Suppressive Effects of Hainosan (Painongsan) against Biofilm Production by Streptococcus mutans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crude Drugs and Extract Preparation
2.2. Bacterial Strains
2.3. In Vitro Growth Study
2.4. Safranin Red Staining
2.5. Confocal Laser Microscopy
2.6. Scanning Electron Microscopic (SEM)
2.7. Bacterial Morphology
2.8. Cell Surface Hydrophobicity
2.9. Quantitative Real-time RT-PCR
2.10. Statistical Analysis
3. Results
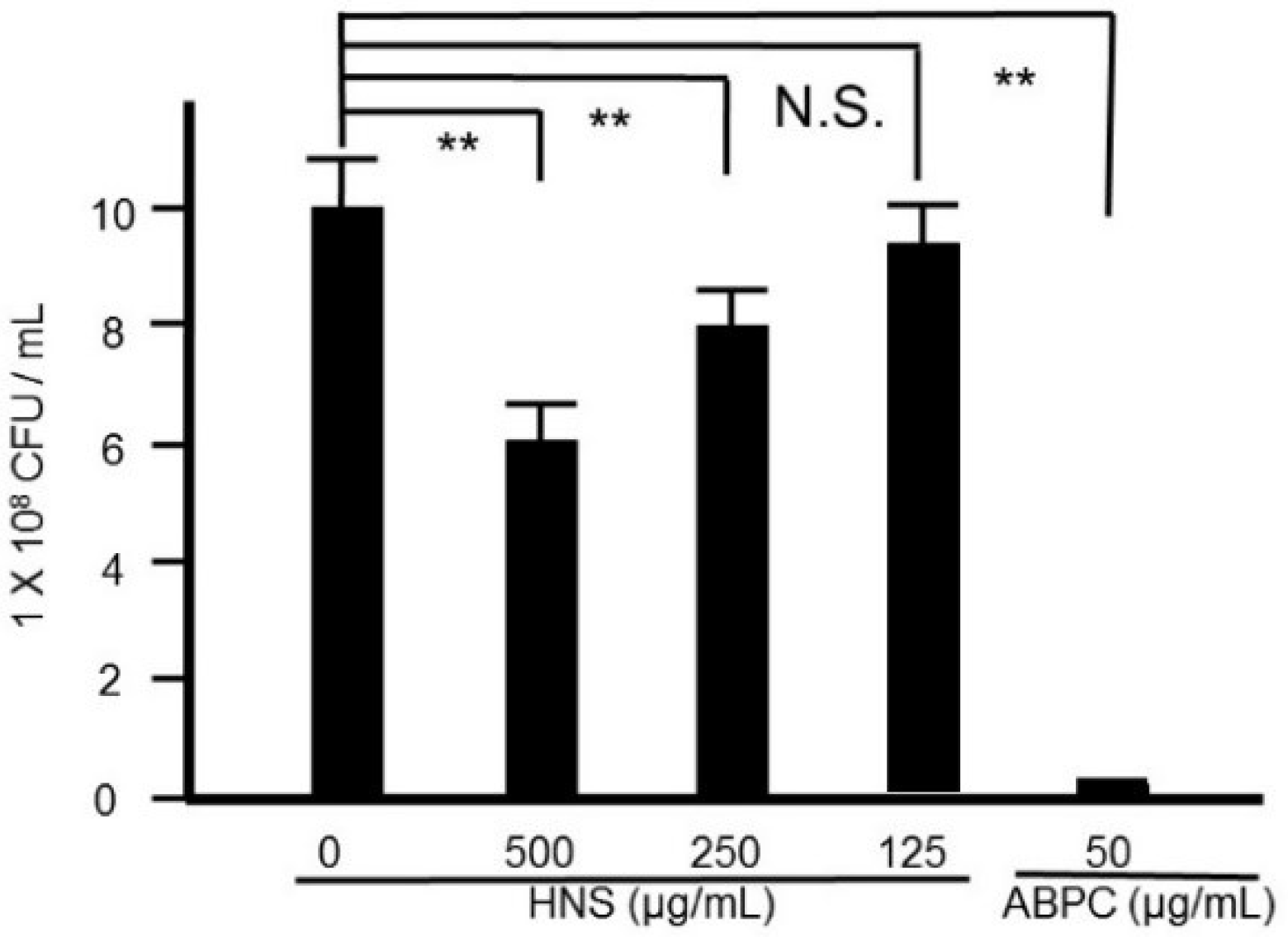
3.1. S. mutans Growth
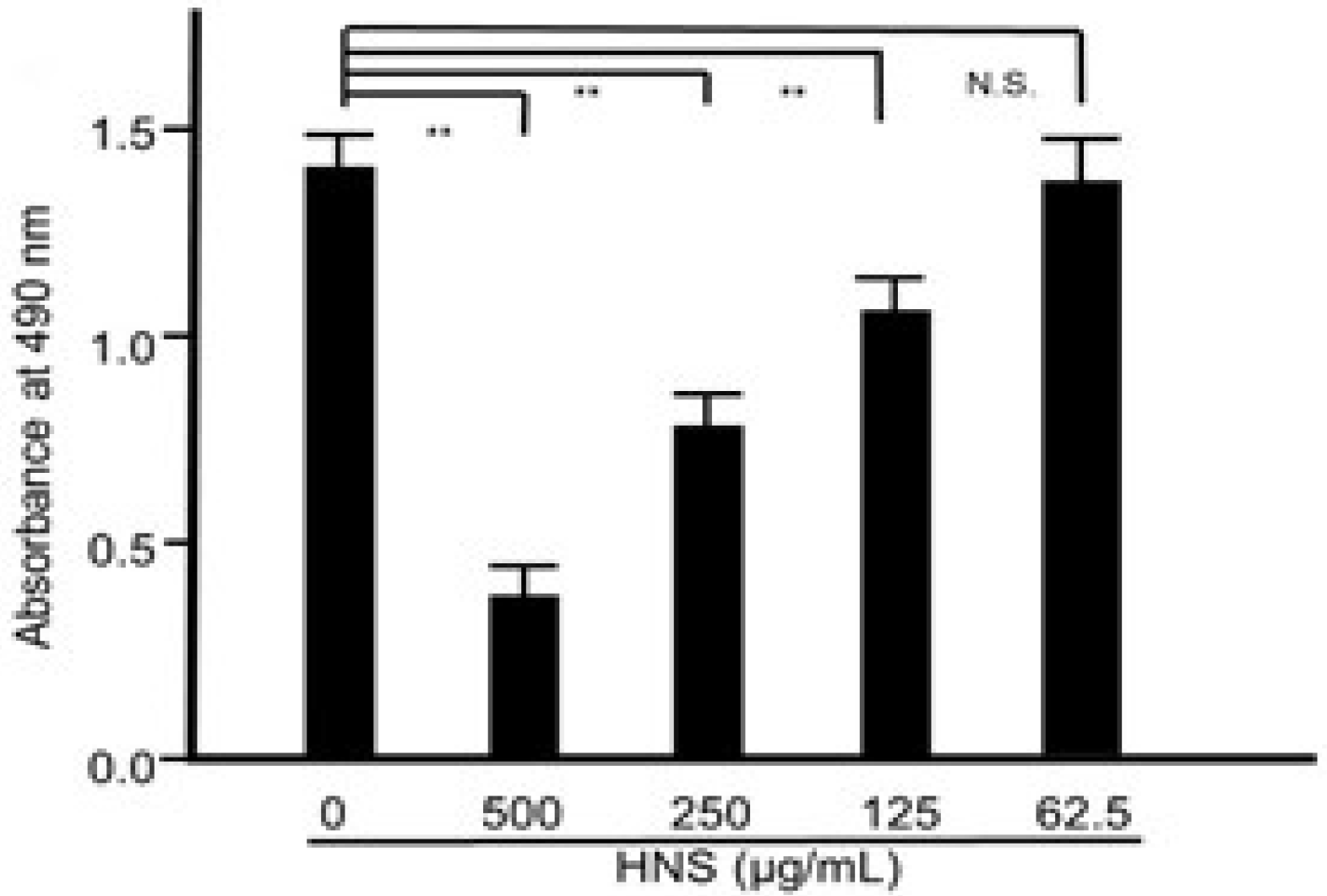
3.2. Safranin Red Staining
3.3. Confocal Laser Scanning Microscopy
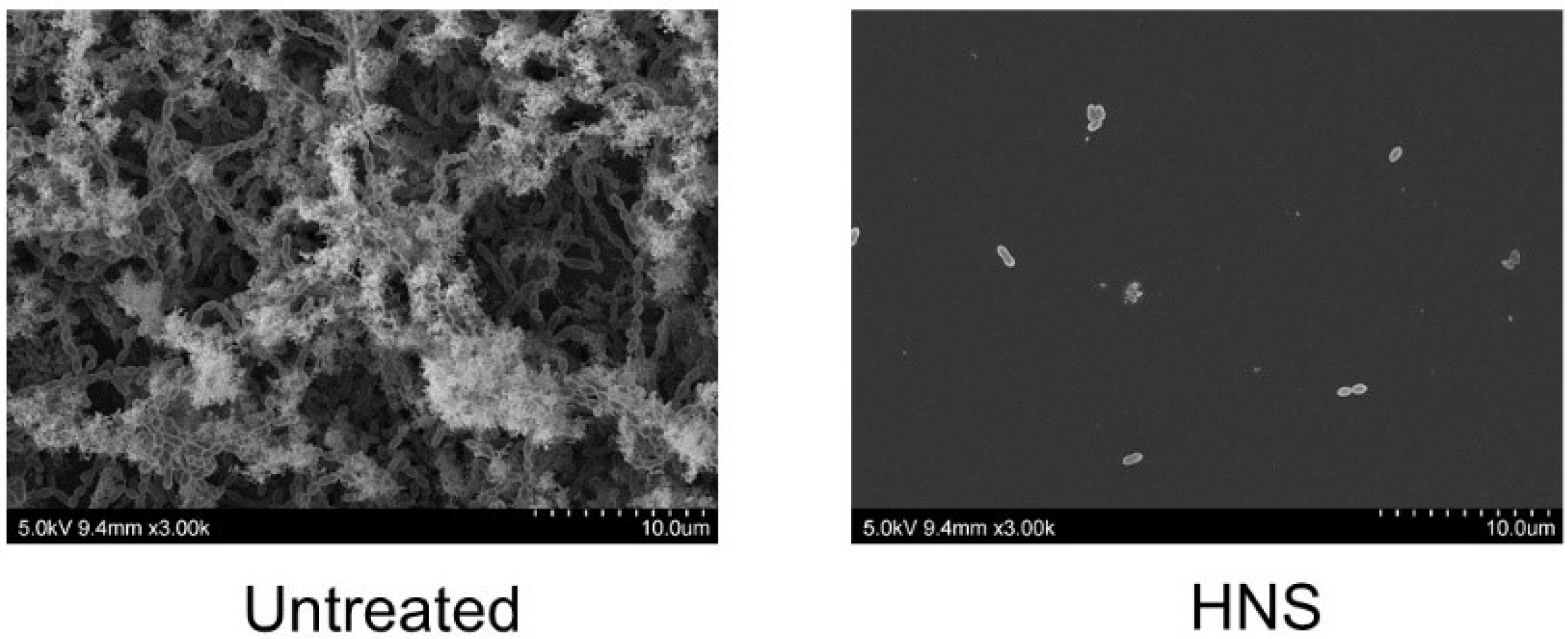
3.4. Scanning Electron Microscopy (SEM)
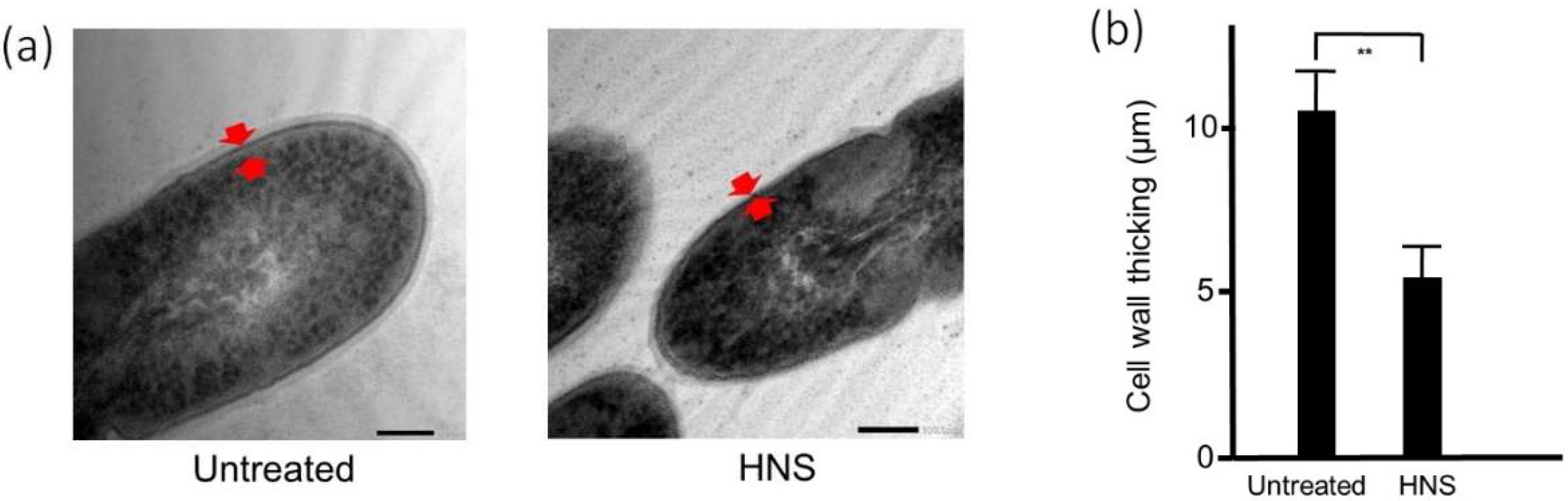
3.5. Transmission Electron Microscopy (TEM)
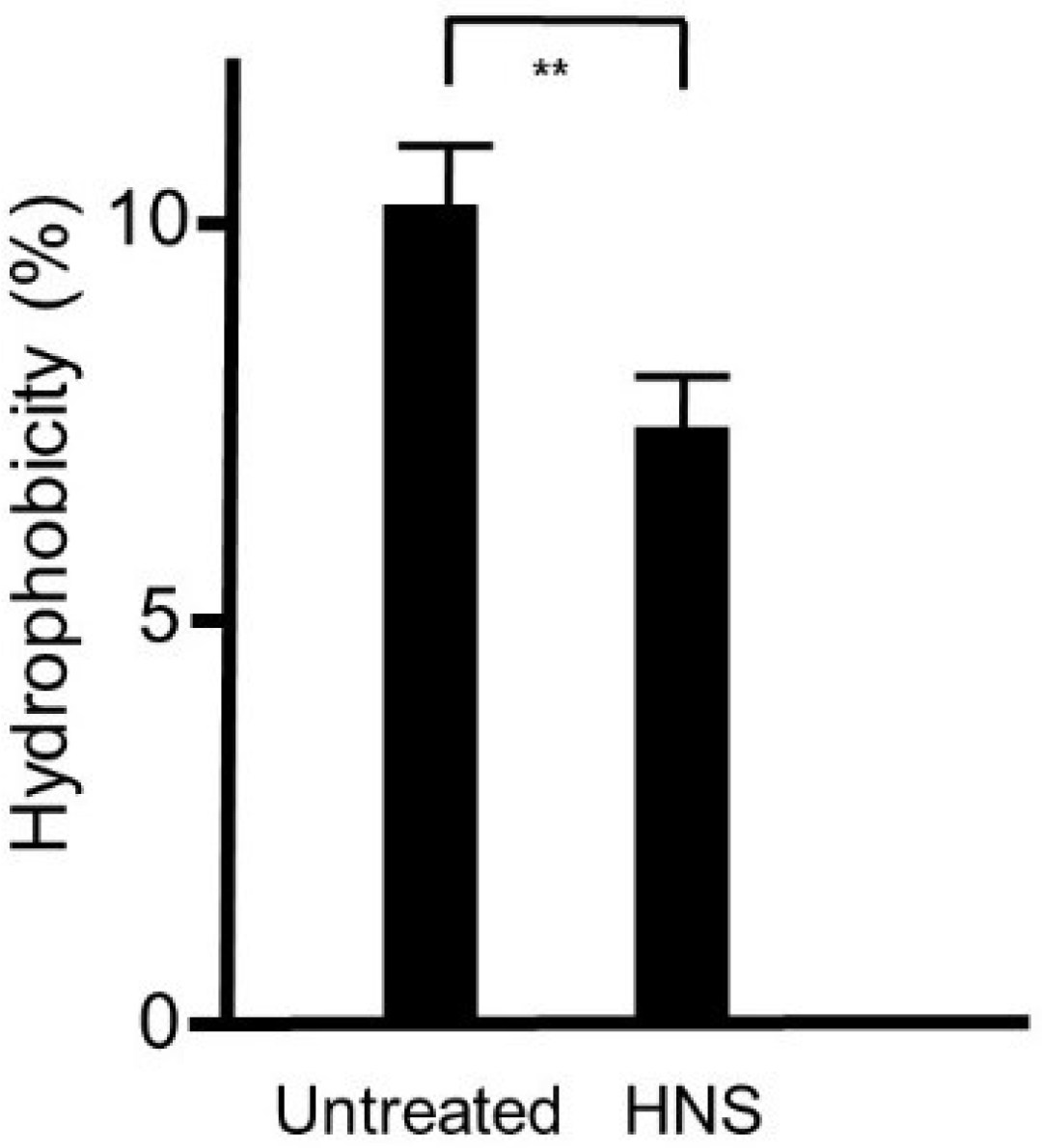
3.6. Hydrophobicity of the Bacterial Cell Surface
3.7. Effects of HNS on Related mRNA Expression of Biofilm Formation
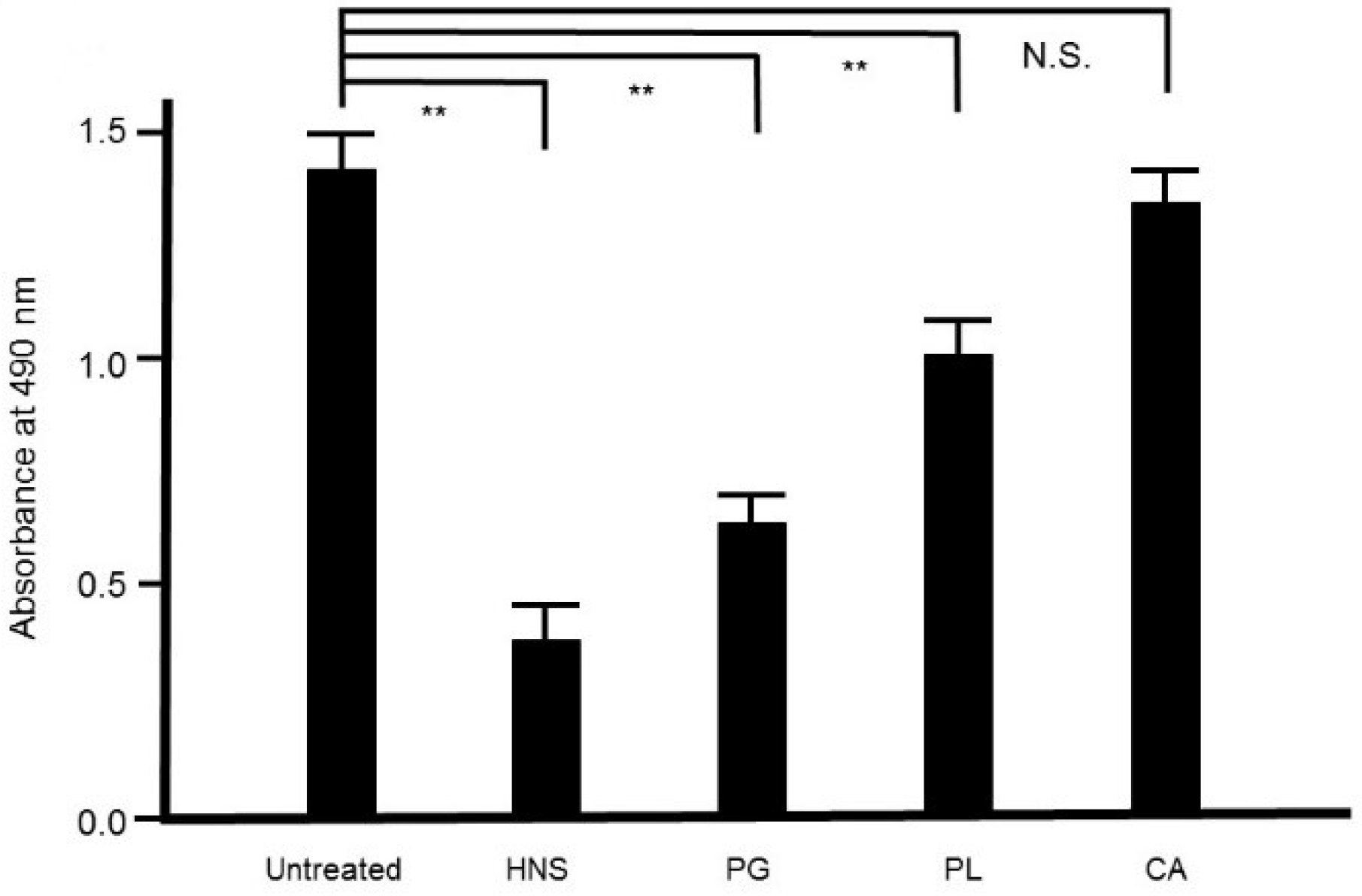
3.8. Effect of the Extracts of each Constituent Crude Drug of HNS on S. mutans Biofilm
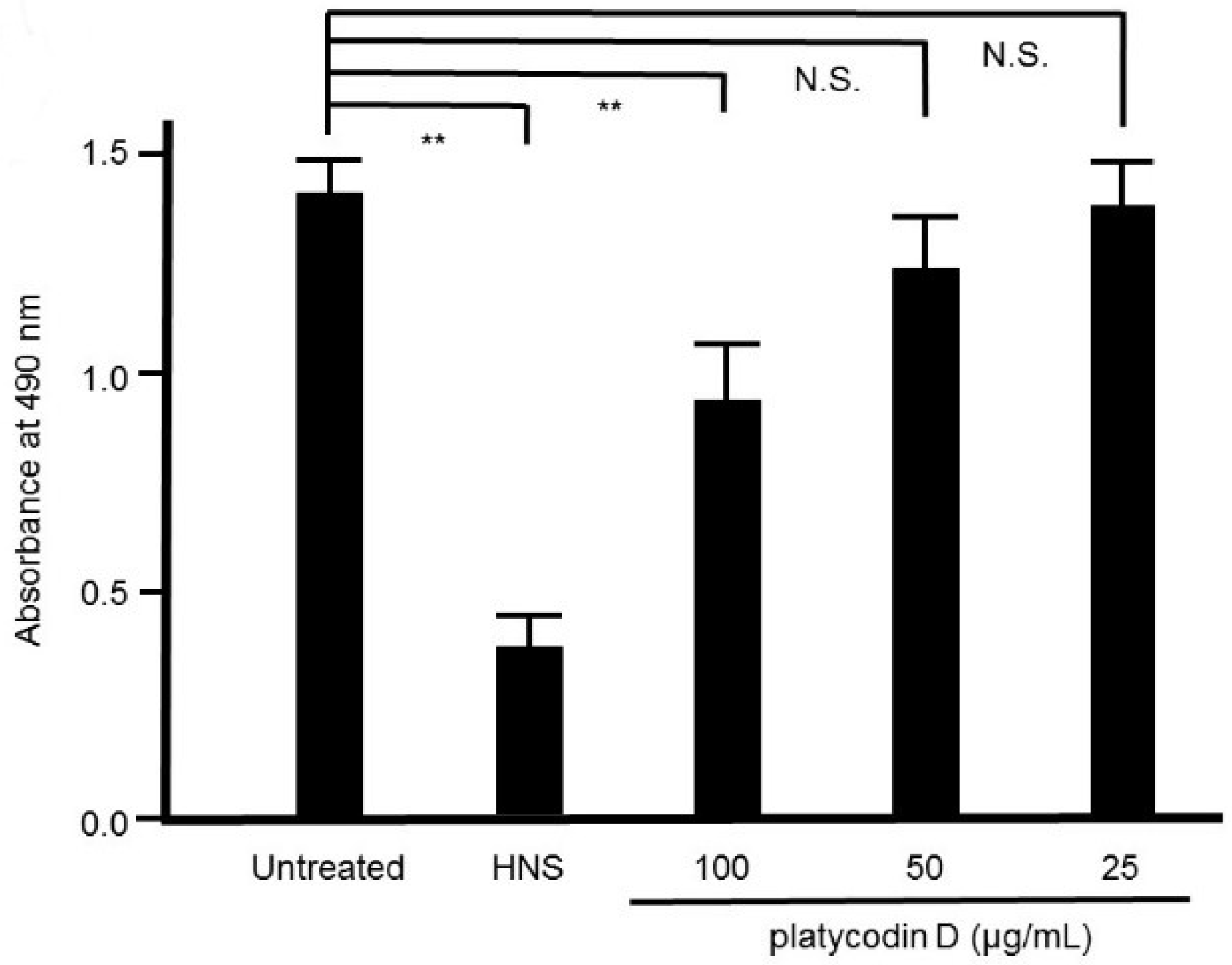
3.9. Effect of Platycodin D on S. mutans Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community-implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Millhouse, E.; Shaw, T.; Lappin, D.F.; Rajendran, R.; Bagg, J.; Lin, H.; Ramage, G. Evaluating Streptococcus mutans strain dependent characteristics in a polymicrobial biofilm community. Front. Microbiol. 2018, 9, 1498. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, S.; Hamada, S. Studying biofilm formation of mutans streptococci. Methods Enzymol. 1999, 310, 513–523. [Google Scholar]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent properties in Streptococcus mutans biofilms are controlled through adhesion force sensing by initial colonizers. mBio 2019, 10, e01908-19. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Abachi, S.; Lee, S.; Rupasinghe, H.P. Molecular mechanisms of inhibition of Streptococcus species by phytochemicals. Molecules 2016, 21, 215. [Google Scholar] [CrossRef] [Green Version]
- Japan Kampo Medicines Manufactures Association. Handbook on OTC Medicinal Product in Kampo; Jiho Inc.: Tokyo, Japan, 2013. [Google Scholar]
- Minami, M.; Takase, H.; Taira, M.; Makino, T. Hainosan (painongsan) suppresses the biofilm formation of Porphyromonas gingivalis and Prevotella intermedia in vitro. Tradit. Kampo Med. 2019, 6, 79–87. [Google Scholar] [CrossRef]
- Minami, M.; Takase, H.; Taira, M.; Makino, T. In vitro effect of the traditional medicine Hainosan (Painongsan) on Porphyromonas gingivalis. Medicines 2019, 6, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ). Japanese Pharmacopoeia Seventeenth Edition (JP XVII); Pharmaceutical and Medical Device Regulatory Science Society of Japan: Tokyo, Japan, 2016. [Google Scholar]
- Minami, M.; Takase, H.; Nakamura, M.; Makino, T. Effect of Loniceracaerulea var. emphyllocalyx fruit on biofilm formed by Porphyromonas gingivalis. BioMed Res. Int. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, M.; Konishi, T.; Takase, H.; Makino, T. Shin’iseihaito (Xinyiqingfeitang) suppresses the biofilm formation of Streptococcus pneumoniae in vitro. BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Savabi, O.; Kazemi, M.; Kamali, S.; Salehi, A.R.; Eslami, G.; Tahmourespour, A.; Salehi, R. Effects of biosurfactant produced by Lactobacillus casei on gtfB, gtfC, and ftf gene expression level in S. mutans by real-time RT-PCR. Adv. Biomed. Res. 2014, 3, 231. [Google Scholar]
- Banas, J.A. Virulence properties of Streptococcus mutans. Front. Biosci. 2004, 9, 1267–1277. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Hanada, N.; Kuramitsu, H.K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect. Immun. 1988, 56, 1999–2005. [Google Scholar] [CrossRef] [Green Version]
- Hanada, N.; Kuramitsu, H.K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 1989, 57, 2079–2085. [Google Scholar] [CrossRef] [Green Version]
- Mandava, K.; Batchu, U.R.; Kakulavaram, S.; Repally, S.; Chennuri, I.; Bedarakota, S.; Sunkara, N. Design and study of anticaries effect of different medicinal plants against S. mutans glucosyltransferase. BMC Complementary Altern. Med. 2019, 19, 197. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Furukawa, S.; Fujita, S.; Mitobe, J.; Kawarai, T.; Narisawa, N.; Sekizuka, T.; Kuroda, M.; Ochiai, K.; Ogihara, H.; et al. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl. Environ. Microbiol. 2011, 77, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [Green Version]
- Tahmourespour, A.; Kasra-Kermanshahi, R.; Salehi, R. Lactobacillus rhamnosus biosurfactant inhibits biofilm formation and gene expression of caries-inducing Streptococcus mutans. Dent. Res. J. 2019, 16, 87–94. [Google Scholar] [CrossRef]
- Yoshida, A.; Ansai, T.; Takehara, T.; Kuramitsu, H.K. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 2005, 71, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leme, A.P.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The role of sucrose in cariogenic dental biofilm formation-new insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative. Appl. Microbiol. Biotechnol. 2016, 100, 857–867. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, M.; Takase, H.; Taira, M.; Makino, T. Suppressive Effects of Hainosan (Painongsan) against Biofilm Production by Streptococcus mutans. Dent. J. 2020, 8, 71. https://doi.org/10.3390/dj8030071
Minami M, Takase H, Taira M, Makino T. Suppressive Effects of Hainosan (Painongsan) against Biofilm Production by Streptococcus mutans. Dentistry Journal. 2020; 8(3):71. https://doi.org/10.3390/dj8030071
Chicago/Turabian StyleMinami, Masaaki, Hiroshi Takase, Masayo Taira, and Toshiaki Makino. 2020. "Suppressive Effects of Hainosan (Painongsan) against Biofilm Production by Streptococcus mutans" Dentistry Journal 8, no. 3: 71. https://doi.org/10.3390/dj8030071




