RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Isolation, Culture and Treatment of GCs
2.3. Identification of GCs by Immunofluorescence
2.4. Immunohistochemistry
2.5. Production of siRNA and Transfection
2.6. Total RNA Extraction and RT-qPCR
2.7. Western Blot
2.8. Estimation of Intracellular ROS
2.9. Estimation of GCs Apoptosis
2.10. Analysis of Cell Cycle
2.11. Mitochondrial Membrane Potential Analysis
2.12. Estimation of Cell Viability
2.13. Determination of E2 and P4 Levels
2.14. Statistical Analysis
3. Results
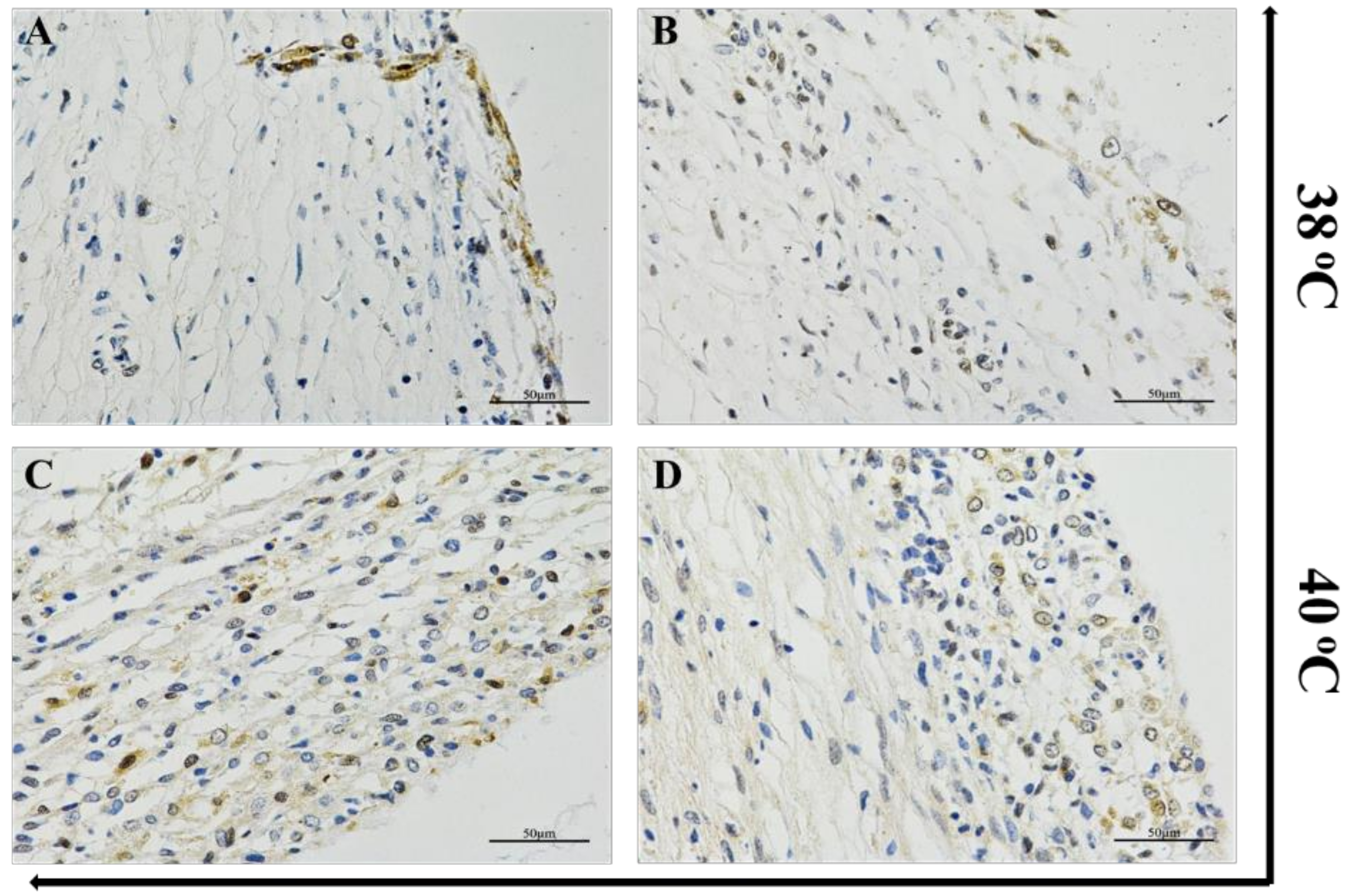
3.1. Heat Stress Induced CAT Expression in Ovarian Follicle Tissues
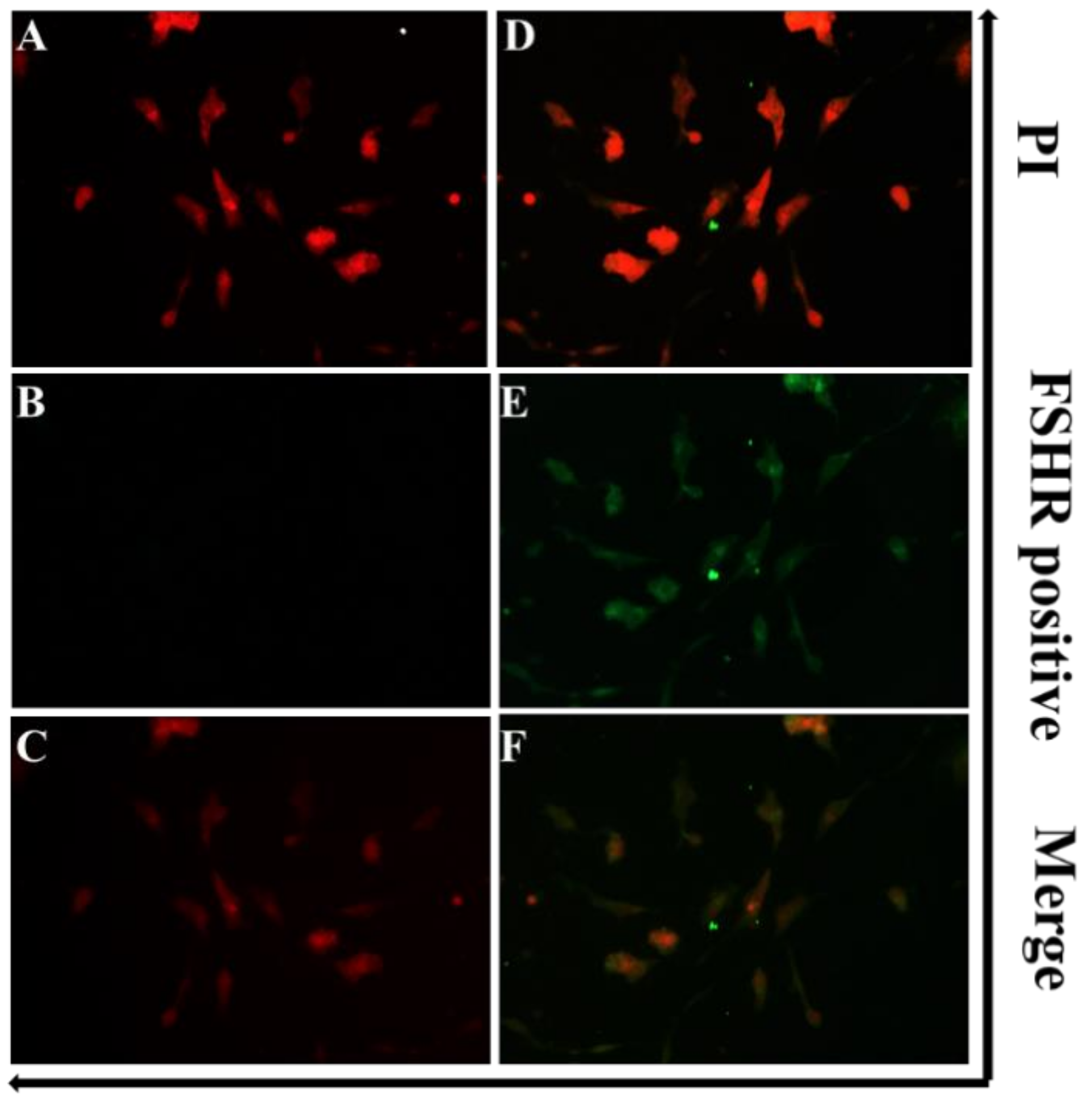
3.2. Identification of GCs
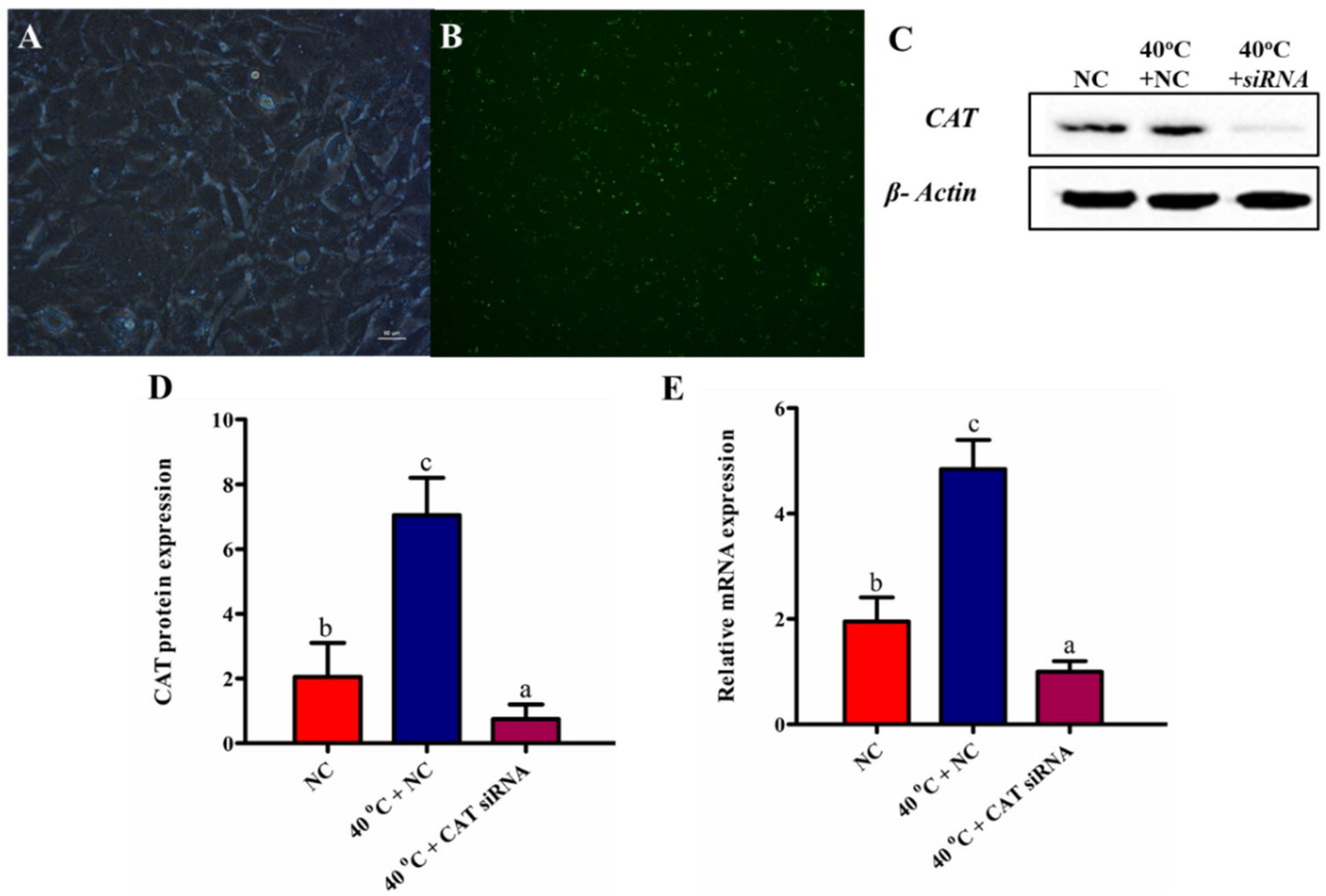
3.3. Efficacy of CAT Transfection
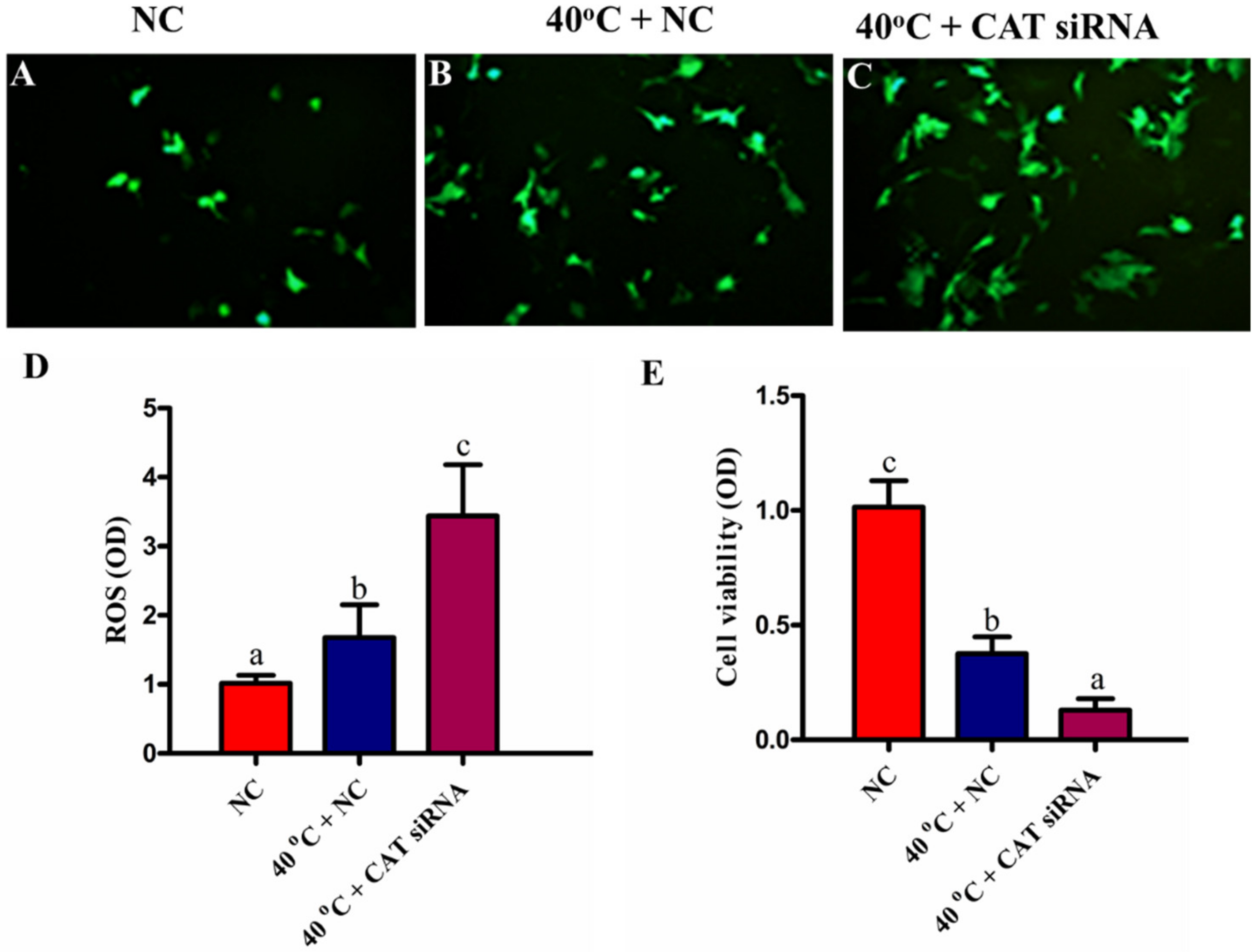
3.4. Silencing of CAT Induced Intracellular ROS Accumulation under HS
3.5. Silencing of CAT Altered Viability of GCs under HS
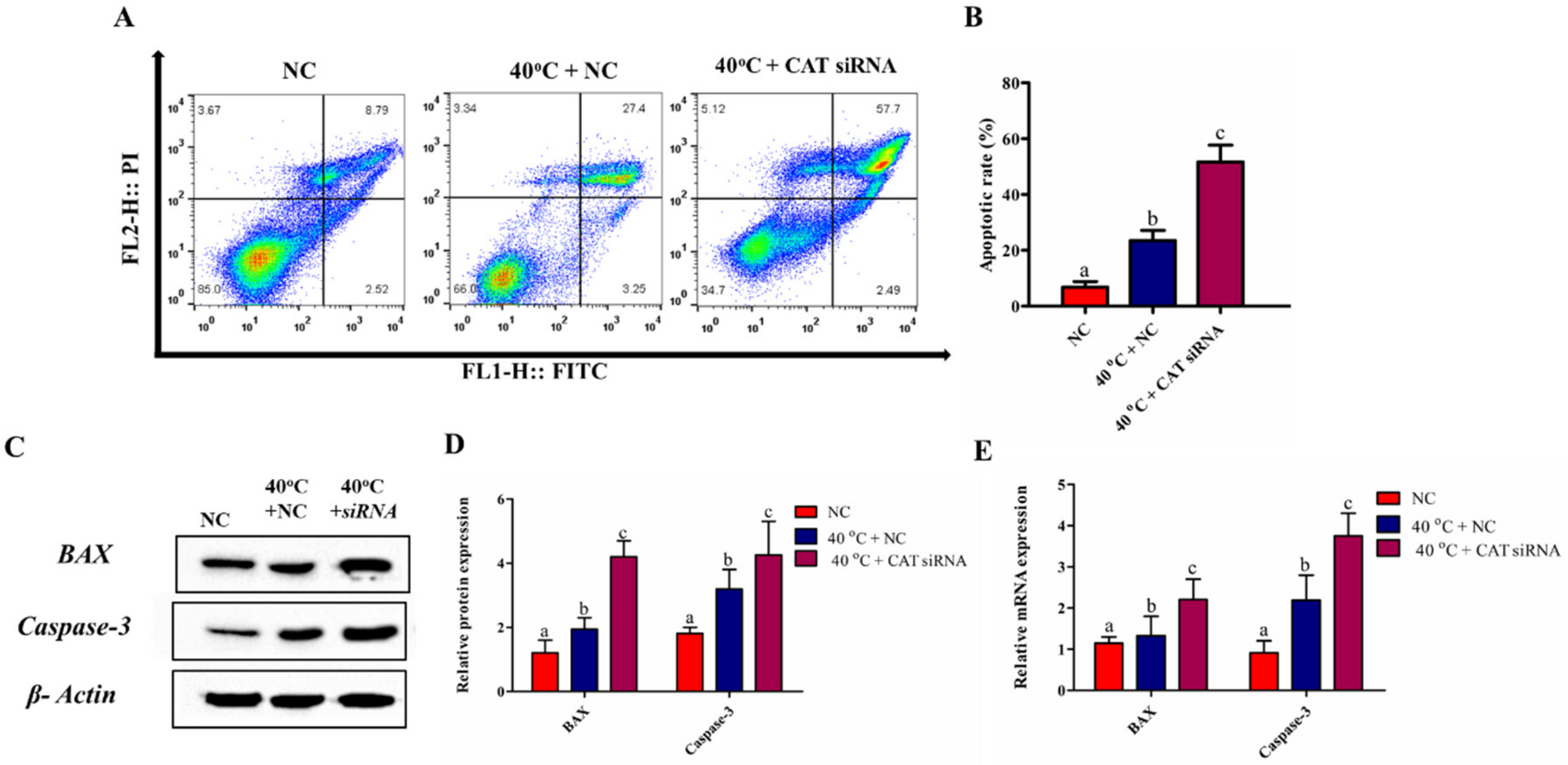
3.6. Silencing of CAT Induced GCs Apoptosis under HS
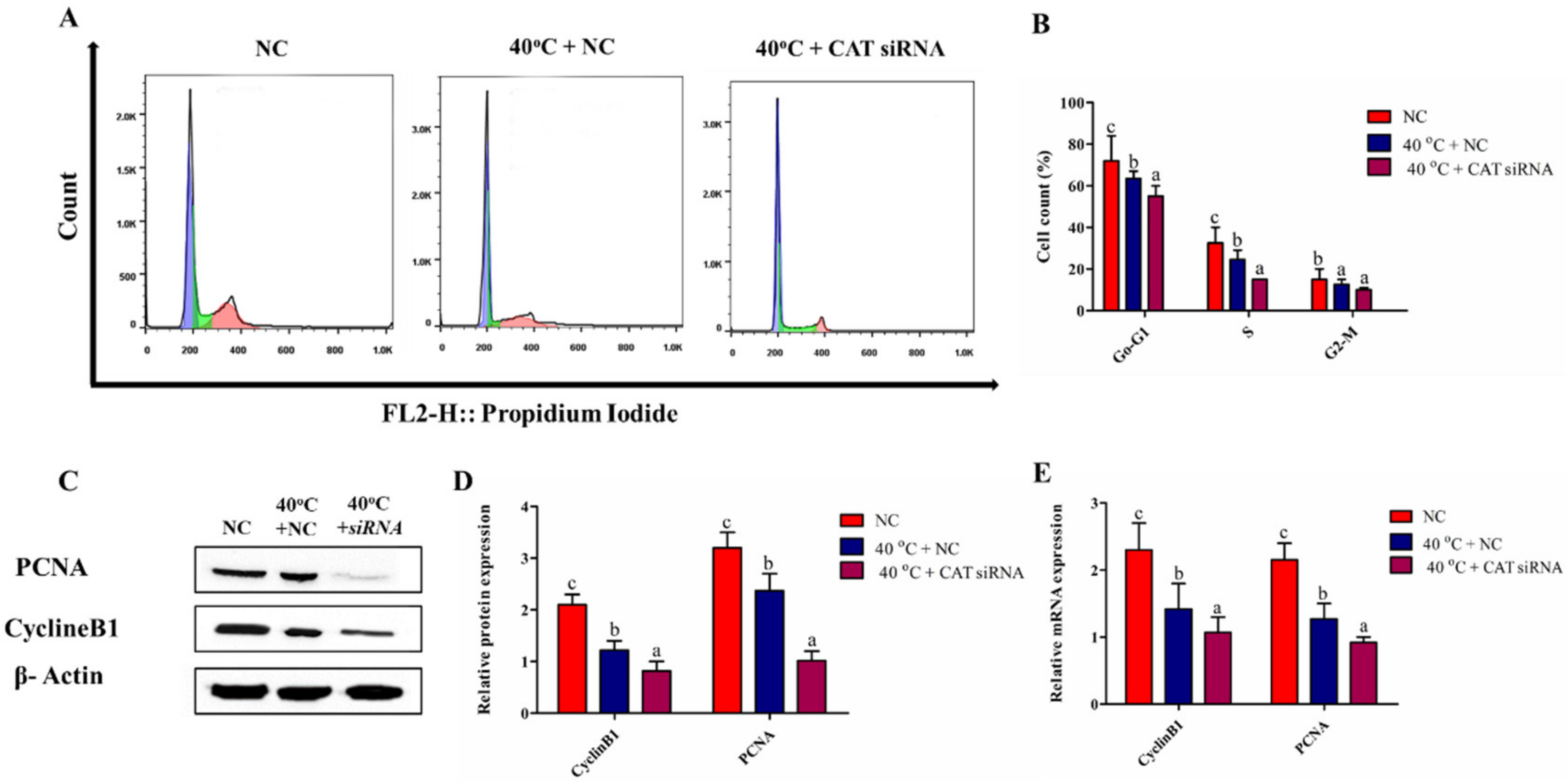
3.7. Silencing of CAT Altered Cell Cycle Transition in GCs under HS
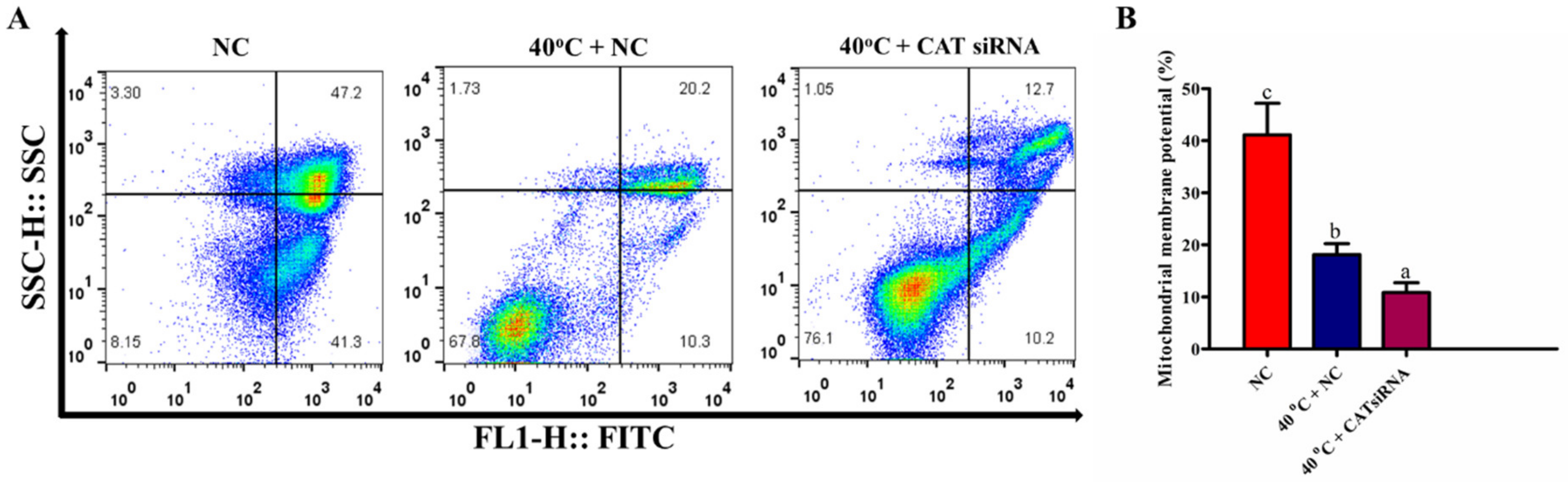
3.8. Silencing of CAT Disrupted Mitochondrial Membrane Potential of GCs under HS
3.9. Silencing of CAT Impaired the Synthesis of P4 and E2 in GCs under HS
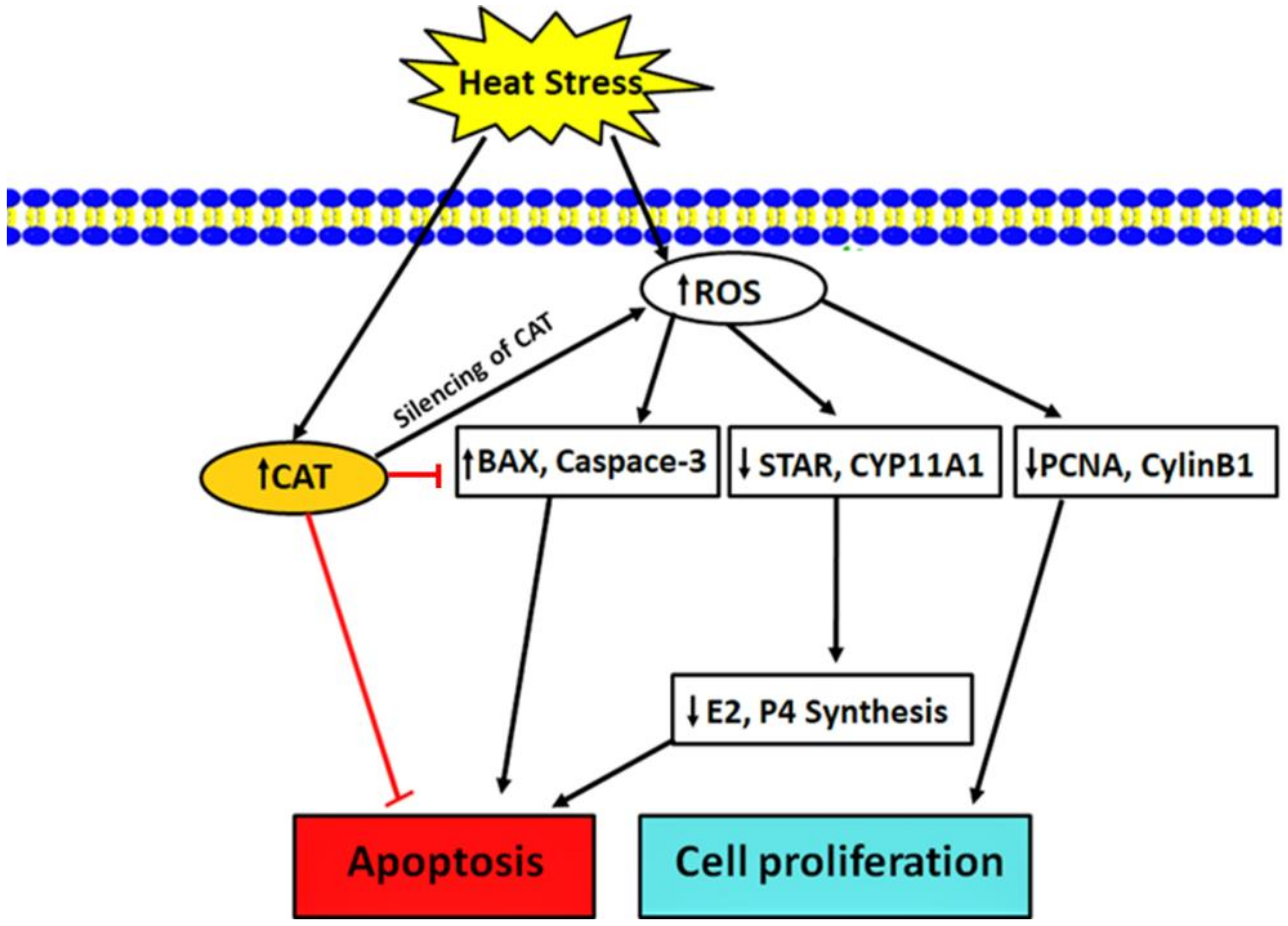
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wegner, K.; Lambertz, C.; Das, G.; Reiner, G.; Gauly, M. Effects of temperature and temperature-humidity index on the reproductive performance of sows during summer months under a temperate climate. Anim. Sci. J. 2016, 87, 1334–1339. [Google Scholar] [CrossRef]
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77, 2–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, Z.; Meiden, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of HS on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M.; Tranter, W.P.; Mayers, D.G.; Jonsson, N.N. Effects of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 2007, 90, 2271–2278. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Effect of short- and long-term HS on the conception risk of dairy cows under natural service and artificial insemination breeding programs. J. Dairy Sci. 2016, 99, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute HS effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and molecular adaptation of bovine granulosa cells and oocytes under HS. Animals 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, Z.; Hansen, P.J. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation 1. Biol. Reprod. 2004, 71, 1898–1906. [Google Scholar] [CrossRef]
- Hooper, L.M.; Payton, R.R.; Rispoli, L.A.; Saxton, A.M.; Edwards, J.L. Impact of HS on germinal vesicle breakdown and lipolytic changes during in vitro maturation of bovine oocytes. J. Reprod. Dev. 2015, 61, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Ascari, I.J.; Alves, N.G.; Jasmin, J.; Lima, R.R.; Quintão, C.C.R.; Oberlender, G.; Moraes, E.A.; Camargo, L.S.A. Addition of insulin-like growth factor I to the maturation medium of bovine oocytes subjected to heat shock: Effects on the production of reactive oxygen species, mitochondrial activity and oocyte competence. Domest. Anim. Endocrinol. 2017, 60, 50–60. [Google Scholar] [CrossRef]
- Payton, R.R.; Rispoli, L.A.; Nagle, K.A.; Gondro, C.; Saxton, A.M.; Voy, B.H.; Edwards, J.L. Mitochondrial-related consequences of HS exposure during bovine oocyte maturation persist in early embryo development. J. Reprod. Dev. 2018, 64, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, Z. Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J. Dairy Sci. 2018, 101, 3642–3654. [Google Scholar] [CrossRef]
- Petro, E.M.L.; Leroy, J.L.M.R.; Van Cruchten, S.J.M.; Covaci, A.; Jorssen, E.P.A.; Bols, P.E.J. Endocrine disruptors and female fertility: Focus on (bovine) ovarian follicular physiology. Theriogenology 2012, 78, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a fever temperature of 40 °C protects cells against hyperthermia-induced apoptosis mediated by death receptor signalling. Biochem. Cell Biol. 2008, 86, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant. Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Jena, K.; Kumar Kar, P.; Kausar, Z.; Babu, C.S. Effects of temperature on modulation of oxidative stress and antioxidant defenses in testes of tropical tasar silkworm Antheraea mylitta. J. Therm. Biol. 2013, 38, 199–204. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant. Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Paul, C.; Teng, S.; Saunders, P.T.K. A Single, Mild, Transient scrotal HS causes hypoxia and oxidative stress in mouse testes, which induces germ cell death1. Biol. Reprod. 2009, 80, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Shen, M.; Wu, W.J.; Li, B.J.; Weng, Q.N.; Li, M.; Liu, H.L. Expression of PUMA in follicular granulosa cells regulated by FoxO1 activation during oxidative stress. Reprod. Sci. 2015, 22, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.W.; Pandey, H.O.; Wondim, D.S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to HS. Theriogenology 2017, 110, 130–141. [Google Scholar] [CrossRef]
- Blondin, P.; Coenen, K.; Sirard, M.A. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J. Androl. 1997, 18, 454–460. [Google Scholar] [PubMed]
- Dawson, N.J.; Storey, K.B. A hydrogen peroxide safety valve: The reversible phosphorylation of catalase from the freeze-tolerant North American wood frog, Rana sylvatica. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, Y.; Zhang, Y.H. Oxidative stress and hepatotoxicity in the frog, Rana chensinensis, when exposed to low doses of trichlorfon. J. Environ. Sci. Heal. Part. B Pestic. Food Contam. Agric. Wastes 2017, 52, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujit, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. J. Natl. Cancer Inst. 2013, 65, 81–93. [Google Scholar]
- Kashiwagi, A.; Kashiwagi, K.; Takase, M.; Hanada, H.; Nakamura, M. Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 499–503. [Google Scholar] [CrossRef]
- Srivastava, S.; Pathak, A.D.; Gupta, P.S.; Kumar, A.; Kumar, A. Hydrogen peroxide-scavenging enzymes impart tolerance to high temperature induced oxidative stress in sugarcane. J. Environ. Biol. 2012, 33, 657–661. [Google Scholar]
- Dai, D.F.; Chiao, Y.A.; Martin, G.M.; Marcinek, D.J.; Basisty, N.; Quarles, E.K.; Rabinovitch, P.S. Mitochondrial-targeted catalase: Extended longevity and the roles in various disease models. Prog. Mol. Biol. Transl. Sci. 2017, 146, 203–241. [Google Scholar]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Scandalios, J.G.; Acevedo, A.; Ruzsa, S. Catalase gene expression in response to chronic high temperature stress in maize. Plant. Sci. 2000, 156, 103–110. [Google Scholar] [CrossRef]
- Dhawan, V. Reactive oxygen and nitrogen species: General considerations. oxidative stress in applied basic research and clinical practice. In Studies on Respiratory Disorders; Humana Press: New York, NY, USA, 2014; pp. 27–47. [Google Scholar]
- Dandekar, S.P.; Nadkarni, G.D.; Kulkarni, V.S.; Punekar, S. Lipid peroxidation and antioxidant enzymes in male infertility. J. Postgrad. Med. 2002, 48, 186–189. [Google Scholar] [PubMed]
- Gao, J.J.; Qin, A.G.; Yu, X.C. Effects of grafting on cucumber leaf SOD and CAT gene expression and activities under low temperature stress. Chinese J. Appl. Ecol. 2009, 20, 213–217. [Google Scholar]
- Cansev, A.; Gulen, H.; Eris, A. The activities of catalase and ascorbate peroxidase in olive (Olea europaea L. cv. Gemlik) under low temperature stress. Hortic. Environ. Biotechnol. 2011, 52, 113–120. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, H.C.; Lin, C.Y. Cloning, expression and physiological analysis of broccoli catalase gene and Chinese cabbage ascorbate peroxidase gene under HS. Plant. Cell Rep. 2010, 29, 575–593. [Google Scholar] [CrossRef]
- Figueroa-Yáñez, L.; Cano-Sosa, J.; Castaño, E.; Arroyo-Herrera, A.L.; Caamal-Velazquez, J.H.; Sanchez-Teyer, F.; López-Gómez, R.; De Los Santos-Briones, C.; Rodríguez-Zapata, L. Phylogenetic relationships and expression in response to low temperature of a catalase gene in banana (Musa acuminata cv. “Grand Nain”) fruit. Plant. Cell. Tissue Organ Cult. 2012, 109, 429–438. [Google Scholar] [CrossRef]
- Hammond, S.M.; Boettcher, S.; Caudy, A.A.; Kobayashi, R.; Hannon, G.J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 2001, 293, 1146–1150. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, D.T.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60, 535–547. [Google Scholar] [CrossRef]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of HS in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef]
- Alves, B.G.; Alves, K.A.; Lúcio, A.C.; Martins, M.C.; Silva, T.H.; Alves, B.G.; Braga, L.S.; Silva, T.V.; Viu, M.A.O.; Beletti, M.E.; et al. Ovarian activity and oocyte quality associated with the biochemical profile of serum and follicular fluid from Girolando dairy cows postpartum. Anim. Reprod. Sci. 2014, 146, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Meidan, R.; Shaham-Albalancy, A.; Braw-Tal, R.; Wolfenson, D. Delayed effect of HS on steroid production in medium-sized and preovulatory bovine follicles. Reproduction 2001, 121, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by HS during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu, A.; Ambrose, J.D.; Kassa, T.; Diaz, T.; Thatcher, M.J.; Thatcher, W.W. Long-term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute HS. Anim. Reprod. Sci. 2001, 66, 15–34. [Google Scholar] [CrossRef]
- De Torres-Júnior, J.R.S.; De Pires, F.A.M.; De Sá, W.F.; Ferreira, A.; Viana, J.H.; Camargo, L.S.; Ramos, A.A.; Folhadella, I.M.; Polisseni, J.; De Freitas, C.; et al. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology 2008, 69, 155–166. [Google Scholar]
- Shimizu, T.; Oshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. HS diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.U.; et al. Effects of chronic HS on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Al-Katanani, Y.M.; Paula-Lopes, F.F.; Hansen, P.J. Effect of season and exposure to HS on oocyte competence in Holstein cows. J. Dairy Sci. 2002, 85, 390–396. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Ayres, H.; Chiaratti, M.R.; Ferraz, M.L.; Araújo, A.B.; Rodrigues, C.A.; Watanabe, Y.F. The low fertility of repeat-breeder cows during summer HS is related to a low oocyte competence to develop into blastocysts. J. Dairy Sci. 2011, 94, 2383–2392. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of HS effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2019, 3, 498–507. [Google Scholar]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. HS induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence ofheat stress. J. Anim. Physiol. Anim. Nutr. 2019, 1–9. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Meriin, A.B.; Sherman, M.Y.; Morimoto, R.I.; Massie, B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 2000, 20, 7146–7159. [Google Scholar] [CrossRef] [Green Version]
- Makarevich, A.V.; Olexiková, L.; Chrenek, P.; Kubovičová, E.; Fréharová, K.; Pivko, J. The effect of hyperthermia in vitro on vitality of rabbit preimplantation embryos. Physiol. Res. 2007, 56, 789–796. [Google Scholar]
- Sirotkin, A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010, 213, 2125–2130. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Li, L.; Xiao, C.; Sun, Y.; Wang, G.L. HS impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 2016, 412, 81–90. [Google Scholar] [CrossRef]
- Walsh, D.; Li, K.; Wass, J.; Dolnikov, A.; Zeng, F.; Zhe, L.; Edwards, M. Heat-shock gene expression and cell cycle changes during mammalian embryonic development. Dev. Genet. 1993, 14, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Tan, K.N.; Reyes-Farias, M.; De La Jara, N.; Ngo, S.T.; Garcia-Diaz, D.F.; Llanos, P.; Cires, M.J.; Borges, K. The deleterious effect of cholesterol and protection by quercetin on mitochondrial bioenergetics of pancreatic β-cells, glycemic control and inflammation: In vitro and in vivo studies. Redox Biol. 2016, 9, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.L.; Zhang, J.; Ping, Z.G.; Wang, C.Q.; Sun, Y.F.; Chen, L.; Li, X.Y.; Li, C.J.; Zhu, X.L.; Liu, Z.; et al. Relationship between apoptosis and proliferation in granulosa and theca cells of cystic follicles in sows. Reprod. Domest. Anim. 2012, 47, 601–608. [Google Scholar] [CrossRef]
- Chen, L.; Yi, K.; Sun, Y.; Sun, Y.; Tang, L.; Zhou, X. Expression of BDNF mRNA in porcine reproductive tissues during follicular phase and luteal phase and oocytes in GV and in vitro matured MII Stage. J. Anim. Vet. Adv. 2011, 10, 2571–2574. [Google Scholar]
- Scott, M.; Bonnefin, P.; Vieyra, D.; Boisvert, F.M.; Young, D.; Bazett-Jones, D.P.; Riabowol, K. UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell Sci. 2001, 114, 3455–3462. [Google Scholar]
- Ghate, N.; Das, A.; Chaudhuri, D.; Panja, S.; Mandal, N. Sundew plant, a potential source of anti-inflammatory agents, selectively induces G2/M arrest and apoptosis in MCF-7 cells through upregulation of p53 and Bax/Bcl-2 ratio. Cell Death Discov. 2016, 2, 15062. [Google Scholar] [CrossRef] [Green Version]
- Del Vesco, A.P.; Gasparino, E. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute HS. J. Anim. Sci. 2013, 91, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Mendelson, C.R.; Jiang, B.; Shelton, J.M.; Richardson, J.A.; Hinshelwood, M.M. Transcriptional regulation of aromatase in placenta and ovary. J. Steroid Biochem. Mol. Biol. 2005, 95, 25–33. [Google Scholar] [CrossRef]
- Mosa, A.; Neunzig, J.; Gerber, A.; Zapp, J.; Hannemann, F.; Pilak, P.; Bernhardt, R. 2β- and 16β-hydroxylase activity of CYP11A1 and direct stimulatory effect of estrogens on pregnenolone formation. J. Steroid Biochem. Mol. Biol. 2015, 150, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wu, Y.; Zhao, S.; Liu, Z.X.; Zeng, S.M.; Zhang, G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (STAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology 2015, 84, 811–817. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekawiecki, R.; Nowik, M.; Kotwica, J. Stimulatory effect of LH, PGE2 and progesterone on STAR protein, cytochrome P450 cholesterol side chain cleavage and 3β hydroxysteroid dehydrogenase gene expression in bovine luteal cells. Prostaglandins Other Lipid Mediat. 2005, 78, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Peluso, J.J.; Pappalardo, A. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-3σ1. Biol. Reprod. 2004, 71, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Accession No. | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|---|
| CAT | NM_001035386.2 | GTTCGCTTCTCCACTGTTGC | AGGTGCGTTTGAGGGTTTCT |
| BAX | XM_003355974.2 | GGCTGGACATTGGACTTCCTTC | TGGTCACTGTCTGCCATGTGG |
| Caspase-3 | XM_005671704.1 | CTGGACTGTGGCATTGAGAC | GCAAAGGGACTGGAGAACC |
| CyclinB1 | NM_001170768.1 | AAGACGGAGCGGATCCAAAC | CCAGTGACTTCACGACCCAT |
| PCNA | NM_001291925.1 | GCGTTCATAGTCGTGTTCCG | TTCAAGATGGAGCCCTGGAC |
| STAR | NM_174189.3 | CCCATGGAGAGGCTTTATGA | TGATGACCGTGTCTTTTCCA |
| Cyp11A1 | NM_176644.2 | CTGGCATCTCCACAAAGACC | GTTCTCGATGTGGCGAAAGT |
| GAPDH | NM_001034034.2 | GGTGCTGAGTATGTGGTGGA | GGCATTGCTGACAATCTTGA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Khan, M.Z.; Dou, J.; Umer, S.; Xu, H.; Sammad, A.; Zhu, H.-B.; Wang, Y. RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress. Animals 2020, 10, 1060. https://doi.org/10.3390/ani10061060
Khan A, Khan MZ, Dou J, Umer S, Xu H, Sammad A, Zhu H-B, Wang Y. RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress. Animals. 2020; 10(6):1060. https://doi.org/10.3390/ani10061060
Chicago/Turabian StyleKhan, Adnan, Muhammad Zahoor Khan, Jinhuan Dou, Saqib Umer, Huitao Xu, Abdul Sammad, Hua-Bin Zhu, and Yachun Wang. 2020. "RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress" Animals 10, no. 6: 1060. https://doi.org/10.3390/ani10061060










