HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition
Abstract
1. Introduction
2. Results
2.1. HNG Increases the Proliferation of Dermal Papilla Cells
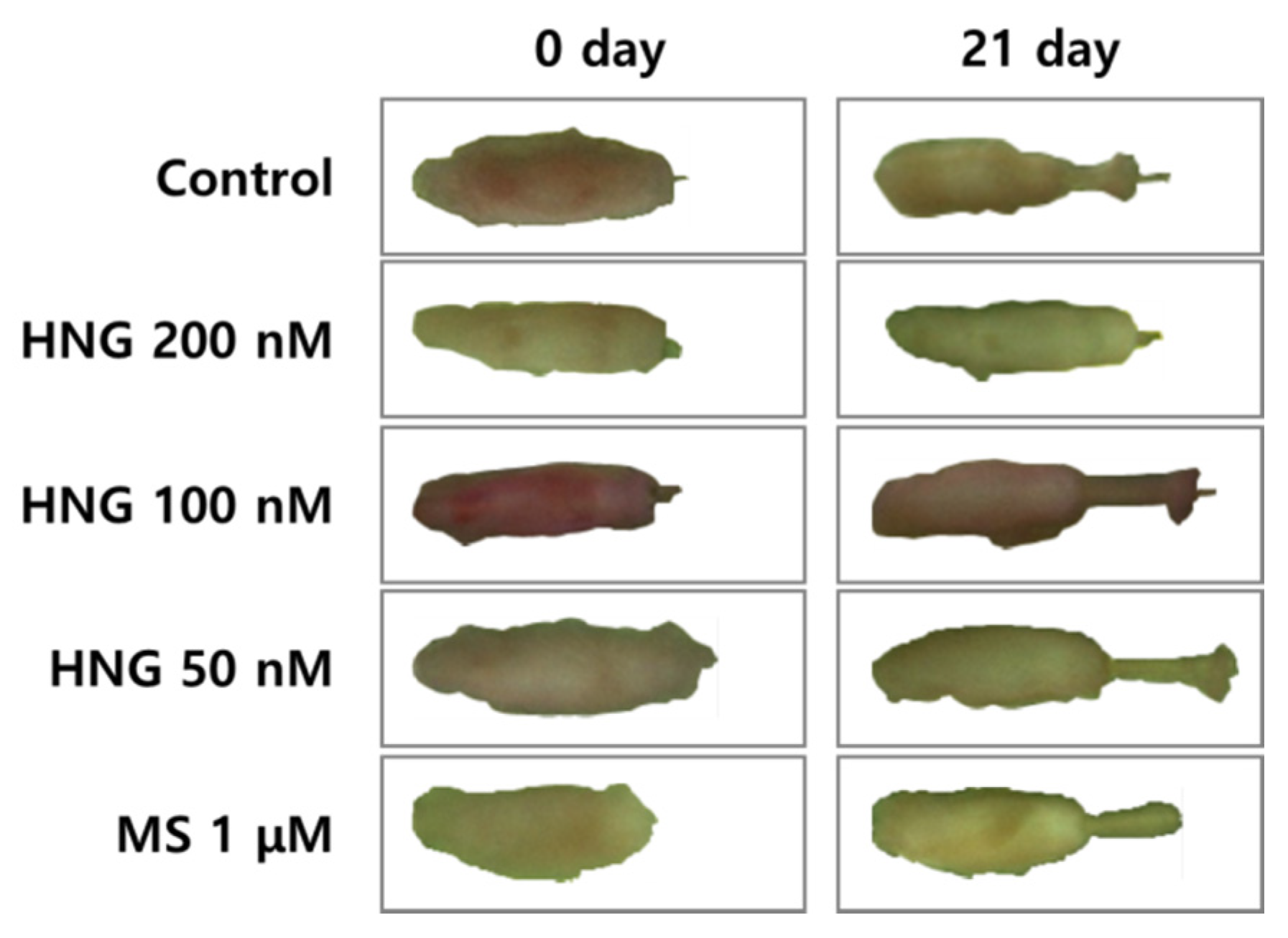
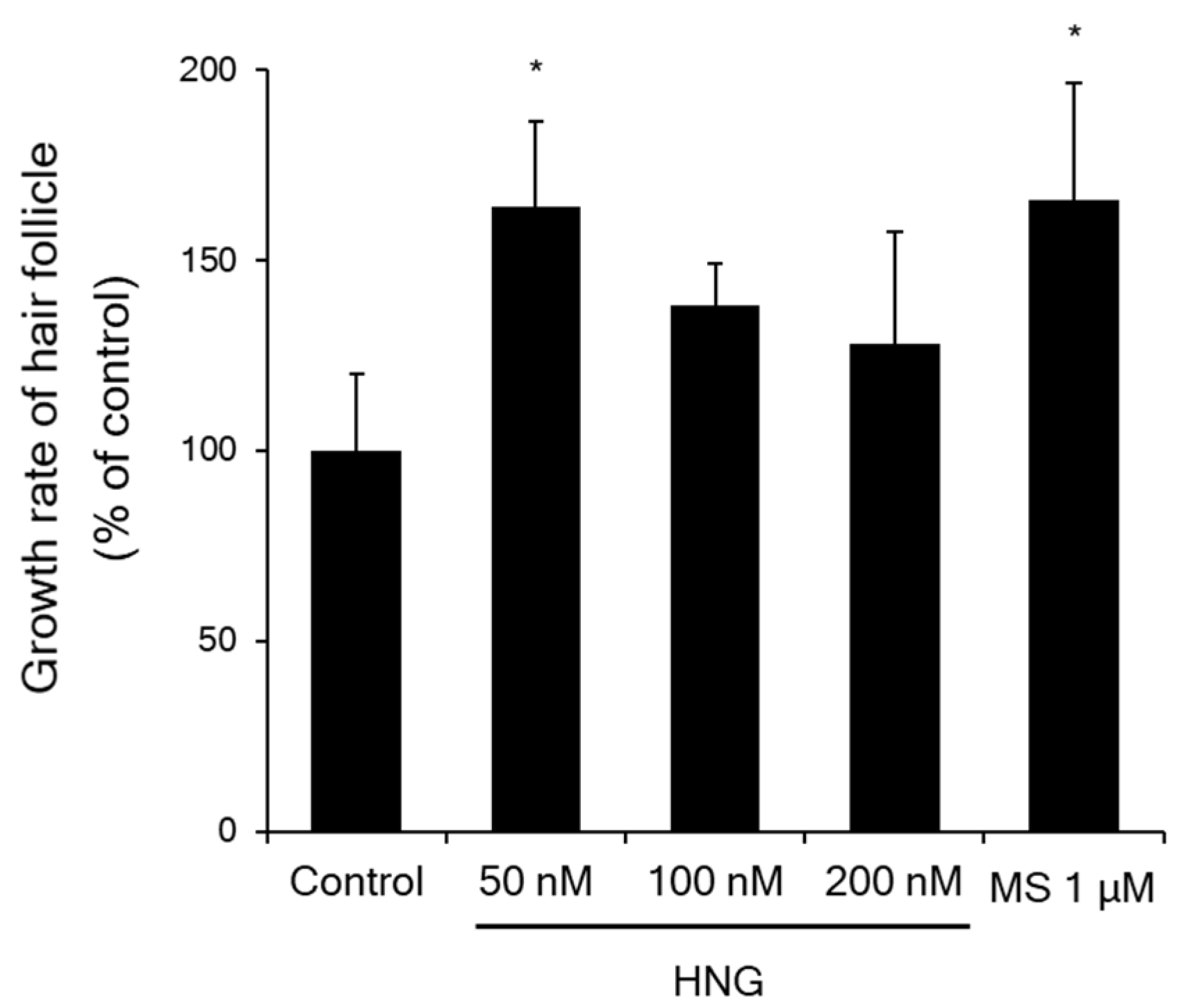
2.2. HNG Increases Hair Shaft Elongation Ex Vivo
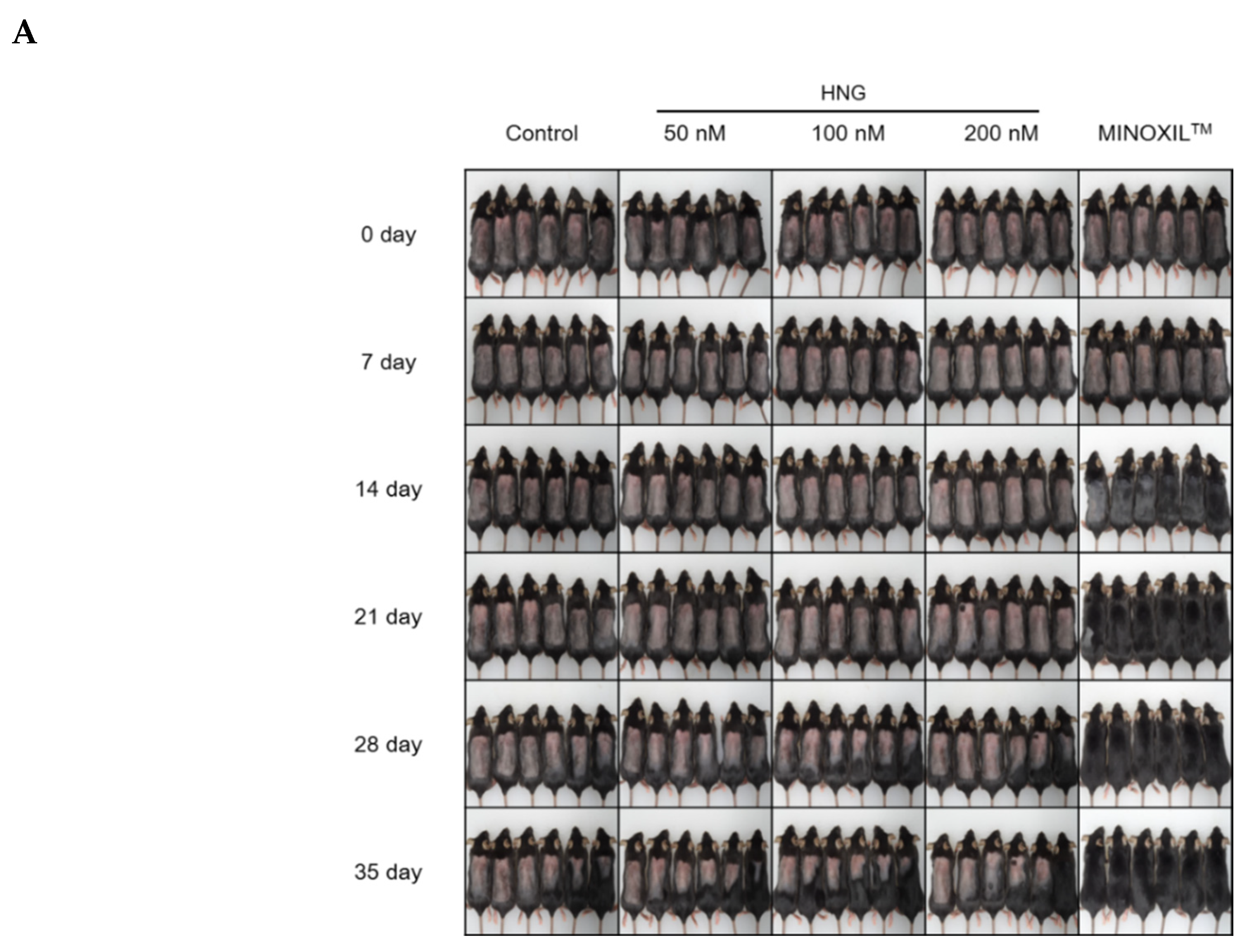
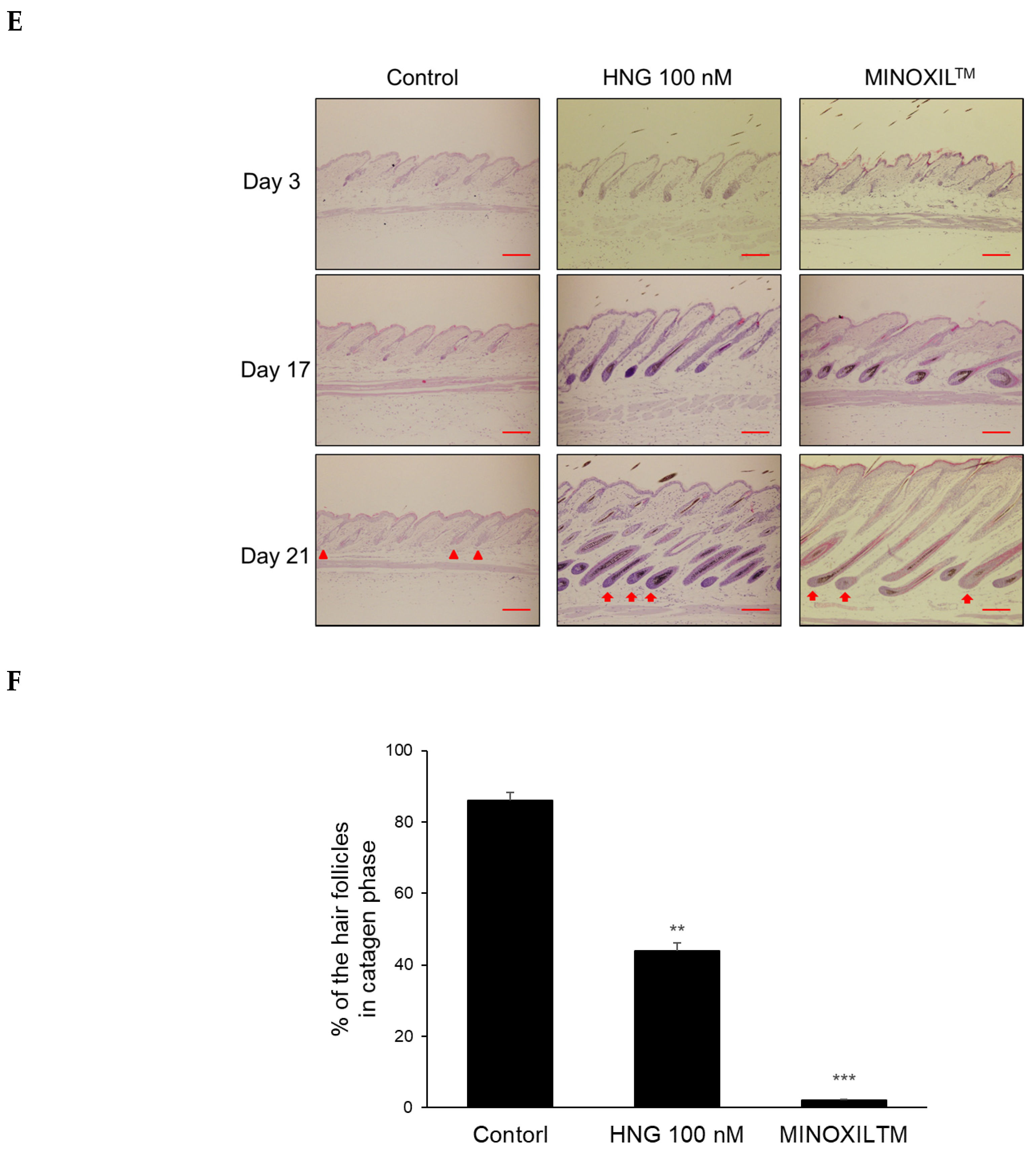
2.3. HNG Promotes In Vivo Hair Growth
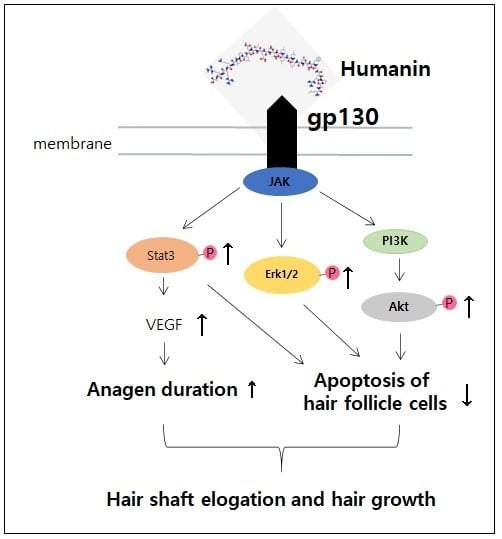
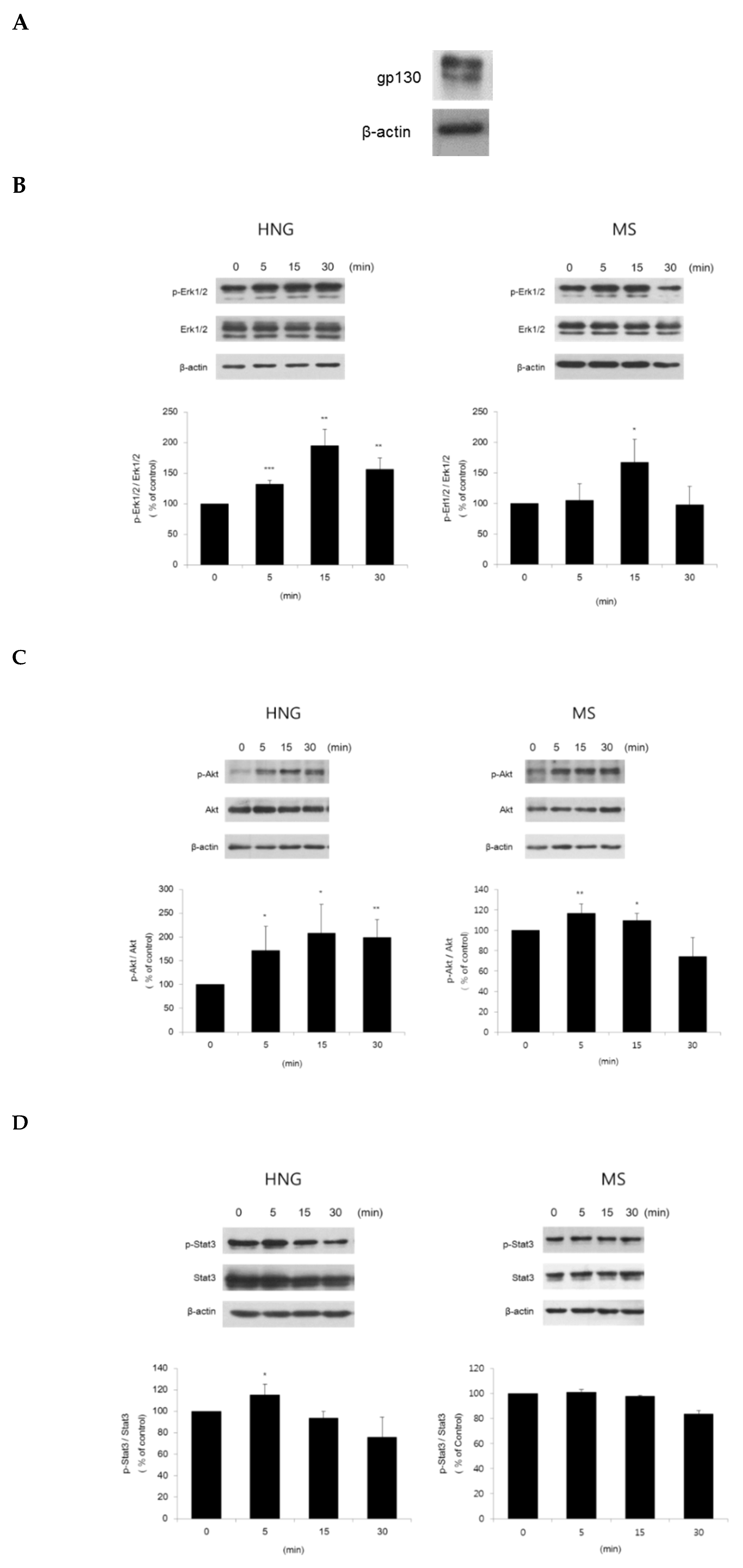
2.4. HNG Activates Erk1/2, Akt, and Stat3 in DPCs
2.5. HNG up-regulates the Expression Level of VEGF mRNA in DPCs
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Treatment
4.3. Cell Proliferation Assay
4.4. Western Blot Analysis
4.5. Real-Time Quantitative Polymerase Chain Reaction (Real-Time PCR)
4.6. Animals
4.7. Isolation and Culture of Rat Vibrissa Follicles
4.8. Hair-Growth In Vivo Experiment
4.9. TUNEL (Tdt-Mediated dUTP-Dig Nick and Labeling) Assay and Hematoxylin and Eosin Staining
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Boil. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [PubMed]
- Pantelireis, N.; Higgins, C.A. A bald statement—Current approaches to manipulate miniaturisation focus only on promoting hair growth. Exp. Dermatol. 2018, 27, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β Signaling Counterbalances BMP-Mediated Repression in Hair Follicle Stem Cell Activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Paus, R.; Foitzik, K. In search of the “hair cycle clock”: A guided tour. Differentiation 2004, 72, 489–511. [Google Scholar] [CrossRef]
- Fujie, T.; Katoh, S.; Oura, H.; Urano, Y.; Arase, S. The chemotactic effect of a dermal papilla cell-derived factor on outer root sheath cells. J. Dermatol. Sci. 2001, 25, 206–212. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A Two-Step Mechanism for Stem Cell Activation During Hair Regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. BioEssays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Boil. 2012, 23, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The Biology of Hair Follicles. New Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Niikura, T.; Tajima, H.; Yasukawa, T.; Sudo, H.; Ito, Y.; Kita, Y.; Kawasumi, M.; Kouyama, K.; Doyu, M.; et al. “A Rescue Factor Abolishing Neuronal Cell Death by a Wide Spectrum of Familial Alzheimer’s Disease Genes and Abeta”. Proc. Natl. Acad. Sci. USA 2001, 98, 6336–6341. [Google Scholar] [CrossRef]
- Yen, K.; Lee, C.; Mehta, H.; Cohen, P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J. Mol. Endocrinol. 2013, 50, R11–R19. [Google Scholar] [CrossRef]
- Lee, C.; Yen, K.; Cohen, P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol. Metab. 2013, 24, 222–228. [Google Scholar] [CrossRef]
- Capt, C.; Passamonti, M.; Breton, S. The human mitochondrial genome may code for more than 13 proteins. Mitochondrial DNA Part A 2015, 27, 3098–3101. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ito, Y.; Niikura, T.; Shao, Z.; Hata, M.; Oyama, F.; Nishimoto, I. Mechanisms of Neuroprotection by a Novel Rescue Factor Humanin from Swedish Mutant Amyloid Precursor Protein. Biochem. Biophys. Res. Commun. 2001, 283, 460–468. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Niikura, T.; Ito, Y.; Sudo, H.; Hata, M.; Arakawa, E.; Abe, Y.; Kita, Y.; Nishimoto, I. Detailed Characterization of Neuroprotection by a Rescue Factor Humanin against Various Alzheimer’s Disease-Relevant Insults. J. Neurosci. 2001, 21, 9235–9245. [Google Scholar] [CrossRef]
- Terashita, K.; Hashimoto, Y.; Niikura, T.; Tajima, H.; Yamagishi, Y.; Ishizaka, M.; Kawasumi, M.; Chiba, T.; Kanekura, K.; Yamada, M.; et al. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factor of Alzheimer’s disease-related neurotoxicity: functional potentiation by isomerization and dimerization. J. Neurochem. 2003, 85, 1521–1538. [Google Scholar] [CrossRef]
- Kim, S.-J.; Guerrero, N.; Wassef, G.; Xiao, J.; Mehta, H.H.; Cohen, P.; Yen, K. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 2016, 7, 46899–46912. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, F.; Langer, P.; Schell, H. Minoxidil Stimulates Mouse Vibrissae Follicles in Organ Culture. J. Investig. Dermatol. 1991, 96, 295–296. [Google Scholar] [CrossRef]
- Langan, E.A.; Philpott, M.P.; Kloepper, J.E.; Paus, R. Human hair follicle organ culture: theory, application and perspectives. Exp. Dermatol. 2015, 24, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; Van Der Veen, C.; Eichmüller, S.B.; McKay, I.A.; Stenn, K.S. A Comprehensive Guide for the Accurate Classification of Murine Hair Follicles in Distinct Hair Cycle Stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; Van Der Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar] [PubMed]
- Kloepper, J.E.; Kawai, K.; Bertolini, M.; Kanekura, T.; Paus, R. Loss of γδ T Cells Results in Hair Cycling Defects. J. Investig. Dermatol. 2013, 133, 1666–1669. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.-W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef]
- Soma, T.; Tajima, M.; Kishimoto, J. Hair cycle-specific expression of versican in human hair follicles. J. Dermatol. Sci. 2005, 39, 147–154. [Google Scholar] [CrossRef]
- Kang, J.I.; Yoo, E.S.; Hyun, J.W.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Ko, C.S.; Kang, H.K. Promotion Effect of Apo-9′-Fucoxanthinone from Sargassum Muticum on Hair Growth Via the Activation of Wnt/Β-Catenin and Vegf-R2. Biol. Pharm. Bull. 2016, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Kim, M.-K.; Lee, J.-H.; Jeon, Y.-J.; Hwang, E.-K.; Koh, Y.-S.; Hyun, J.-W.; Kwon, S.-Y.; Yoo, E.-S.; Kang, H.-K. Undariopsis peterseniana Promotes Hair Growth by the Activation of Wnt/β-Catenin and ERK Pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.S.; Li, Y.; Zhai, H.; Bi, J.W.; Zhang, F.S.; Zhang, X.Y.; Fan, S.H. Humanin Analogue, S14g-Humanin, Has Neuroprotective Effects against Oxygen Glucose Deprivation/Reoxygenation by Reactivating Jak2/Stat3 Signaling through the Pi3k/Akt Pathway. Exp. Ther. Med 2017, 14, 3926–3934. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-Inducible IL-6 Inhibits Elongation of Human Hair Shafts by Suppressing Matrix Cell Proliferation and Promotes Regression of Hair Follicles in Mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Hashimoto, Y. Humanin and the Receptors for Humanin. Mol. Neurobiol. 2009, 41, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef]
- Willard, F.S.; Crouch, M.F. MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell. Signal. 2001, 13, 653–664. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.; Franklin, R.A.; McCubrey, J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef]
- Roche, S.; Koegl, M.; Courtneidge, S.A. The phosphatidylinositol 3-kinase alpha is required for DNA synthesis induced by some, but not all, growth factors. Proc. Natl. Acad. Sci. USA 1994, 91, 9185–9189. [Google Scholar] [CrossRef]
- Zhou, B.P.; Liao, Y.; Xia, W.; Zou, Y.; Spohn, B.; Hung, M.C. Her-2/Neu Induces P53 Ubiquitination Via Akt-Mediated Mdm2 Phosphorylation. Nat. Cell Biol. 2001, 3, 973–982. [Google Scholar] [CrossRef]
- Fukumoto, S.; Hsieh, C.-M.; Maemura, K.; Layne, M.D.; Yet, S.-F.; Lee, K.-H.; Matsui, T.; Rosenzweig, A.; Taylor, W.G.; Rubin, J.S.; et al. Akt Participation in the Wnt Signaling Pathway through Dishevelled. J. Boil. Chem. 2001, 276, 17479–17483. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: an updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-S.; Kang, J.-I.; Kim, S.M.; Ko, A.; Koh, Y.-S.; Hyun, J.-W.; Yoon, S.-P.; Ahn, M.J.; Kim, Y.H.; Kang, J.-H.; et al. Norgalanthamine Stimulates Proliferation of Dermal Papilla Cells via Anagen-Activating Signaling Pathways. Boil. Pharm. Bull. 2019, 42, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, S.; Kim, S.; Park, D.; Jung, E. Effect of sinapic acid on hair growth promoting in human hair follicle dermal papilla cells via Akt activation. Arch. Dermatol. Res. 2017, 309, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, S.; Imai, R.; Ogawa, H. Dermal papilla cells express hepatocyte growth factor. J. Dermatol. Sci. 1994, 7, S79–S83. [Google Scholar] [CrossRef]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouet, J. Vascular Endothelial Growth Factor Is an Autocrine Growth Factor for Hair Dermal Papilla Cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef]
- Man, X.-Y.; Yang, X.-H.; Cai, S.-Q.; Bu, Z.-Y.; Wu, X.-J.; Lu, Z.-F.; Zheng, M. Expression and localization of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 in human epidermal appendages: a comparison study by immunofluorescence. Clin. Exp. Dermatol. 2009, 34, 396–401. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Messenger, A.; Rundegren, J. Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Buhl, A.E.; Waldon, D.J.; Baker, C.A.; Johnson, G.A. Minoxidil Sulfate Is the Active Metabolite that Stimulates Hair Follicles. J. Investig. Dermatol. 1990, 95, 553–557. [Google Scholar] [CrossRef]
- Filsell, W.; Little, J.C.; Stones, A.J.; Granger, S.P.; A Bayley, S. Transfection of rat dermal papilla cells with a gene encoding a temperature-sensitive polyomavirus large T antigen generates cell lines retaining a differentiated phenotype. J. Cell Sci. 1994, 107, 1761–1772. [Google Scholar] [PubMed]
- Philpott, M.P.; Kealey, T. Cyclical Changes in Rat Vibrissa Follicles Maintained In Vitro. J. Investig. Dermatol. 2000, 115, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Ohnemus, U.; Uenalan, M.; Conrad, F.; Handjiski, B.; Mecklenburg, L.; Nakamura, M.; Inzunza, J.; Gustafsson, J.-A.; Paus, R. Hair Cycle Control by Estrogens: Catagen Induction via Estrogen Receptor (ER)-α Is Checked by ERβ Signaling. Endocrinology 2005, 146, 1214–1225. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Sequence (from 5′ to 3′) |
|---|---|
| VEGF forward | 5′-AAC GAA AGC GCA AGA AAT CC-3′ |
| VEGF reverse | 5′-GCT CAC AGT GAA CGC TCC AG-3′ |
| β-actin forward | 5′-TCC TGG CCT CAC TGT CCA C-3′ |
| β-actin reverse | 5′-GGG CCG GAC TCA TCG TAC T-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Kang, J.-I.; Yoon, H.-S.; Choi, Y.K.; Go, J.S.; Oh, S.K.; Ahn, M.; Kim, J.; Koh, Y.S.; Hyun, J.W.; et al. HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. Int. J. Mol. Sci. 2020, 21, 4553. https://doi.org/10.3390/ijms21124553
Kim SM, Kang J-I, Yoon H-S, Choi YK, Go JS, Oh SK, Ahn M, Kim J, Koh YS, Hyun JW, et al. HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. International Journal of Molecular Sciences. 2020; 21(12):4553. https://doi.org/10.3390/ijms21124553
Chicago/Turabian StyleKim, Sung Min, Jung-Il Kang, Hoon-Seok Yoon, Youn Kyung Choi, Ji Soo Go, Sun Kyung Oh, Meejung Ahn, Jeongtae Kim, Young Sang Koh, Jin Won Hyun, and et al. 2020. "HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition" International Journal of Molecular Sciences 21, no. 12: 4553. https://doi.org/10.3390/ijms21124553
APA StyleKim, S. M., Kang, J.-I., Yoon, H.-S., Choi, Y. K., Go, J. S., Oh, S. K., Ahn, M., Kim, J., Koh, Y. S., Hyun, J. W., Yoo, E.-S., & Kang, H.-K. (2020). HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. International Journal of Molecular Sciences, 21(12), 4553. https://doi.org/10.3390/ijms21124553