Induction of CD4+CD25+ Regulatory T Cells from In Vitro Grown Human Mononuclear Cells by Sparteine Sulfate and Harpagoside
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Blood
2.2. Reagents
2.3. Natural Molecule Discovery
2.4. Real-Time q-PCR
2.5. Flow Cytometry
2.6. Cytokine Quantitation
2.7. Allogeneic Mixed Lymphocyte Reaction (MLR)
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
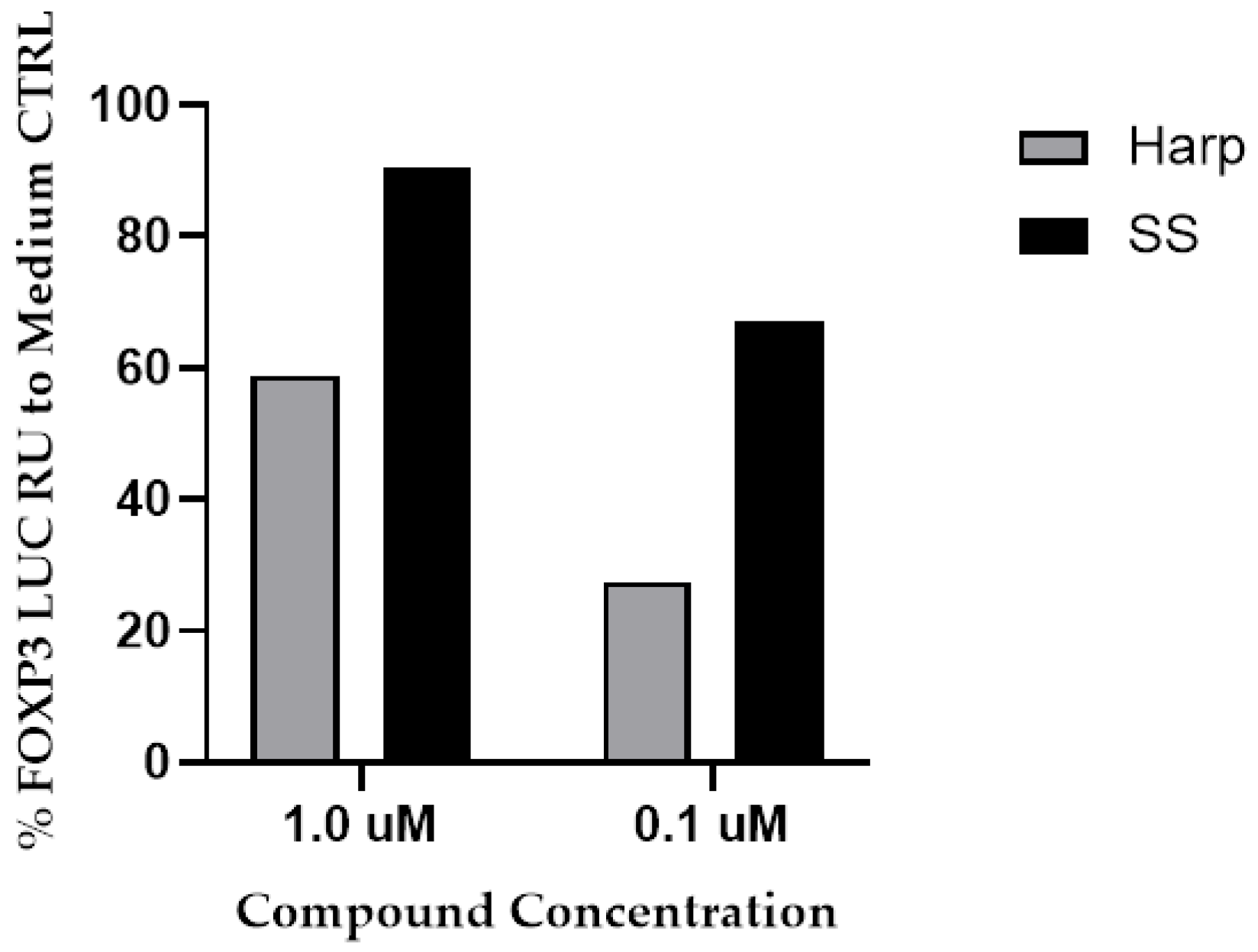
3.1. SS and Harp Induced the Expression of Luciferase in the FoxP3 Reporter Cell Line
3.2. Cytotoxicity of SS and Harp on PBMCs In Vitro
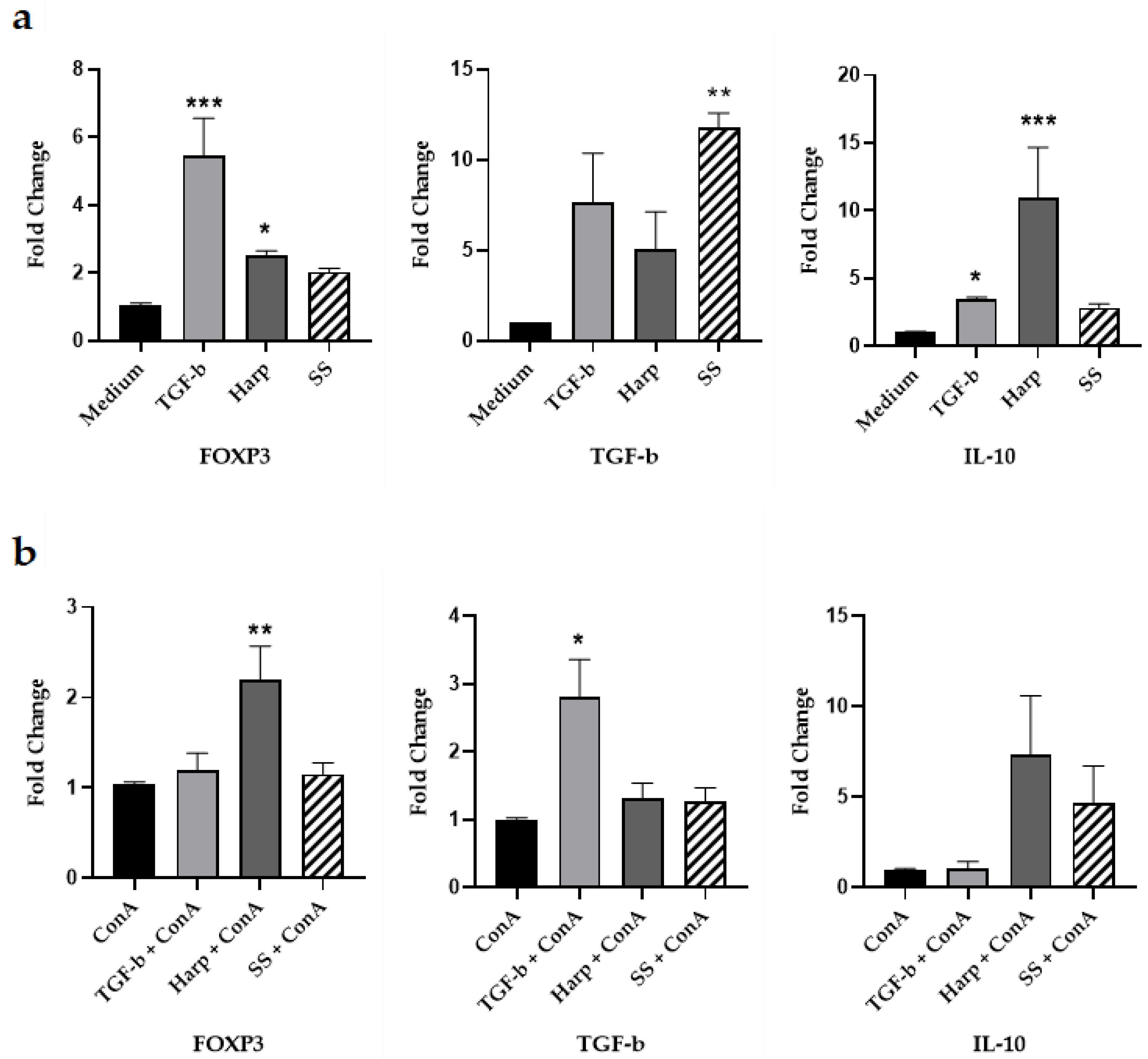
3.3. Harp and SS Upregulate Expression of mRNA for Treg Cell Markers In Vitro
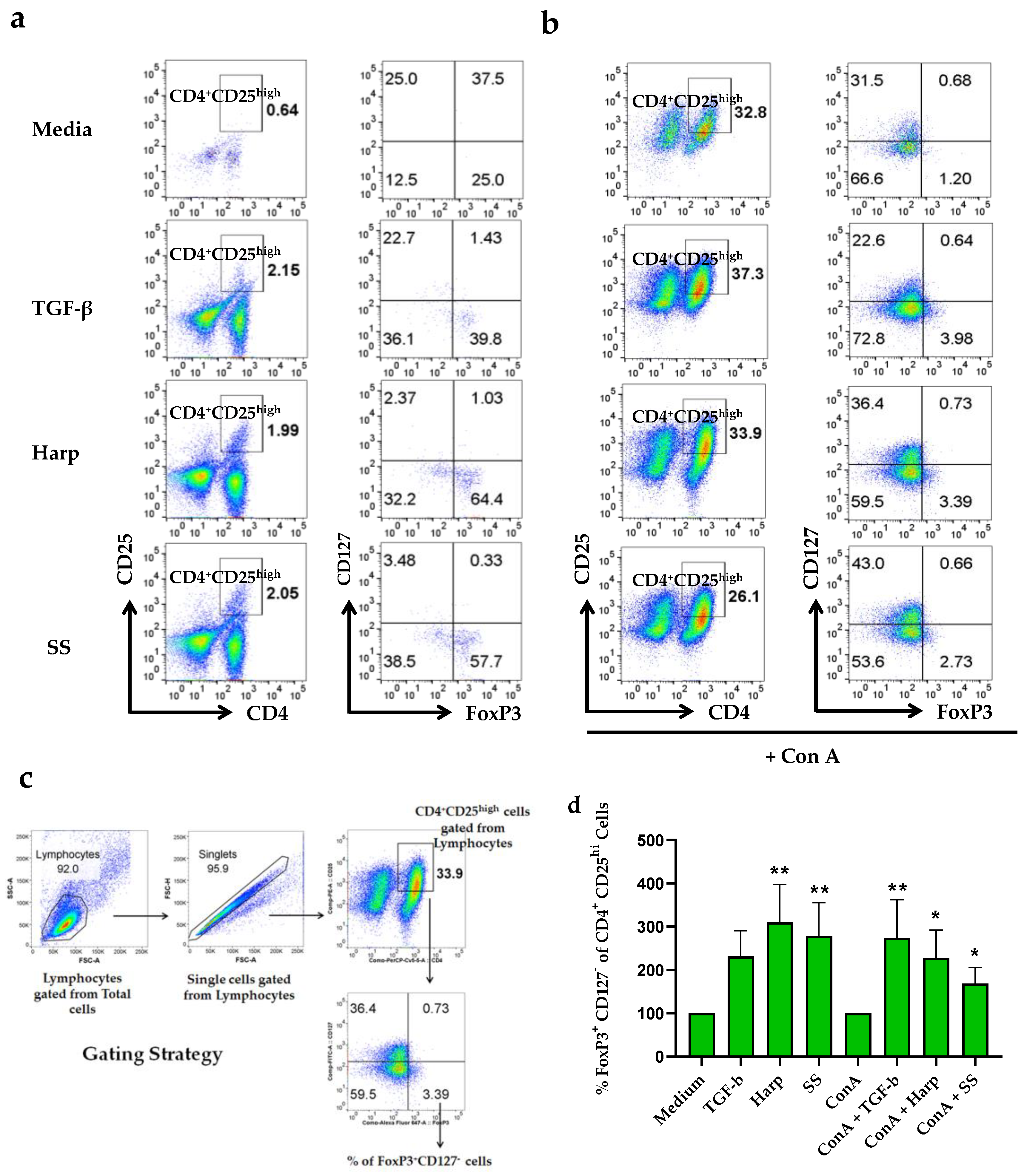
3.4. Harp and SS Increase Percentege of CD4+CD25+ Treg Cells in PBMCs In Vitro
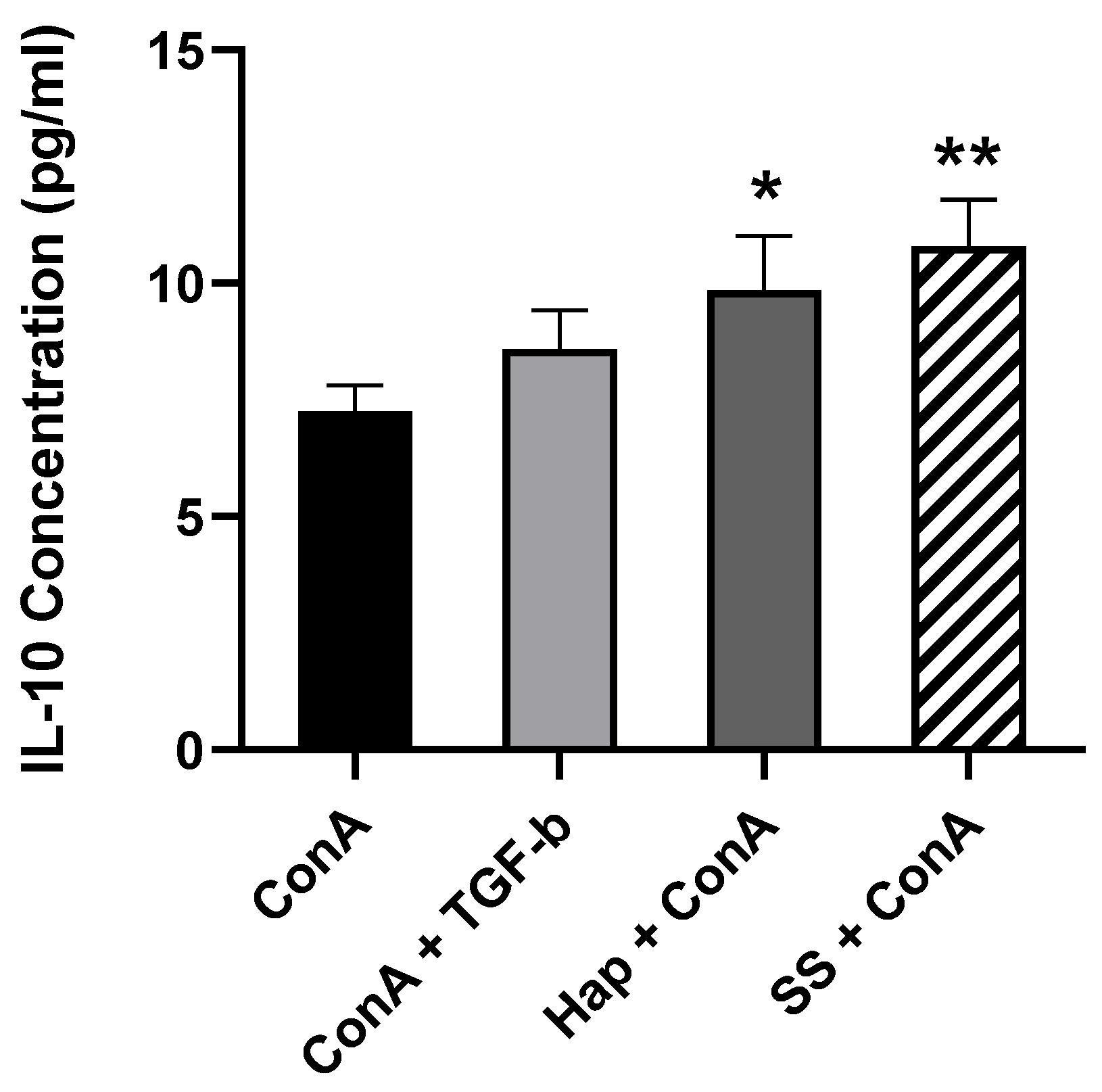
3.5. SS and Harp Induced the Production of the Anti-Inflammatory Cytokine IL-10
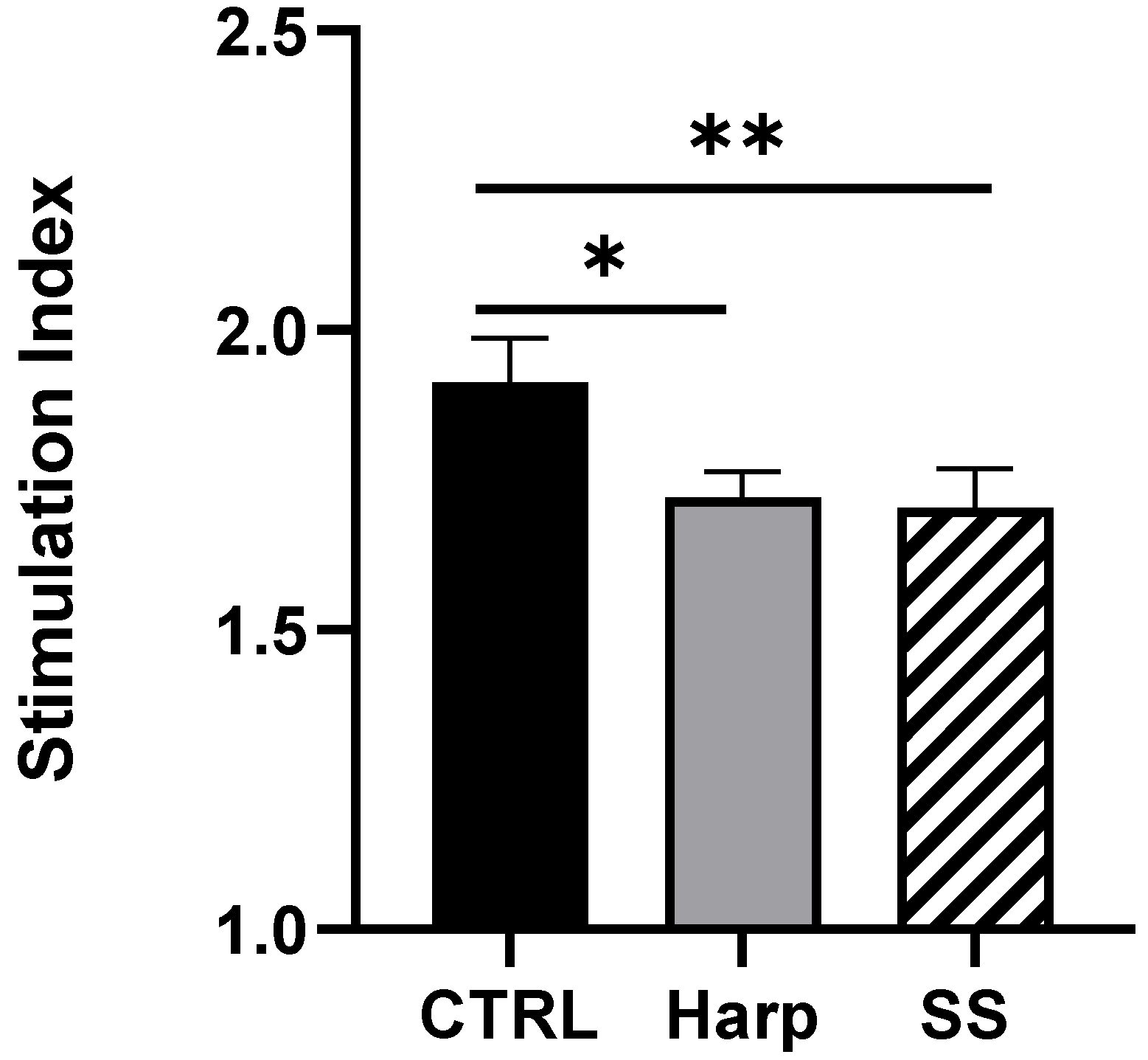
3.6. Harp and SS Are Able to Suppress T Cell-Mediated Allogeneic MLR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SS | Sparteine Sulfate |
| Harp | Harpagoside |
| PBMCs | Peripheral Blood Mononuclear Cells |
| ConA | Concanavalin A |
| FoxP3 | Fork Head Box P3 |
| TGF-β | Transformation Growth Factor-β |
| IL-10 | Interleukin-10 |
| MLR | Mixed Lymphocyte Reaction |
References
- Nishizuka, Y.; Sakakura, T. Thymus and reproduction: Sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 1969, 166, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Noelle, R.J.; Blazar, B.R. Cd4+ Cd25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001, 193, 1311–1318. [Google Scholar] [CrossRef]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Srivastava, S.; Stolley, J.M.; Campbell, D.J. Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol. Rev. 2014, 259, 40–59. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in auto-immune diseases: Challenges, controversies and yet unanswered questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Blue-stone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH-17 cells in auto-immune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Acharya, S.; Fathman, C.G. CD4+ CD25+ Regulatory T Cells as Adoptive Cell Therapy for Autoimmune Disease and for the Treatment of Graft-Versus-Host Disease. In Regulatory T Cells and Clinical Application; Springer: New York, NY, USA, 2008; pp. 231–252. [Google Scholar]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The de-velopment and function of regulatory T cells. Cell. Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor B and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Malmström, V.; Powrie, F. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of Cd25+ Cd4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000, 192, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.; Nolan, D.; Reed, A. Altered lymphocyte markers and blastogenic responses associated with 24 hour delay in processing of blood samples. J. Immunol. Methods 1982, 50, 187–191. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef]
- Cohen, J.L.; Trenado, A.; Vasey, D.; Klatzmann, D.; Salomon, B.L. CD4+ CD25+ immunoregulatory T cells: New therapeutics for graft-versus-host disease. J. Exp. Med. 2002, 196, 401–406. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Boehm, F.; Martin, M.; Kesselring, R.; Schiechl, G.; Geissler, E.; Schlitt, H.J.; Fichtner–Feigl, S. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012, 12, 97. [Google Scholar] [CrossRef]
- Yu, N.; Li, X.; Song, W.; Li, N.; Yu, D.; Zeng, X.; Li, M.; Leng, X.; Li, X. CD4+ CD25+ CD127 low/− T cells: A more specific treg population in human peripheral blood. Inflammation 2012, 35, 1773–1780. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Chen, D.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-β induces Foxp3+ T-regulatory cells from CD4+ CD25− precursors. Am. J. Transplant. 2004, 4, 1614–1627. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Li, M. Baicalin, a natural compound, promotes regulatory T cell differentiation. BMC Complement. Altern. Med. 2012, 12, 64. [Google Scholar] [CrossRef]
- Guo, A.; He, N.; Xu, H.-B.; Geng, C.-A.; Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 2015, 5, 14046. [Google Scholar] [CrossRef] [PubMed]
- Raschack, M. Actions of sparteine and sparteine derivatives on the heart and circulation. Arzneimittelforschung 1974, 24, 753–759. [Google Scholar] [PubMed]
- Park, K.S. A systematic Review on anti-inflammatory activity of harpagoside. J. Biochem. Mol. Boil. Res. 2016, 2, 166–169. [Google Scholar] [CrossRef]
- Clemo, G.R.; Raper, R.; Short, W.S. 142. The lupin alkaloids. J. Chem. Soc. 1949, 14, 663–665. [Google Scholar] [CrossRef]
- Vargas, F.V.V.; Medina-Ceja, L. Sparteine as an anticonvulsant drug: Evidence and possible mechanism of action. Seizure 2016, 39, 49–55. [Google Scholar] [CrossRef]
- Newton, B.W.; Benson, R.C.; McCorriston, C.C. Sparteine sulfate: A potent, capricious oxytocic. Am. J. Obstet. Gynecol. 1966, 94, 234–241. [Google Scholar] [CrossRef]
- Mendel, E.B.; Buring, D.M. Use of oxytocin and sparteine in postpartum hemorrhage caused by uterine atony. Tex. Med. 1972, 68, 101–103. [Google Scholar]
- Gaze, S.; McSorley, H.J.; Daveson, J.; Jones, D.; Bethony, J.M.; Oliveira, L.M.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; Croese, J.; et al. Characterizing the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012, 8, e1002520. [Google Scholar] [CrossRef]
- Kruisbeek, A.M.; Shevach, E.M.; Thornton, A.M. Proliferative assays for T cell function. Curr. Protoc. Immunol. 2004, 60, 3–12. [Google Scholar] [CrossRef]
- Jurukovski, V.; Dabovic, B.; Todorovic, V.; Chen, Y.; Rifkin, D.B. Methods for Measuring TGF-β Using Antibodies, Cells, and Mice; Varga, J., Brenner, D.A., Phan, S.H., Eds.; Humana Press: Totowa, NJ. USA, 2005; Volume 117. [Google Scholar]
- Robertson, I.; Rifkin, D.B. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb. Perspect. Boil. 2016, 8, a021907. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Heiden, M.G.; Harris, M.H.; Frauwirth, K.A.; Thompson, C.B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell 2000, 6, 683–692. [Google Scholar] [CrossRef]
- Haller, D.; Blum, S.; Bode, C.; Hammes, W.P.; Schiffrin, E.J. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: Evidence of NK cells as primary targets. Infect. Immun. 2000, 68, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Adair, P.R.; Kim, Y.C.; Zhang, A.-H.; Yoon, J.; Scott, D.W. Human tregs made antigen specific by gene modification: The power to treat autoimmunity and antidrug antibodies with precision. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, Y.; Feng, X.; Jiang, J.; Chen, T.; Xie, H.; Zhou, L.; Zheng, S. Hepatic stellate cells promote immunotolerance following orthotopic liver transplantation in rats via induction of T cell apoptosis and regulation of Th2/Th3 like cell cytokine production. Exp. Ther. Med. 2013, 5, 165–169. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Zhou, J.; He, W.; Luo, G. Mixed lymphocyte reaction induced by multiple alloantigens and the role for IL-10 in proliferation inhibition. Burn. Trauma 2014, 2, 24–28. [Google Scholar] [CrossRef]
- Yu, P.; Gregg, R.K.; Bell, J.J.; Ellis, J.S.; Divekar, R.; Lee, H.-H.; Jain, R.; Waldner, H.; Hardaway, J.C.; Collins, M.; et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J. Immunol. 2005, 174, 6772–6780. [Google Scholar] [CrossRef]
- Huang, T.H.; Tran, V.H.; Duke, R.K.; Tan, S.; Chrubasik, S.; Roufogalis, B.; Duke, C.C. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-κB activation. J. Ethnopharmacol. 2006, 104, 149–155. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atwany, N.Z.; Hashemi, S.-K.; Jayakumar, M.N.; Nagarkatti, M.; Nagarkatti, P.; Hassuneh, M.R. Induction of CD4+CD25+ Regulatory T Cells from In Vitro Grown Human Mononuclear Cells by Sparteine Sulfate and Harpagoside. Biology 2020, 9, 211. https://doi.org/10.3390/biology9080211
Atwany NZ, Hashemi S-K, Jayakumar MN, Nagarkatti M, Nagarkatti P, Hassuneh MR. Induction of CD4+CD25+ Regulatory T Cells from In Vitro Grown Human Mononuclear Cells by Sparteine Sulfate and Harpagoside. Biology. 2020; 9(8):211. https://doi.org/10.3390/biology9080211
Chicago/Turabian StyleAtwany, Nour Z., Seyedeh-Khadijeh Hashemi, Manju Nidagodu Jayakumar, Mitzi Nagarkatti, Prakash Nagarkatti, and Mona Rushdi Hassuneh. 2020. "Induction of CD4+CD25+ Regulatory T Cells from In Vitro Grown Human Mononuclear Cells by Sparteine Sulfate and Harpagoside" Biology 9, no. 8: 211. https://doi.org/10.3390/biology9080211
APA StyleAtwany, N. Z., Hashemi, S.-K., Jayakumar, M. N., Nagarkatti, M., Nagarkatti, P., & Hassuneh, M. R. (2020). Induction of CD4+CD25+ Regulatory T Cells from In Vitro Grown Human Mononuclear Cells by Sparteine Sulfate and Harpagoside. Biology, 9(8), 211. https://doi.org/10.3390/biology9080211






