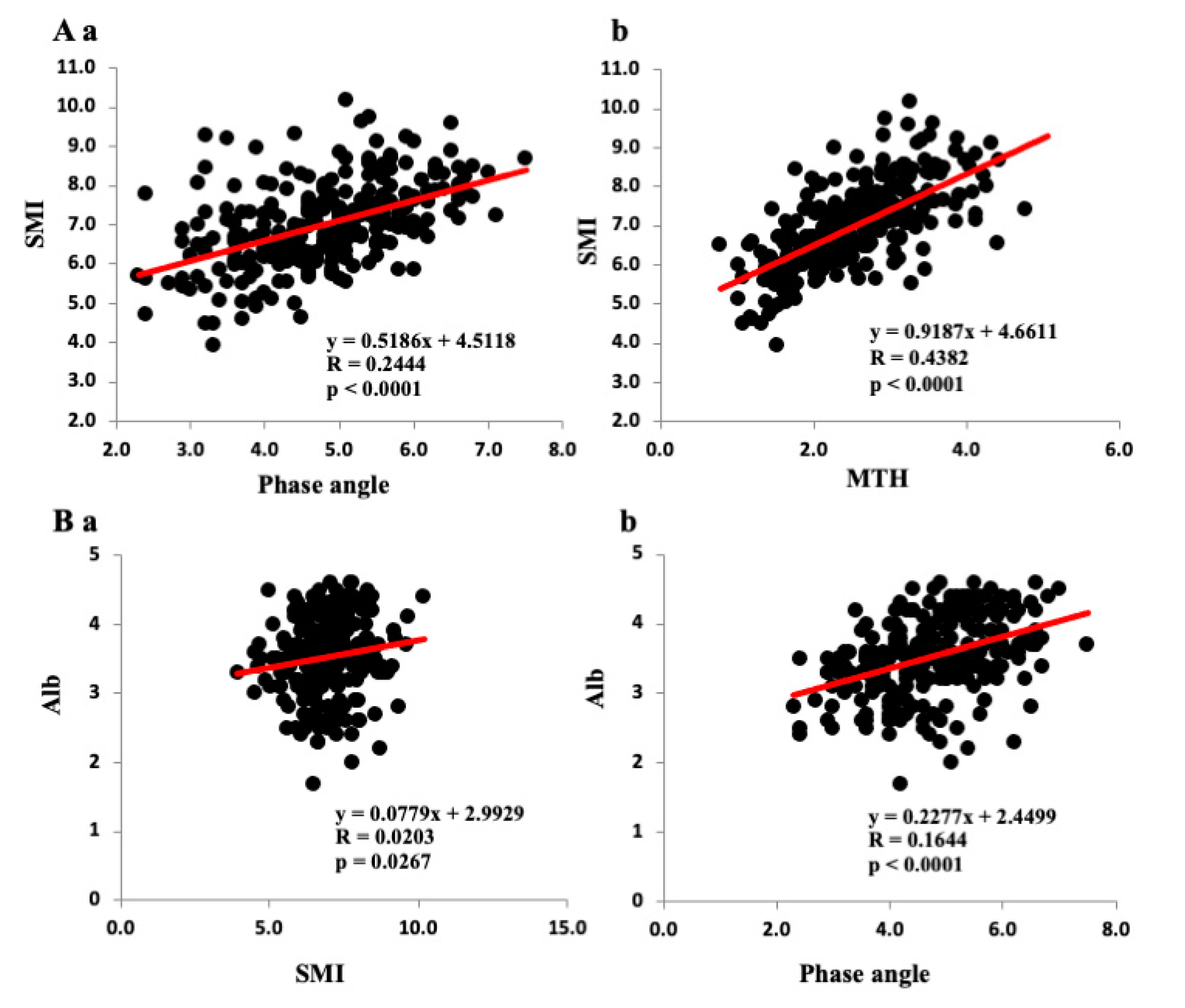
3.2. Correlations among Various Parameters and SMI, PhA, and Anterior MTH
3.3. Multivariate Regression Analysis of the PhA/SMI and Clinical Parameters
3.4. Relationships Between Sarcopenia and Various Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | heart failure |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| BIA | bioelectrical impedance analysis |
| PhA | phase angle |
| BMI | body mass index |
| CHF | chronic heart failure |
| BNP | brain natriuretic peptide |
| eGFR | estimated glomerular filtration rate |
| Hb | hemoglobin |
| Alb | albumin |
| SPPB | short physical performance battery |
| SMI | smooth muscle index |
| CRP | C-reactive protein |
| CONUT | controlling nutritional status |
| MTh | muscle thickness |
| ROC | receiver operating characteristic |
References
- Sorensen, J.; Kondrup, J.; Prokopowicz, J.; Schiesser, M.; Krähenbühl, L.; Meier, R.; Liberda, M.; EuroOOPS Study Group. EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin. Nutr. 2008, 27, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Bulj, N.; Kolačević Zeljković, M.; Radeljić, V.; Krcmar, T.; Trbusic, M.; Delić-Brkljačić, D.; Alebic, T.; Vcev, A. Nutritional considerations of cardiovascular diseases and treatments. Nutr. Metab. Insights 2019, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Sharma, Y.; Miller, M.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Horwood, C.; Thompson, C. Factors influencing early and late readmissions in Australian hospitalised patients and investigating role of admission nutrition status as a predictor of hospital readmissions: A cohort study. BMJ Open 2018, 8, e022246. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Direct. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Kamiya, K.; Hamazaki, N.; Natsuzawa, R.; Nozaki, K.; Tanaka, S.; Ichinosawa, Y.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Matsunaga, A.; et al. Sarcopenia: Prevalence and prognostic implications in elderly patients with cardiovascular disease. JCSM Clin. Rep. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Chin, S.O.; Rhee, S.Y.; Chon, S.; Hwang, Y.C.; Jeong, I.K.; Oh, S.; Ahn, K.J.; Chung, H.Y.; Woo, J.T.; Kim, S.W.; et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 2013, 8, e60119. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Rodriguez-Miguelez, P.; Kirkman, D.L.; Garten, R.; Franco, R.L.; Lee, D.C.; Lavie, C.J. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr. Probl. Cardiol. 2019, 28, 100417. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Bekfani, T.; Pellicori, P.; Morris, D.A.; Ebner, N.; Valentova, M.; Steinbeck, L.; Wachter, R.; Elsner, S.; Sliziuk, V.; Schefold, J.C.; et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int. J. Cardiol. 2016, 222, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Rossi, A.P.; Corzato, F.; Bambace, C.; Mazzali, G.; Fantin, F. Sarcopenia, cachexia and congestive heart failure in the elderly. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Masson, S.; Barlera, S.; Girerd, N.; Castelnovo, A.; Zannad, F.; Clemenza, F.; Tognoni, G.; Anand, I.S.; Cohn, J.N.; et al. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: Insights from the GISSI-HF and Val-HeFT trials. Eur. J. Heart Fail. 2015, 17, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Springer, J.; Anker, M.; Doehner, W.; Lainscak, M. Cardiac cachexia: Hic et nunc. J. Cachexia Sarcopenia Muscle 2016, 7, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Bioelectric impedance phase angle and body composition. Am. J. Clin. Nutr. 1988, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Máttar, J.A. Application of total body bioimpedance to the critically ill patient. Brazilian Group for Bioimpedance Study. New Horiz. 1996, 4, 493–503. [Google Scholar] [PubMed]
- Pirlich, M.; Schütz, T.; Spachos, T.; Ertl, S.; Weiss, M.L.; Lochs, H.; Plauth, M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000, 32, 1208–1215. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Bassani, L.; Nunes, F.F.; Aydos, M.E.; Alves, A.V.; Marroni, C.A. Nutritional assessment in patients with cirrhosis. Arq. Gastroenterol. 2012, 49, 19–27. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Bragagnolo, R.; Caporossi, F.S.; Dock-Nascimento, D.B.; De Aguilar-Nascimento, J.E. Adductor pollicis muscle thickness: A fast and reliable method for nutritional assessment in surgical patients. Rev. Col. Bras. Cir. 2009, 36, 371–376. [Google Scholar] [CrossRef]
- Llames, L.; Baldomero, V.; Iglesias, M.L.; Rodota, L.P. Values of the phase angle by bioelectrical impedance; nutritional status and prognostic value. Nutr. Hosp. 2013, 28, 286–295. [Google Scholar] [PubMed]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nakajima, T.; Sawaguchi, T.; Nozawa, N.; Arakawa, T.; Takahashi, R.; Mizushima, Y.; Katayanagi, S.; Matsumoto, K.; Toyoda, S.; et al. Short physical performance battery for cardiovascular disease inpatients: Implications for critical factors and sarcopenia. Sci. Rep. 2017, 7, 17425. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Shibasaki, I.; Sawaguchi, T.; Haruyama, A.; Kaneda, H.; Nakajima, T.; Hasegawa, T.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Growth differentiation factor-15 (GDF-15) is a biomarker of muscle wasting and renal dysfunction in preoperative cardiovascular surgery patients. J. Clin. Med. 2019, 8, 1576. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Kyle, U.G.; Pirlich, M.; Lochs, H.; Schuetz, T.; Pichard, C. Increased length of hospital stay in underweight and overweight patients at hospital admission: A controlled population study. Clin. Nutr. 2005, 24, 133–142. [Google Scholar] [CrossRef]
- Ignacio, I.; De Ulíbarri, J.; González-Madroño, A.; De Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Composition of the ESPEN Working Group. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Buffa, R.; Floris, G.; Marini, E. Migration of the bioelectrical impedance vector in healthy elderly subjects. Nutrition 2003, 19, 917–921. [Google Scholar] [CrossRef]
- Gunn, S.M.; Halbert, J.A.; Giles, L.C.; Stepien, J.M.; Miller, M.D.; Crotty, M. Bioelectrical phase angle values in a clinical sample of ambulatory rehabilitation patients. Dyn. Med. 2008, 10, 7–14. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Kubrusly, M.; Mota, R.S.; Silva, C.A.; Choukroun, G.; Oliveira, V.N. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J. Ren. Nutr. 2010, 20, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Della-Morte, D.; Cacciatore, F.; Gargiulo, G.; Galizia, G.; Roselli, M.; Curcio, F.; Bonaduce, D.; Abete, P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp. Gerontol. 2014, 58, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.M.; Pinho, C.P.S.; Da Silva, C.P.; Dos Santos, N.F.; Mendes, R.M.L. Phase angle as a sarcopenia marker in hospitalized elderly patients. Nutr. Clin. Pr. 2018, 33, 232–237. [Google Scholar] [CrossRef]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef]
- Barak, N.; Wall-Alonso, E.; Cheng, A.; Sitrin, M.D. Use of bioelectrical impedance analysis to predict energy expenditure of hospitalized patients receiving nutrition support. J. Parenter. Enter. Nutr. 2003, 27, 43–46. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Danielzik, S.; Dörhöfer, R.P.; Later, W.; Wiese, S.; Müller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. J. Parenter. Enter. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef]
- Kilic, M.K.; Kizilarslanoglu, M.C.; Arik, G.; Bolayir, B.; Kara, O.; Dogan Varan, H.; Sumer, F.; Kuyumcu, M.E.; Halil, M.; Ulger, Z. Association of bioelectrical impedance analysis-derived phase angle and sarcopenia in older adults. Nutr. Clin. Pr. 2017, 32, 103–109. [Google Scholar] [CrossRef]
- Colín-Ramírez, E.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Durán, M.; Rodríguez, A.E.; Keirns-Davis, C. Bioelectrical impedance phase angle as a prognostic marker in chronic heart failure. Nutrition 2012, 28, 901–905. [Google Scholar] [CrossRef]
- Castillo Martínez, L.; Colín Ramírez, E.; Orea Tejeda, A.; Asensio Lafuente, E.; Bernal Rosales, L.P.; Rebollar González, V.; Narváez David, R.; Dorantes García, J. Bioelectrical impedance and strength measurements in patients with heart failure: Comparison with functional class. Nutrition 2007, 23, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Cabre, M.; Ferreiro, C.; Arus, M.; Roca, M.; Palomera, E.; Serra-Prat, M. Evaluation of CONUT for clinical malnutrition detection and short-term prognostic assessment in hospitalized elderly people. J. Nutr. Health Aging 2015, 19, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Ueno, Y.; Kawagishi, N.; Kondo, Y.; Inoue, J.; Kakazu, E.; Ninomiya, M.; Wakui, Y.; Saito, N.; Satomi, S.; et al. The nutritional index ‘CONUT’ is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J. Exp. Med. 2014, 224, 215–219. [Google Scholar] [CrossRef]
- Nakagomi, A.; Kohashi, K.; Morisawa, T.; Kosugi, M.; Endoh, I.; Kusama, Y.; Atarashi, H.; Shimizu, W. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J. Atheroscler. Thromb. 2016, 23, 713–727. [Google Scholar] [CrossRef]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Chesworth, B.; Madill, J. Is phase angle an appropriate indicator of malnutrition in different disease states? A systematic review. Clin. Nutr. ESPEN 2019, 29, 1–14. [Google Scholar] [CrossRef]
- Schalk, B.W.; Deeg, D.J.; Penninx, B.W.; Bouter, L.M.; Visser, M. Serum albumin and muscle strength: A longitudinal study in older men and women. J. Am. Geriatr. Soc. 2005, 53, 1331–1338. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009, 119, e391–e479. [Google Scholar]
- Christensen, H.M.; Kistorp, C.; Schou, M.; Keller, N.; Zerahn, B.; Frystyk, J.; Schwarz, P.; Faber, J. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine 2013, 43, 626–634. [Google Scholar] [CrossRef]
- Argilés, J.M.; Anker, S.D.; Evans, W.J.; Morley, J.E.; Fearon, K.C.; Strasser, F.; Muscaritoli, M.; Baracos, V.E. Consensus on cachexia definitions. J. Am. Med. Direct. Assoc. 2010, 11, 229–230. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Paiva, I.S.; Borges, R.L.; Halpern-Silveira, D.; Assuncao, F.M.C.; Barros, J.D.A.; Gonzalez, C.M. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support. Care Cancer 2011, 19, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Toso, S.; Piccoli, A.; Gusella, M.; Menon, D.; Bononi, A.; Crepaldi, G.; Ferrazzi, E. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition 2000, 16, 120–124. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, G.C.; Dahlk, L.S.; Vashi, G.P.; Grutsch, F.J.; Lammersfeld, A.C. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br. J. Nutr. 2004, 92, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Heymsfield, S.B. Lean tissue imaging: A new era for nutritional assessment and intervention. J. Parenter. Enter. Nutr. 2014, 38, 940–953. [Google Scholar] [CrossRef] [PubMed]

| Males (n = 277) | Females (n = 135) | |
|---|---|---|
| Specific diseases, n (%) | ||
| Surgical disease | ||
| CABG | 52 (18.8%) | 7 (5.6%) |
| Valve surgery | 36 (13.0%) | 26 (19.3%) |
| Aortic surgery | 35 (12.6%) | 15 (6.4%) |
| ASO | 6 (2.1%) | 2 (1.5%) |
| TAVI | 8 (3.0%) | 18 (13.3%) |
| Others | 20 (7.2%) | 3 (2.2%) |
| Internal disease | ||
| CHF | 70 (25.3%) | 47 (34.8%) |
| IHD | 75 (27.1%) | 20 (14.8%) |
| Others | 1 (0.3%) | 0 (0%) |
| Risk factor | ||
| HT | 177 (64.0%) | 94 (69.6%) |
| HL | 155 (56.0%) | 54 (40.0%) |
| DM | 124 (44.8%) | 30 (22.2%) |
| CKD | 53 (19.1%) | 17 (13.0%) |
| Age, years | 67.7 (12.5) *** | 74.6 (11.4) |
| Standing height, cm | 164.7 (6.4) *** | 149.0 (7.5) |
| Body weight, kg | 64.8 (12.5) *** | 49.0 (11.5) |
| BMI, kg/m2 | 23.6 (3.8) *** | 22.1 (4.3) |
| % body fat | 27.7 (7.6) *** | 33.3 (10.1) |
| Body fat mass, kg | 18.3 (7.4) | 17.3 (8.1) |
| Systolic BP, mmHg | 113.9 (16.6) | 118.3 (18.9) |
| Diastolic BP, mmHg | 64.5 (11.9) | 66.0 (11.6) |
| BNP, pg/mL | 464.0 (639.4) | 455.2 (550.1) |
| eGFR, ml/min/1.73 m2 | 60.9 (27.1) | 61.3 (28.1) |
| Hb, g/dL | 12.4 (2.2) *** | 11.5 (1.5) |
| Alb, g/dL | 3.5 (0.6) | 3.6 (0.6) |
| TChol, mg/dL | 160.9 (40.6) *** | 184.8 (47.1) |
| CRP, mg/dL | 2.00 (3.91) ** | 1.38 (3.09) |
| Hand grip, kgf | 28.9 (8.6) *** | 16.1 (4.5) |
| Knee extension, kgf | 27.2 (11.7) *** | 14.5 (6.0) |
| CONUT score | 3.21 (2.62) | 2.81 (2.36) |
| LMI, kg/m2 | 16.7 (2.0) *** | 14.4 (1.5) |
| SMI, kg/m2 | 6.99 (1.06) *** | 5.20 (0.96) |
| Phase angle, ° | 4.79 (1.02) *** | 3.99 (0.89) |
| MTH, cm | 2.55 (0.78) *** | 2.04 (0.66) |
| SPPB (total) | 10.5 (2.2) *** | 8.9 (3.1) |
| Balance (score) | 3.68 (0.83) ** | 3.31 (1.17) |
| Gait speed, s/5 m | 1.04 (0.38) *** | 0.91 (0.90) |
| Chair stand, s/5 reps | 11.7 (5.1) *** | 14.4 (7.6) |
| Variable | Males (n = 277) SMI r-Value (p-Value) | Phase Angle r-Value (p-Value) | MTH r-Value (p-Value) | Females (n = 135) SMI r-Value (p-Value) | Phase Angle r-Value (p-Value) | MTH r-Value (p-Value) |
|---|---|---|---|---|---|---|
| Age | −0.3387 **** | −0.3120 **** | −0.3681 **** | −0.4590 **** | −0.3230 **** | −0.3900 **** |
| BMI | 0.7290 **** | 0.3310 **** | 0.5768 **** | 0.6076 **** | 0.3115 *** | 0.4840 **** |
| Hand grip | 0.5948 **** | 0.6713 **** | 0.5542 **** | 0.5551 **** | 0.5989 **** | 0.4726 **** |
| Knee extension MVC | 0.5523 **** | 0.6053 **** | 0.5968 **** | 0.4818 **** | 0.5260 **** | 0.4344 **** |
| CONUT | −0.2088 * | −0.4190 **** | −0.2753 *** | −0.0715 | −0.2198 | −0.2687 * |
| Hb | 0.2385 *** | 0.4668 **** | 0.4067 **** | 0.1343 | 0.2066 * | 0.1071 |
| Alb | 0.1424 * | 0.4055 **** | 0.2467 **** | 0.1509 | 0.3115 *** | 0.4840 ** |
| eGFR | 0.1679 ** | 0.4183 **** | 0.2360 *** | 0.0671 | 0.2549 ** | 0.1167 |
| BNP | −0.2397 ** | −0.3527 **** | −0.3858 **** | −0.1992 | −0.3268 ** | −0.1849 |
| SMI | – | 0.4944 **** | 0.6620 **** | – | 0.4438 **** | 0.5958 *** |
| Phase angle | 0.4944 **** | – | 0.6683 **** | 0.4438 **** | – | 0.5942 **** |
| MTH | 0.6620 **** | 0.6683 **** | – | 0.5958 **** | 0.5942 **** | – |
| SPPB (total) | 0.2088 * | 0.4293 **** | 0.3197 **** | 0.3826 **** | 0.5325 **** | 0.3237 *** |
| Balance | 0.1457 * | 0.2553 **** | 0.0910 | 0.4017 **** | 0.3977 **** | 0.2559 ** |
| Gait speed | 0.2402 *** | 0.4112 **** | 0.3070 **** | 0.1404 | 0.0764 | 0.2932 ** |
| Chair stand | −0.2403 *** | −0.4666 **** | −0.3840 **** | −0.2773 *** | −0.3652 **** | −0.3376 *** |
| Dependent Variable: Phase Angle/SMI | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Independent Variable | β-Value (p) Males/Females | β-Value (p) Males/Females | β-Value (p) Males/Females |
| Hand grip | 0.378 (0.000)/0.453 (0.001) 0.554 (0.000)/0.593 (0.000) | 0.328 (0.000)/0.370 (0.006) 0.400 (0.000)/0.373 (0.003) | 0.316 (0.000)/0.320 (0.020) 0.376 (0.000)/0.266 (0.024) |
| Knee extension (weight) | 0.049(0.539)/0.057 (0.630) −0.097 (0.287)/−0.169 (0.202) | 0.105 (0.198)/0.136 (0.273) 0.071 (0.311)/0.039 (0.735) | 0.104 (0.200)/0.144 (0.242) 0.077 (0.263)/0.056 (0.599) |
| BNP log | −0.239 (0.004)/−0.163 (0.141) −0.311 (0.001)/−0.070 (0.565) | −0.206 (0.011)/−0.137 (0.206) −0.203 (0.004)/−0.002 (0.983) | −0.206 (0.012)/−0.152 (0.161) −0.198 (0.005)/−0.034 (0.712) |
| eGFR | 0.052 (0.489)/0.022 (0.836) −0.100 (0.252)/−0.129 (0.269) | 0.065 (0.374)/0.108 (0.340) −0.054 (0.404)/0.099 (0.345) | 0.064 (0.377)/0.092 (0.412) −0.057 (0.368)/0.065 (0.715) |
| CRP log | 0.176 (0.035)/0.000 (0.997) 0.057 (0.618)/0.233 (0.076) | 0.138 (0.093)/−0.060 (0.614) −0.079 (0.273)/0.076 (0.493) | 0.136 (0.099)/−0.091 (0.448) −0.088 (0.214)/0.008 (0.937) |
| Hb | 0.291 (0.000)/0.230 (0.038) 0.128 (0.153)/−0.028 (0.815) | 0.239 (0.003)/0.207 (0.058) −0.027 (0.694)/−0.091 (0.362) | 0.227 (0.005)/0.209 (0.054) −0.048 (0.483)/−0.087 (0.342) |
| Alb log | 0.160 (0.065)/−0.062 (0.607) 0.038 (0.702)/−0.109 (0.416) | 0.134 (0.115)/−0.059 (0.619) −0.053 (0.481)/−0.100 (0.364) | 0.140 (0.101)/−0.066 (0.576) −0.047 (0.520)/−0.114 (0.256) |
| Variable | Males (n = 234) Sarcopenia n = 74 | Others n = 160 | Females (n = 114) Sarcopenia n = 37 | Others n = 77 |
|---|---|---|---|---|
| Age, years | 74.9 (9.2) **** | 64.9 (11.2) | 79.4 (8.1) **** | 71.7 (12.1) |
| BMI, kg/m2 | 21.1 (2.9) **** | 24.7 (3.5) | 21.4 (3.8) | 22.8 (4.5) |
| % body fat | 27.0 (8.3) | 27.7 (7.1) | 34.5 (10.6) | 32.9 (9.8) |
| BNP, pg/mL | 683 (815) *** | 346 (489) | 613 (640) | 371 (405) |
| eGFR, pg/mL | 51.5 (28.2) *** | 66.0 (24.7) | 59.0 (33.4) | 62.2 (24.8) |
| Hand grip, kgf | 21.1 (4.2) **** | 32.8 (7.1) | 12.8 (3.3) **** | 18.2 (3.8) |
| Knee extension, kgf | 18.1 (6.1) **** | 31.3 (10.8) | 11.0 (4.4) **** | 16.5 (5.6) |
| CONUT score | 4.3 (2.6) **** | 2.5 (2.3) | 3.2 (2.4) | 2.3 (2.0) |
| Hb, g/dL | 11.7 (1.8) **** | 12.8 (2.2) | 11.1 (1.4) * | 11.8 (1.6) |
| Alb, g/dL | 3.4 (0.5) *** | 3.6 (0.6) | 3.4 (0.6) * | 3.7 (0.6) |
| SMI, kg/m2 | 5.92 (0.57) **** | 7.54 (0.84) | 4.58 (0.61) **** | 5.64 (0.85) |
| LMI, kg/m2 | 15.1 (1.5) **** | 17.6 (2.0) | 13.7 (1.1) **** | 14.9 (1.4) |
| Phase angle | 4.05 (0.79) **** | 5.19 (0.87) | 3.62 (0.69) **** | 4.30 (0.88) |
| MTH, cm | 1.96 (0.64) **** | 2.91 (0.67) | 1.75 (0.57) **** | 2.28 (0.64) |
| SPPB (total) | 8.9 (2.3) **** | 11.1 (1.6) | 6.3 (2.6) **** | 10.1 (2.2) |
| Balance (score) | 3.54 (0.97) ** | 3.78 (0.67) | 2.64 (1.34) **** | 3.69 (0.78) |
| Gait speed, s/5 m | 0.83 (0.23) **** | 1.14 (0.39) | 0.56 (0.13) **** | 1.08 (1.06) |
| Chair stand, s/5 reps | 15.4 (6.7) **** | 10.5 (3.6) | 18.8 (8.4) **** | 12.6 (4.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2554. https://doi.org/10.3390/jcm9082554
Hirose S, Nakajima T, Nozawa N, Katayanagi S, Ishizaka H, Mizushima Y, Matsumoto K, Nishikawa K, Toyama Y, Takahashi R, et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. Journal of Clinical Medicine. 2020; 9(8):2554. https://doi.org/10.3390/jcm9082554
Chicago/Turabian StyleHirose, Suguru, Toshiaki Nakajima, Naohiro Nozawa, Satoshi Katayanagi, Hayato Ishizaka, Yuta Mizushima, Kazuhisa Matsumoto, Kaori Nishikawa, Yohei Toyama, Reiko Takahashi, and et al. 2020. "Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases" Journal of Clinical Medicine 9, no. 8: 2554. https://doi.org/10.3390/jcm9082554
APA StyleHirose, S., Nakajima, T., Nozawa, N., Katayanagi, S., Ishizaka, H., Mizushima, Y., Matsumoto, K., Nishikawa, K., Toyama, Y., Takahashi, R., Arakawa, T., Yasuda, T., Haruyama, A., Yazawa, H., Yamaguchi, S., Toyoda, S., Shibasaki, I., Mizushima, T., Fukuda, H., & Inoue, T. (2020). Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. Journal of Clinical Medicine, 9(8), 2554. https://doi.org/10.3390/jcm9082554