Cardiac Rehabilitation and Endothelial Function
Abstract
1. Introduction
2. Endothelial Function and Dysfunction
3. Assessment of Endothelial Function
3.1. Peripheral Circulation
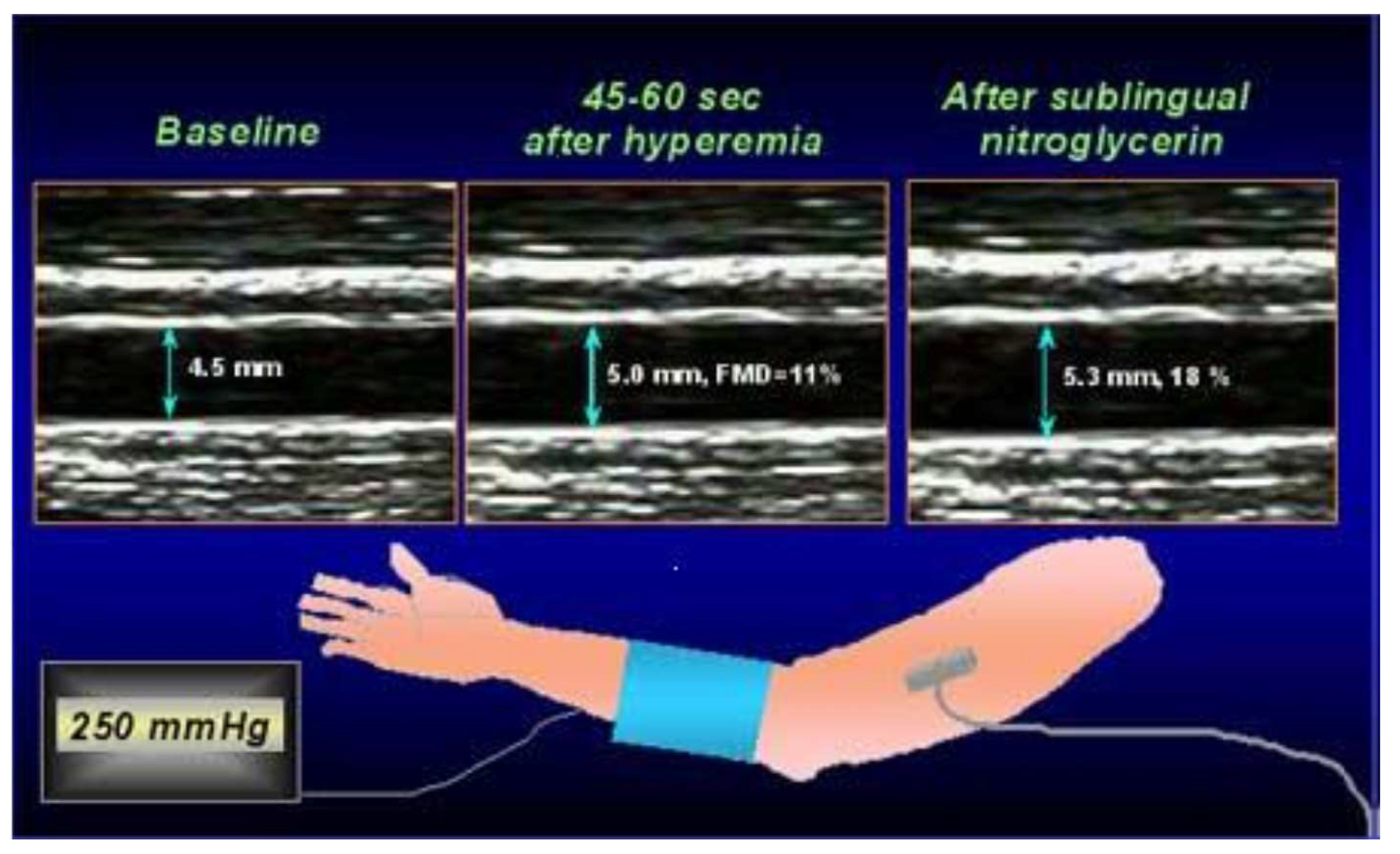
3.1.1. Flow-Mediated Dilatation
3.1.2. Peripheral Arterial Tonometry
3.1.3. Laboratory Markers
3.2. Coronary Circulation
4. Cardiac Rehabilitation and Endothelial Function
5. Acute Myocardial Infarction
6. Stable Coronary Artery Disease
7. Heart Failure
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K. Exercise-based rehabilitation for coronary heart disease (Review). J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.R.; Ades, P.A.; Thompson, P.D. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc. Med. 2017, 27, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.; Schmid, J.P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol 2020, (in press). [CrossRef] [PubMed]
- Prescott, E.; Mikkelsen, N.; Holdgaard, A.; Eser, P.; Marcin, T.; Wilhelm, M.; Gil, C.P.; González-Juanatey, J.R.; Moatemri, F.; Iliou, M.C.; et al. Cardiac rehabilitation in the elderly patient in eight rehabilitation units in Western Europe: Baseline data from the EU-CaRE multicentre observational study. Eur. J. Prev. Cardiol. 2019, 26, 1052–1063. [Google Scholar] [CrossRef]
- van Halewijn, G.; Deckers, J.; Tay, H.Y.; van Domburg, R.; Kotseva, K.; Wood, D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 232, 294–303. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011, 162, 571–584. [Google Scholar] [CrossRef]
- Heinl, R.E.; Dhindsa, D.S.; Mahlof, E.N.; Schult, W.M.; Ricketts, J.C. Comprehensive cardiovascular risk reduction and cardiac rehabilitation in diabetes and the metabolic syndrome. Can. J. Cardiol. 2016, 32 (Suppl. 2), S349–S357. [Google Scholar] [CrossRef]
- Kotseva, K.; Wood, D.; de Bacquer, D. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROSPIRE IV survey. Eur. J. Prev. Cardiol. 2018, 25, 1242–1251. [Google Scholar] [CrossRef]
- Lavie, C.J.; Menezes, A.R.; de Schutter, A.; Milani, R.V.; Blumenthal, J.A. Impact of cardiac rehabilitation and exercise training on psychological risk factors and subsequent prognosis in patients with cardiovascular disease. Can. J. Cardiol. 2016, 31 (Suppl. 2), S365–S373. [Google Scholar] [CrossRef]
- Leon, A.S.; Franklin, B.A.; Costa, F.; Balady, G.J.; Berra, K.A.; Stewart, K.J.; Thompson, P.D.; Williams, M.A.; Lauer, M.S. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American Heart Association Scientific Statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in Collaboration With the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005, 111, 369–376. [Google Scholar]
- Balady, G.J.; Ades, P.A.; Bittner, V.A.; Franklin, B.A.; Gordon, N.F.; Thomas, R.J.; Tomaselli, G.F.; Yancy, C.W. Referral, enrollment and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: A presidential advisory from the American Heart Association. Circulation 2011, 124, 2951–2960. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; John, G.F.C.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial functions. Arter. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar]
- Shi, Y.; Vanhoutte, P.M. Macro and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arter. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial dysfunction in hypercholesterolemia: Mechanisms, pathophysiological importance, and therapeutic interventions. Semin. Thromb. Hemost. 2000, 26, 529–537. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Issachar, A.; Marai, I.; Koren-Morag, N.; Freinark, D.; Shahar, Y.; Shechter, A.; Feinberg, M.S. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int. J. Cardiol. 2009, 134, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: A systematic review and metaanalysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Gokce, N.; Keaney, J.F.J.; Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via non invasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef]
- Schächinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic impact of coronary vasodilator dysfunction and adverse long-term outcome of coronary heart disease. Circulation 2000, 101, 1899–1906. [Google Scholar] [CrossRef]
- Halcox, J.P.J.; Schenk, W.H.; Zalos, G.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.A.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial function. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef]
- Neunteufl, T.; Heher, S.; Katzenschlager, R.; Wölfl, G.; Kostner, K.; Maurer, G.; Weidinger, F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am. J. Cardiol. 2000, 86, 207–210. [Google Scholar] [CrossRef]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef]
- Anderson, T.J.; Meredith, I.T.; Yeung, A.C.; Frei, B.; Selwyn, A.P.; Ganz, P. The effect of cholesterol-lowering and antioxidant therapy on endothelium dependent coronary vasomotion. N. Engl. J. Med. 1995, 332, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004, 561 Pt 1, 1–25. [Google Scholar] [CrossRef]
- Phillips, S.A.; Mahmoud, A.M.; Brown, M.D.; Haus, J.M. Exercise interventions and peripheral arterial function: Implications for cardiac metabolic disease. Progr. Cardiovasc. Dis. 2015, 57, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Majerczak, J.; Duda, K.; Chłopicki, S. Endurance training increases exercise-induced prostacyclin release in young, healthy men-relationship with VO2max. Pharmacol. Rep. 2010, 62, 494–502. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.J.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Takase, B.; Uehata, A.; Akima, T.; Nagai, T.; Nishioka, T.; Hamabe, A.; Satomura, K.; Ohsuzu, F.; Kurita, A. Endothelium-dependent flow mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am. J. Cardiol. 1998, 82, 1535–1539. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Luscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function. From Research Into Clinical Practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Storch, A.S.; de Mattos, J.D.; Alves, R.; Dos Santos Galdino, I.; Rocha, H.N.M. Methods of endothelial function assessment: Description and applicaions. Int. J. Card. Sci. 2017, 30, 262–273. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 3, 291–308. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, P.; Brodmann, M.; Cífková, R.; Cosentino, F.; de Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar]
- Ghiadoni, L.; Faita, F.; Salvetti, M.; Cordiano, C.; Biggi, A.; Puato, M.; di Monaco, A.; de Siati, L.; Volpe, M.; Ambrosio, G.; et al. Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. J. Hyperten. 2012, 30, 1399–1405. [Google Scholar] [CrossRef]
- Nerla, R.; Tarzia, P.; Sestito, A.; di Monaco, A.; Infusino, F.; Matera, D.; Greco, F.; Tacchino, R.M.; Lanza, G.A.; Crea, F. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 626–634. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Flammer, A.J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Phillips, S.A. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 2015, 57, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrinton, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Villano, A.; Mencarelli, E.; Melita, V.; Rizzi, A.; Lamendola, P.; de Vita, A.; Manfredonia, L.; Ravenna, S.E.; Pitocco, D.; Lanza, G.A.; et al. Endothelial dysfunction and cardiovascular outcome in asymptomatic patients with type 2 diabetes: A pilot study. Diabetes Metab. Res. Rev. 2020, 36, e3215. [Google Scholar] [CrossRef] [PubMed]
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.J.; Brandock, D.; Burggraaf, J. Evaluation of the EndoPAT as a tool to assess endothelial function. Int. J. Vasc. Med. 2012, 2012, 904141. [Google Scholar] [CrossRef]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008, 117, 2467–2474. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Mammen, A.; Mooney, P.; Alsheikh-Ali, A.A.; Karas, R.H. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc. Med. 2007, 12, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart. J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.B.; Vun, S.V.; Spark, J.I. A comparison of measures of endothelial function in patients with peripheral arterial disease and age and gender matched controls. Int. J. Cardiovasc. Med. 2016, 2016, 2969740. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Jang, W.S.; Kim, H.S.; Lee, J.H.; Choi, E.Y. Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron. Artery Dis. 2014, 25, 421–426. [Google Scholar] [CrossRef]
- Weisrock, F.; Fritschka, M.; Beckmann, S.; Litmeier, S.; Wagner, J.; Tahirovic, E.; Radenovic, S.; Zelenak, C.; Hashemi, D.; Busjahn, A.; et al. Reliability of peripheral arterial tonometry in patients with heart failure, diabetic nephropathy and arterial hypertension. Vasc. Med. 2017, 22, 292–300. [Google Scholar] [CrossRef]
- Nil, M.; Schafer, D.; Radtke, T.; Saner, H.; Wilhelm, M.; Eser, P. Reproducibility of peripheral arterial tonometry measurements in male cardiovascular patients. Eur. J. Clin. Investig. 2014, 44, 1065–1071. [Google Scholar] [CrossRef]
- Fujita, Y.; Asahara, T. Evaluation of circulating endothelial progenitor cells in cardiovascular risk. Circ. J. 2011, 75, 2541–2542. [Google Scholar] [CrossRef]
- Marin, V.; Kaplanski, G.; Gres, S.; Farnarier, C.; Bongrand, P. Endothelial cell culture: Protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods 2001, 254, 183–190. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Dakak, N.; Mulcahy, D.; Andrews, N.P.; Husain, S.; Panza, J.A.; Cannon, R.O. Nitric oxide activity in the atherosclerotic human coronary circulation. J. Am. Coll. Cardiol. 1997, 29, 308–317. [Google Scholar] [CrossRef]
- Joye, J.D.; Schulman, D.S. Clinical application of coronary flow reserve using an intracoronary Doppler guide wire. Cardiol. Clin. 1997, 15, 101–129. [Google Scholar] [CrossRef]
- Fearon, W.M.; Kobayashi, Y. Invasive assessment of the coronary microvasculature. The index of microcirculatory resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Randè, J.L.; Dupouy, P.; Aptecar, E.; Bhati, A.; Teiger, E.; Hittinger, L.; Berdeaux, A.; Castaigne, A.; Geschwind, H. Comparison of the effects of exercise and cold pressure test on the vasomotor response of normal and atherosclerotic coronary arteries and their relation to the flow-mediated mechanism. Am. J. Cardiol. 1995, 76, 467–473. [Google Scholar] [CrossRef]
- Raizner, A.E.; Chahine, R.A.; Ishimori, T.; Verani, M.S.; Zacca, N.; Jamal, N.; Miller, R.R.; Luchi, R.J. Provocation of coronary artery spasm by the cold pressor test. Hemodynamic, arteriographic and quantitative angiographic observations. Circulation 1980, 62, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Pham, I.; Nguyen, M.T.; Valensi, P.; Rousseau, H.; Nitenberg, A.; Vicaut, E.; Cosson, E. Noninvasive study of coronary microcirculation response to a cold pressure test. Eur. J. Clin. Investig. 2015, 45, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Dibben, G.O.; Dalal, H.M.; Taylor, R.S.; Doherty, P.; Tang, L.H.; Hillsdon, M. Cardiac rehabilitation and physical activity: Systematic review and meta-analysis. Heart 2018, 104, 1394–1402. [Google Scholar] [CrossRef]
- Rauch, B.; Davos, C.H.; Doherty, P.; Saure, D.; Metzendorf, M.I.; Salzwedel, A.; Völler, H.; Jensen, K.; Schmid, J.P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 2016, 23, 1914–1939. [Google Scholar] [CrossRef]
- Salzwedel, A.; Jensen, K.; Rauch, B.; Doherty, P.; Metzendorf, M.I.; Hackbusch, M.; Völler, H.; Schmid, J.P.; Davos, C.H. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur. J. Prev. Cardiol 2020, (in press). [CrossRef]
- Vona, M.; Rossi, A.; Capodaglio, P.; Rizzo, S.; Servi, P.; de Marchi, M.; Cobelli, F. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am. Heart J. 2004, 147, 1039–1046. [Google Scholar] [CrossRef]
- Lee, K.W.; Blann, A.D.; Jolly, K.; Lip, G.Y. BRUM Investigators. Plasma haemostatic markers, endothelial function and ambulatory blood pressure changes with home versus hospital cardiac rehabilitation: The Birmingham Rehabilitation Uptake Maximisation Study. Heart 2006, 92, 1732–1738. [Google Scholar] [CrossRef]
- Peller, M.; Balsam, P.; Główczyńska, R.; Ossoliński, K.; Gilarowska, A.; Kołtowski, Ł.; Grabowski, M.; Filipiak, K.J.; Opolski, G. The impact of physical training on endothelial function in myocardial infarction survivors: Pilot study. Kardiol. Pol. 2016, 74, 439–446. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Ribeiro, F.; Silva, G.; Alves, A.J.; Silva, N.; Guimarães, J.T.; Teixeira, M.; Oliveira, J. Effect of exercise-based cardiac rehabilitation on arterial stiffness and inflammatory and endothelial dysfunction biomarkers: A randomized controlled trial of myocardial infarction patients. Atherosclerosis 2015, 239, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Onkelinx, S.; Goetschalckx, K.; Thomaes, T.; Janssens, S.; Fagard, R.; Verhamme, P.; Vanhees, L. Exercise-based cardiac rehabilitation improves endothelial function assessed by flow-mediated dilation but not by pulse amplitude tonometry. Eur. J. Prev. Cardiol. 2014, 21, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.G.; Schofield, R.S.; Lennon, S.L.; Pierce, G.L.; Nichols, W.W.; Braith, R.W. Effect of Exercise Training on Endothelial Function in Men with Coronary Artery Disease. Am. J. Cardiol. 2004, 93, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Vita, J.A.; Bader, D.S.; Sherman, D.L.; Hunter, L.M.; Holbrook, M.; O’Malley, C.; Keaney, J.F.J.; Balady, G.J. Effect of Exercise on Upper and Lower Extremity Endothelial Function in Patients with Coronary Artery Disease. Am. J. Cardiol. 2002, 90, 124–127. [Google Scholar] [CrossRef]
- Gagliardi, J.A.; Maciel, N.; Castellano, J.L.; Masoli, O.; Miksztowicz, V.; Berg, G.; Bermejo, E.; Lazzari, M.; Gelpi, R.J. Relationship between endothelial progenitor cells and vascular endothelial growth factor and its variation with exercise. Thromb. Res. 2016, 137, 92–96. [Google Scholar] [CrossRef]
- Conraads, V.M.; Pattyn, N.; de Maeyer, C.; Beckers, P.J.; Coeckelberghs, E.; Cornelissen, V.A.; Denollet, J.; Frederix, G.; Goetschalckx, K.; Hoymans, V.Y.; et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. Int. J. Cardiol. 2015, 179, 203–210. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Frederix, G.; Pattyn, N.; Beckers, P.; van Craenenbroeck, A.H.; Gavaert, A.; Possemiers, N.; Cornelissen, V.; Goetschalckx, K.; Vrints, C.J.; et al. Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: A SAINTEX-CAD substudy. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1876–H1882. [Google Scholar] [CrossRef]
- Hambrecht, R.; Wolf, A.; Gielen, S.; Linke, A.; Hofer, J.; Erbs, S.; Schoene, N.; Schuler, G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N. Engl. J. Med. 2000, 342, 454–460. [Google Scholar] [CrossRef]
- Tanaka, S.; Sanuki, Y.; Ozumi, K.; Harada, T.; Tasaki, H. Heart failure with preserved vs reduced ejection fraction following cardiac rehabilitation: Impact of endothelial function. Heart Vessel. 2018, 33, 886–892. [Google Scholar] [CrossRef]
- Legallois, D.; Belin, A.; Nesterov, S.V.; Milliez, P.; Parienti, J.J.; Knuuti, J.; Abbas, A.; Tirel, O.; Agostini, D.; Manrique, A. Cardiac rehabilitation improves coronary endothelial function in patients with heart failure due to dilated cardiomyopathy: A positron emission tomography study. Eur. J. Prev. Cardiol. 2016, 23, 129–136. [Google Scholar] [CrossRef]
- Katz, S.D.; Schwarz, M.; Yuen, J.; LeJemtel, T.H. Impaired acetylcholine mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation 1993, 88, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.H.; Rector, T.S.; Bank, A.J.; Williams, R.E.; Heifetz, S.M. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 1991, 84, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.D.; Hryniewicz, K.; Hriljac, I.; Balidemaj, K.; Dimayuga, C.; Hudaihed, A.; Yasskiy, A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005, 111, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Mörtl, D.; Strecker, K.; Hülsmann, M.; Kulemann, V.; Neunteufl, T.; Pacher, R.; Berger, R. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: Comparison with B-type natriuretic peptide. J. Am. Coll. Cardiol. 2005, 46, 1011–1018. [Google Scholar] [CrossRef]
- Bjarnason-Wehrens, B.; Nebel, R.; Jensen, K.; Hackbusch, M.; Grilli, M.; Gielen, S.; Schwaab, B.; Rauch, B. German Society of Cardiovascular Prevention and Rehabilitation (DGPR). Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2020, 27, 929–952. [Google Scholar]
- De Arauro Pio, C.S.; Marzolini, S.; Pakosh, M.; Grace, S.L. Effect of cardiac rehabilitation dose on mortality and morbidity: A Systematic Review and Meta-regression Analysis. Mayo Clin. Proc. 2017, 92, 1644–1659. [Google Scholar]


| Study | Population | No. Patients | Study Design | Assessment of ED | Exercise Program | Results |
|---|---|---|---|---|---|---|
| Vona [68] | Recent AMI | 52 | RCT | FMD, CPT | 3 months of moderate aerobic ET | CR significantly improved ED vasodilation (p < 0.01) |
| Lee [69] | Previous AMI or coronary revascularization | 81 | RCT | FMD, vWF | 3 months of home/hospital-based CR program | CR improved FMD and reduced vWF (all p ≤ 0.001) |
| Peller [70] | Recent AMI | 25 | Prospective uncontrolled | RHI-PAT | 4 weeks (12–24 ET session) | CR did not significantly improve endothelial function (p = 0.14) |
| Oliveira [71] | Recent AMI | 96 | RCT | Endothelial biomarkers | 8 weeks of aerobic ET at 70–85% of maximal HR during 3 weekly sessions | CR did not reduce the markers of ED |
| Cornelissen [72] | Stable CAD | 146 | Prospective uncontrolled | FMD, RHI-PAT | 12 weeks (3 sessions per week at an intensity of 80% of HR reserve | CR improved FMD (p < 0.0001), but not RHI (p = 0.47) |
| Edwards [73] | Stable CAD | 18 | Prospective controlled | FMD, nitrites, nitrates | 12 weeks (3 times per week of treadmill walking and stationary cycling at an intensity of 40–50%, to a maximum 70–85% of HR reserve. | CR improved FMD and increase nitrate and nitrite levels |
| Gocke [74] | Stable CAD | 58 | Prospective controlled | FMD | 10 weeks of leg exercise of moderate intensity (30 min 3 times per week) | CR improved FMD (p = 0.02), in particular in the exercised limbs |
| Gagliardi [75] | Stable CAD | 21 | RCT | VEGF, EPCs | 12 weeks (three weekly exercise bout) | CR determined a reduction of EPC and an increase in VEGF |
| SAINTEX-CAD study [76,77] | Stable CAD | 200 | RCT | FMD, EPCs | 12 weeks of aerobic interval vs. continuous ET on a bicycle | Both ET programs improved FMD, but had no effect on EPCs |
| Hambrect [78] | Stable CAD | 19 | RCT | Coronary epicardial and MV response to Ach | 4 weeks (10 min 6 time a day on a bicycle ergometer to the 80% of HR) | CR improved epicardial and MV endothelial response to Ach |
| Tanaka [79] | HFrEF/HFpEF | 78 | Retrospective study | FMD | 5 months (2–3 times per week of training on a cycle ergometer for 20 min at anaerobic threshold level) | CR did not improve FMD, but improved exercise capacity in patients with ED at baseline |
| Legallois [80] | HF in DCM | 29 | Prospective study | MBF response to CPT | 12 weeks (36 aerobic ET sessions on the basis of ventilatory threshold) | CR improved MBF response to CPT (p < 0.001) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, G.A.; Golino, M.; Villano, A.; Lanza, O.; Lamendola, P.; Fusco, A.; Leggio, M. Cardiac Rehabilitation and Endothelial Function. J. Clin. Med. 2020, 9, 2487. https://doi.org/10.3390/jcm9082487
Lanza GA, Golino M, Villano A, Lanza O, Lamendola P, Fusco A, Leggio M. Cardiac Rehabilitation and Endothelial Function. Journal of Clinical Medicine. 2020; 9(8):2487. https://doi.org/10.3390/jcm9082487
Chicago/Turabian StyleLanza, Gaetano Antonio, Michele Golino, Angelo Villano, Oreste Lanza, Priscilla Lamendola, Augusto Fusco, and Massimo Leggio. 2020. "Cardiac Rehabilitation and Endothelial Function" Journal of Clinical Medicine 9, no. 8: 2487. https://doi.org/10.3390/jcm9082487
APA StyleLanza, G. A., Golino, M., Villano, A., Lanza, O., Lamendola, P., Fusco, A., & Leggio, M. (2020). Cardiac Rehabilitation and Endothelial Function. Journal of Clinical Medicine, 9(8), 2487. https://doi.org/10.3390/jcm9082487






