SnS2 and SnO2 Nanoparticles Obtained from Organotin(IV) Dithiocarbamate Complex and Their Photocatalytic Activities on Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Sodium p-Methylphenyldithiocarbamate (NaL)
2.2. Synthesis of the Diphenyltin(IV) p-Methylphenyldithiocarbamate Complex [(C6H5)2SnL2]
2.3. Synthesis of Tin Disulfide Nanoparticles (SnS2)
2.4. Synthesis of Tin Dioxide Nanoparticles (SnO2)
2.5. Evaluation of the Photocatalytic Activities of the Nanoparticles
3. Results
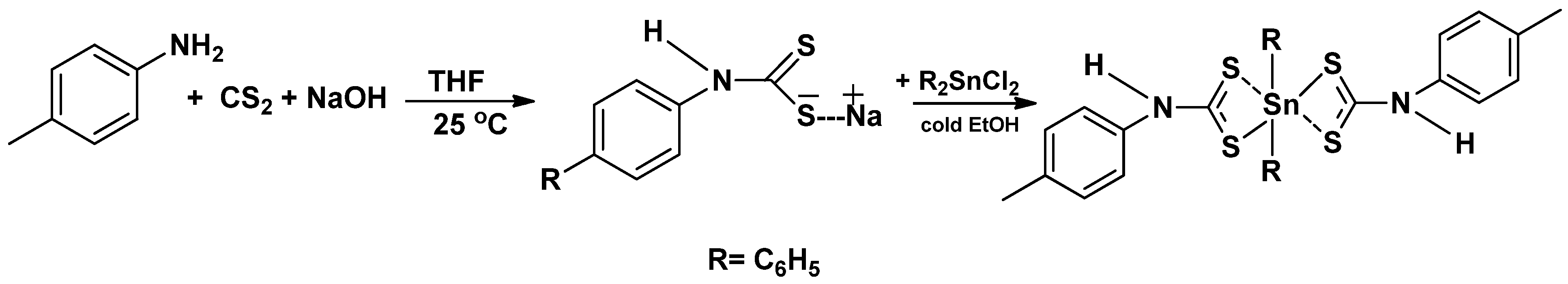
3.1. Synthesis of the Ligand (L) and Complex [(C6H5)2SnL2]
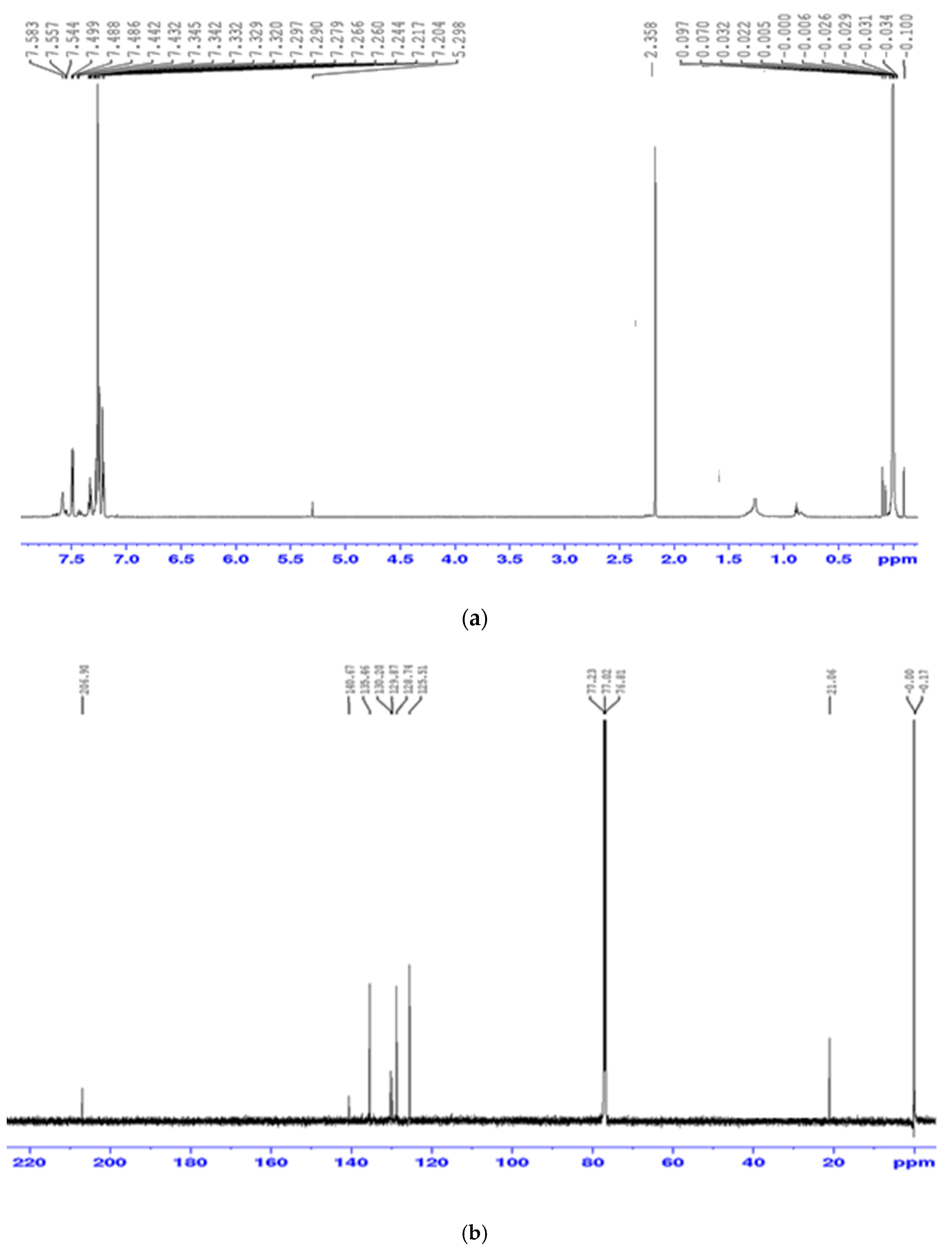
3.2. Spectroscopic Studies of the Precursor Complex [(C6H5)2Sn(L)2]
3.3. Thermogravimetric Analysis (TGA) of Diphenyltin(IV) and p-Methylphenyldithiocarbamate [(C6H5)2SnL2]
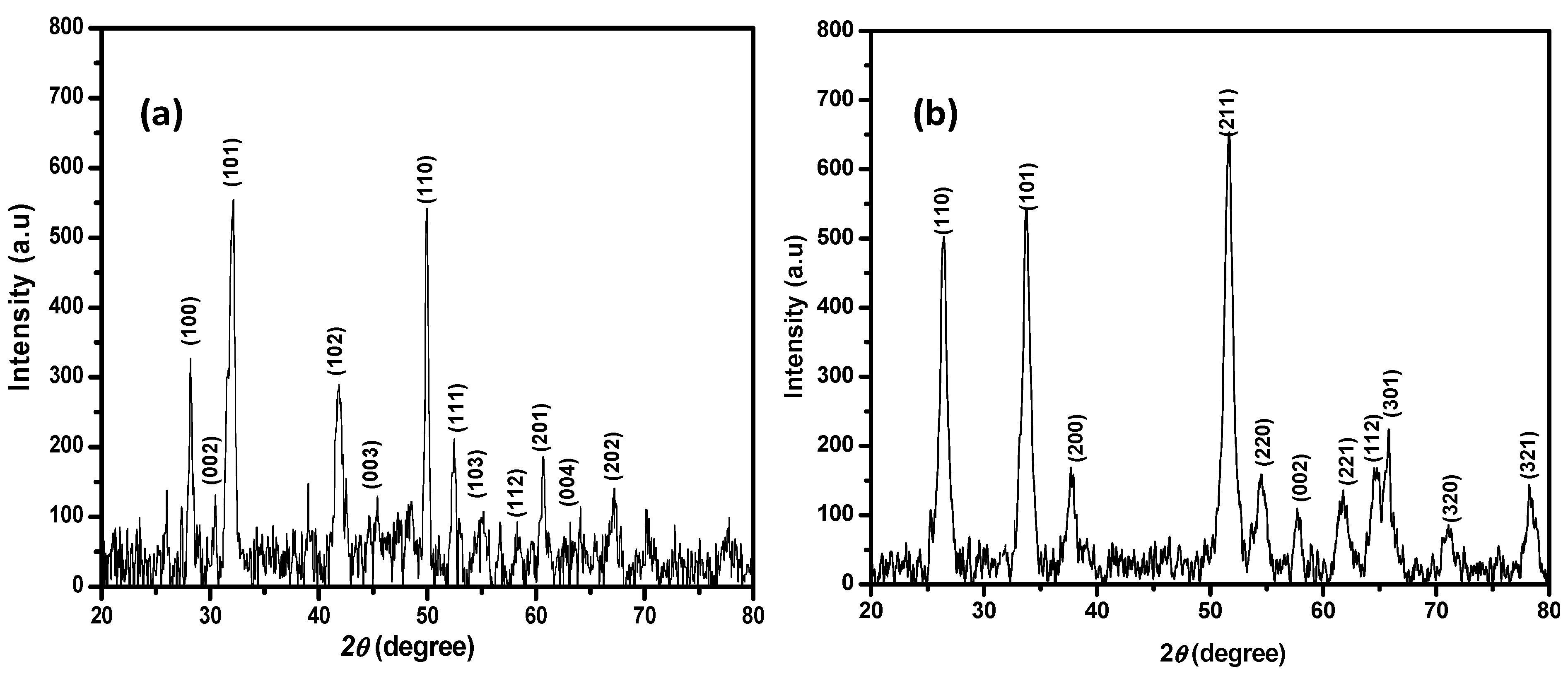
3.4. X-ray Diffraction Study of the Synthesized SnS2 and SnO2 Nanoparticles
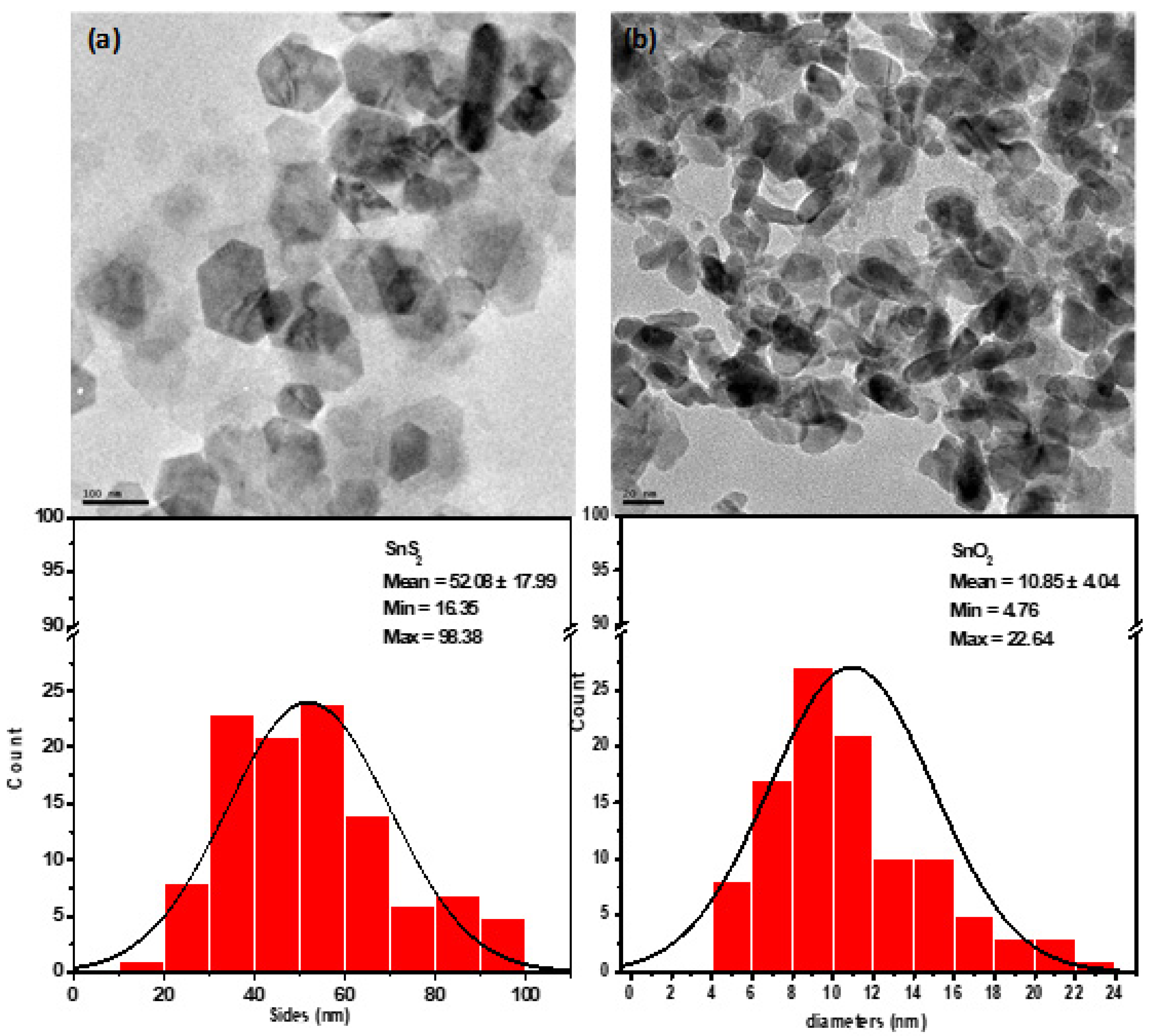
3.5. Morphology of the Synthesize SnS2 and SnO2
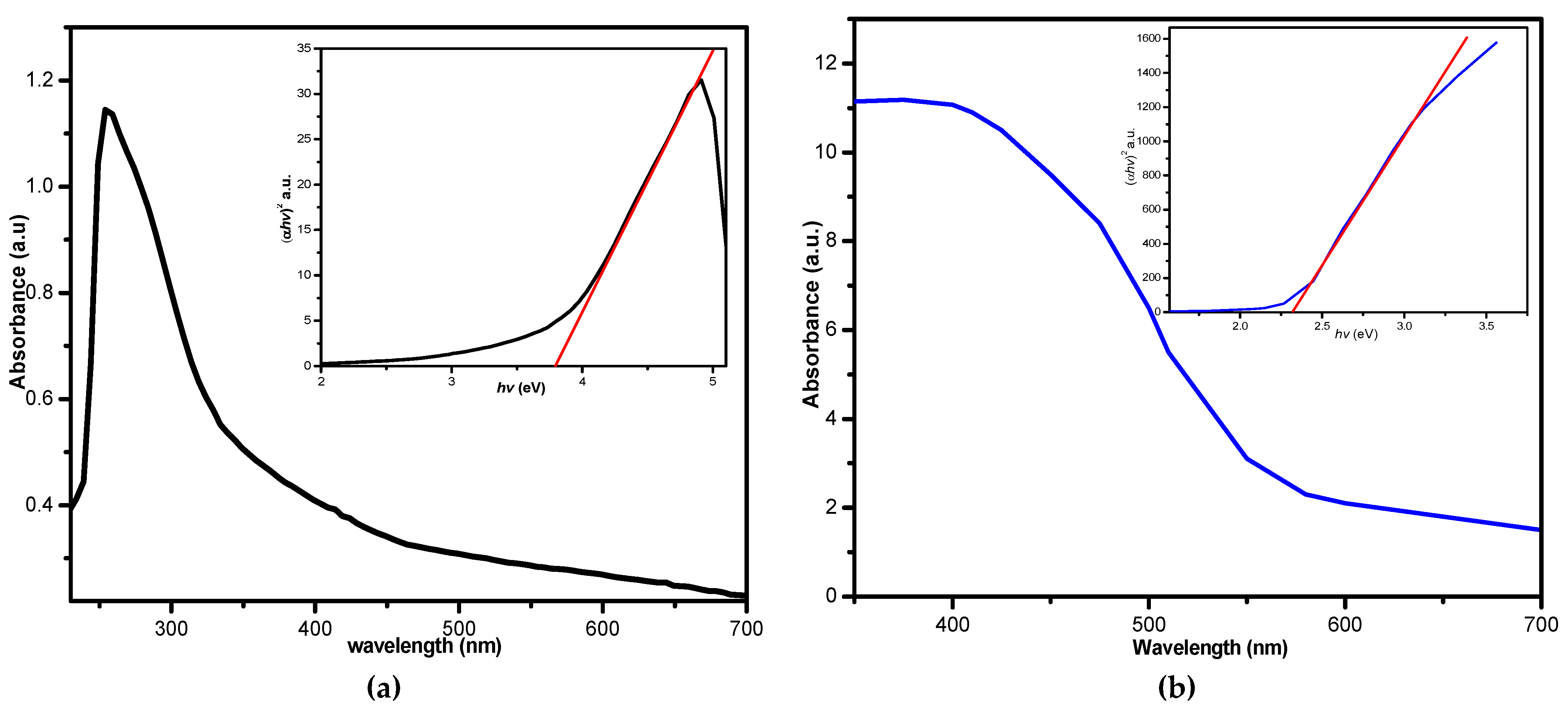
3.6. Ultraviolet-Visible Absorption Spectra
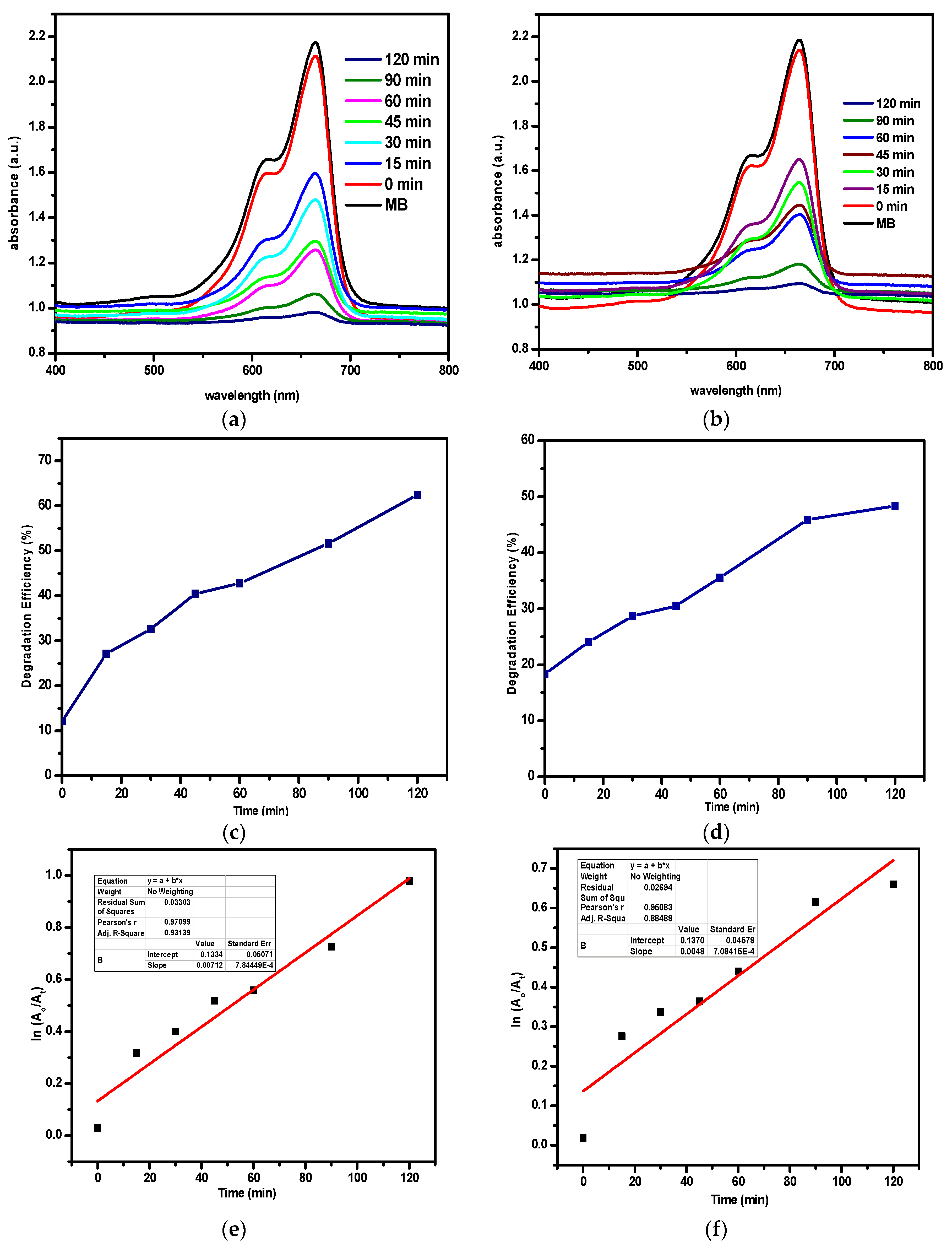
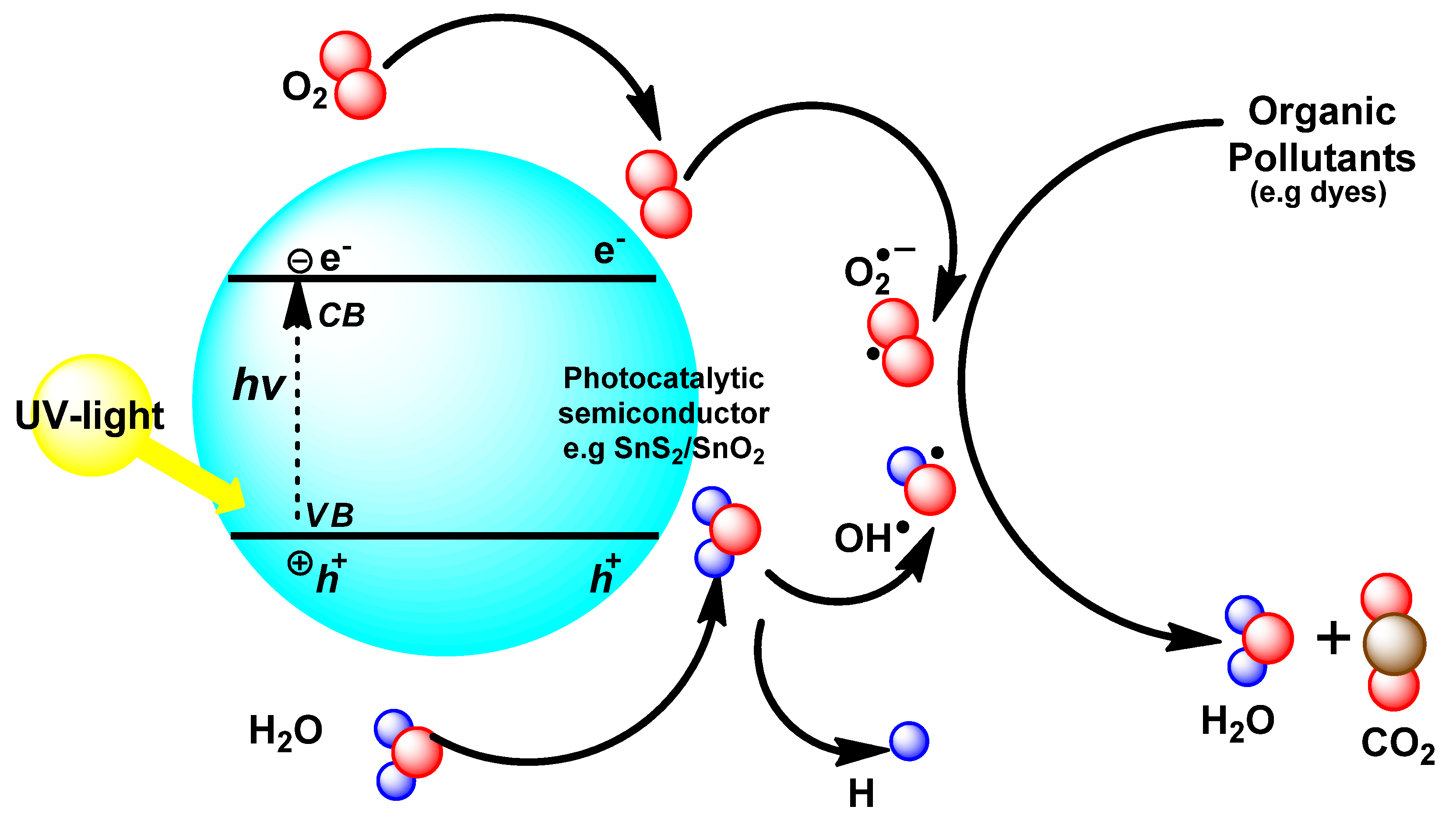
3.7. Photocatalytic Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Facile One-Pot Synthesis of ZnO/SnO2 Heterojunction Photocatalysts with Excellent Photocatalytic Activity and Photostability. Chempluschem 2012, 77, 217–223. [Google Scholar] [CrossRef]
- Cui, L.; Meng, J.; Gan, Y.; Li, Y. Synthesis and structure of an organobismuth (V) dithiocarbamate polymer [PhBiS2CN(CH3)2 Cl]n and its use as a high-efficiency photocatalysis for organic dyes degradation. Inorg. Nano Metal Chem. 2017, 47, 1537–1541. [Google Scholar] [CrossRef]
- Chowdhury, A.P.; Shambharkar, B.H.; Ghugal, S.G.; Umare, S.S.; Shende, A.G. Ethylene glycol mediated synthesis of SnS quantum dots and their application towards degradation of eosin yellow and brilliant green dyes under solar irradiation. RSC Adv. 2016, 6, 108290–108297. [Google Scholar] [CrossRef]
- Kurra, S.; Venkataswamy, P.; Ravi, G.; Sudhakar Reddy, C.; Jaganmohan Reddy, B.; Vithal, M. Enhancement of Photocatalytic Activity of Sodium Bismuth Titanate by Doping with Copper, Silver, and Tin Ions. Z. Anorg. Allg. Chem. 2019, 645, 1–9. [Google Scholar] [CrossRef]
- Pamecha, K.; Mehta, V.; Kabra, B.V. Photocatalytic Degradation of Commercial Textile Azo Dye Reactive Blue 160 by Heterogeneous Photocatalysis. Adv. Appl. Sci. Res. 2016, 7, 95–101. [Google Scholar]
- Balu, S.; Uma, K.; Pan, G.-T.; Yang, T.; Ramaraj, S. Degradation of Methylene Blue Dye in the Presence of Visible Light Using SiO2@α-Fe2O3 Nanocomposites Deposited on SnS2 Flowers. Materials 2018, 11, 1030. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O.P. Photocatalytic degradation of organic dyes under UV—Visible light using capped ZnS nanoparticles. Sol. Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B Biol. 2018, 180, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pal, P. Treatment and Disposal of Pharmaceutical Wastewater: Toward the Sustainable Strategy. Sep. Purif. Rev. 2018, 47, 179–198. [Google Scholar] [CrossRef]
- Tang, P.; Chen, H.; Cao, F.; Pan, G.; Wang, K.; Xu, M.; Tong, Y. Nanoparticulate SnS as an efficient photocatalyst under visible-light irradiation. Mater. Lett. 2011, 65, 450–452. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Wang, X. Preparation and characterization of ZnO/ZnS hybrid photocatalysts via microwave-hydrothermal method. Front. Environ. Sci. Eng. China 2008, 2, 415–420. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G. Role of nanoparticles in photocatalysis. J. Nanopart. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Fakhri, A.; Behrouz, S.; Pourmand, M. Synthesis, photocatalytic and antimicrobial properties of SnO2, SnS2 and SnO2/SnS2 nanostructure. J. Photochem. Photobiol. B Biol. 2015, 149, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Kevin, P.; Bakr, O.; Muryn, C.A.; Malik, M.A.; O’Brien, P. Routes to tin chalcogenide materials as thin films or nanoparticles: A potentially important class of semiconductor for sustainable solar energy conversion. Inorg. Chem. Front. 2014, 1, 577–598. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Du, Z.N.; Li, S.Y.; Zhang, M. Novel synthesis and high visible light photocatalytic activity of SnS2 nanoflakes from SnCl2·2H2O and S powders. Appl. Catal. B Environ. 2010, 95, 153–159. [Google Scholar] [CrossRef]
- Wang, C.; Tang, K.; Yang, Q.; Qian, Y. Raman scattering, far infrared spectrum and photoluminescence of SnS2 nanocrystallites. Chem. Phys. Lett. 2002, 357, 371–375. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Xue, D.; Zhang, D.; Yang, H. Preparation of nanoporous tin oxide by electrochemical anodization in alkaline electrolytes. Electrochim. Acta 2011, 56, 8797–8801. [Google Scholar] [CrossRef]
- Elango, G.; Kumaran, S.M.; Kumar, S.S.; Muthuraja, S.; Roopan, S.M. Green synthesis of SnO2 nanoparticles and its photocatalytic activity of phenolsulfonphthalein dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 176–180. [Google Scholar] [CrossRef]
- Luwang, M.N.; Ningthoujam, R.S.; Singh, N.S.; Tewari, R.; Srivastava, S.K.; Vatsa, R.K. Surface chemistry of surfactant AOT-stabilized SnO2 nanoparticles and effect of temperature. J. Colloid Interface Sci. 2010, 349, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, G.; Concina, I.; Comini, E.; Falasconi, M.; Ferroni, M.; Sberveglieri, V. Synthesis and integration of tin oxide nanowires into an electronic nose. Vacuum 2012, 86, 532–535. [Google Scholar] [CrossRef]
- Zamand, N.; Nakhaei Pour, A.; Housaindokht, M.R.; Izadyar, M. Size-controlled synthesis of SnO2 nanoparticles using reverse microemulsion method. Solid State Sci. 2014, 33, 6–11. [Google Scholar] [CrossRef]
- Thirumoorthi, M.; Prakash, J.T.J. Effect of F doping on physical properties of (211) oriented SnO2 thin films prepared by jet nebulizer spray pyrolysis technique. Superlattices Microstruct. 2016, 89, 378–389. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Heng, B.T.; Adnan, R. Surfactant-controlled aqueous synthesis of SnO2 nanoparticles via the hydrothermal and conventional heating methods. Turk. J. Chem. 2010, 34, 537–550. [Google Scholar]
- Talebian, N.; Jafarinezhad, F. Morphology-controlled synthesis of SnO2 nanostructures using hydrothermal method and their photocatalytic applications. Ceram. Int. 2013, 39, 8311–8317. [Google Scholar] [CrossRef]
- Etefagh, R.; Pirposhte, M.A.; Radfar, R.; Azhir, E.; Shahtahmasebi, N.; Tabasi, E. Fabrication and characterization of SnO2 and SnS2 nanobiosensor in the Presence of Aspergillus Niger Fungi. Nanomed Res. J. 2019, 4, 35–39. [Google Scholar]
- Lin, C.; Zhu, M.; Zhang, T.; Liu, Y.; Lv, Y.; Li, X.; Liu, M. Cellulose/SnS2 composite with enhanced visible-light photocatalytic activity prepared by microwave-assisted ionic liquid method. RSC Adv. 2017, 7, 12255–12264. [Google Scholar] [CrossRef]
- Niwate, Y.S.; Garje, S.S. Preparation of tin chalcogenide nanoparticles using tribenzyltin (IV) semi-and thiosemicarbazone precursors. Synth. React. Inorg. Met. Nano Metal Chem. 2011, 41, 36–43. [Google Scholar]
- Onwudiwe, D.C.; Strydom, C.A. Colloidal-route synthesis of N-butylaniline capped ZnS and CdS nanoparticles. Mater. Lett. 2013, 92, 71–74. [Google Scholar] [CrossRef]
- Chunggaze, M.; Azad Malik, M.; O’Brien, P. Studies of the thermal decomposition of some diselenocarbamato complexes of cadmium or zinc: Molecular design for the deposition of MSe films by CVD. J. Mater. Chem. 1999, 9, 2433–2437. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-Temperature Synthesis of ZnS Nanoparticles Using Zinc Xanthates as Molecular Precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Huynh, L.; Diverchy, C.; Hermans, S.; Devillers, M.; Dikarev, E.V. Bismuth-Palladium Heterometallic Carboxylate as a Single-Source Precursor for the Carbon-Supported Pd-Bi/C Catalysts. Inorg. Chem. 2009, 48, 6152–6158. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.F.; Bhatti, M.H.; Aamir, M.; Ch, M.A.; Tahir, M.N.; Sher, M.; Ahmed, M.J.; Akhtar, J. A Facile Synthesis of Organotin (IV) Carboxylates: Application as Single Source Precursor for Deposition of Tin Oxide Thin Films and Evaluation of Biological Activities. ChemistrySelect 2018, 3, 10325–10332. [Google Scholar] [CrossRef]
- Mishra, S.; Jeanneau, E.; Berger, M.-H.; Hochepied, J.-F.; Daniele, S. Novel Heteroleptic Heterobimetallic Alkoxide Complexes as Facile Single-Source Precursors for Ta 5+ Doped TiO2-SnO2 Nanoparticles. Inorg. Chem. 2010, 49, 11184–11189. [Google Scholar] [CrossRef] [PubMed]
- Roffey, A.R. Dithiocarbamate Complexes as Single Source Precursors to Metal Sulfide Nanoparticles for Applications in Catalysis; University College London: London, UK, 2012. [Google Scholar]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin (IV) dithiocarbamate complexes: Chemistry and biological activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oyewo, O.A.; Onwudiwe, D.C. Optical and Structural Properties of Tin Sulfide Nanoparticles Obtained via Solvothermal Routes. Z. Anorg. Allg. Chem. 2019, 645, 1–7. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Synthesis, characterization and the use of organotin (IV) dithiocarbamate complexes as precursor to tin sulfide nanoparticles by heat up approach. J. Mol. Struct. 2019, 1195, 395–402. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Arfin, T.; Strydom, C.A.; Kriek, R.J. Synthesis, spectroscopic characterization and behavior of AC impedance spectroscopy of Cd(II) bis(N-para-methylphenyl dithiocarbamate). Electrochim. Acta 2013, 104, 19–25. [Google Scholar] [CrossRef]
- Hrubaru, M.; Onwudiwe, D.C.; Hosten, E. Synthesis and properties of ZnS nanoparticles by solvothermal and pyrolysis routes using the Zn dithiocarbamate complex as novel single source precursor. J. Sulfur Chem. 2016, 37, 37–47. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C. ZnO nanoparticles mediated by aqueous extracts of Dovyalis caffra fruits and the photocatalytic evaluations. Mater. Res. Express 2019, 6, 125091. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Aqueous extract of broccoli mediated synthesis of CaO nanoparticles and its application in the photocatalytic degradation of bromocrescol green. IET Nanobiotechnol. 2018, 12, 888–894. [Google Scholar] [CrossRef]
- Hogarth, G. Transition Metal Dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; Wiley: Hoboken, NJ, USA, 2005; Volume 53, pp. 71–561. ISBN 9780471725589. [Google Scholar]
- Hogarth, G. Metal-dithiocarbamate complexes: Chemistry and biological activity. Mini Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Ahmad, S.; Ahmad, S.; Ali, S.; Awan, S.A. Copper (II) complexes of pyrrolidine dithiocarbamate. Transit. Met. Chem. 2007, 32, 199–203. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Tahir, M.N. Pharmacological investigation of mono-, di- and tri-organotin (IV) derivatives of carbodithioates: Design, spectroscopic characterization, interaction with SS-DNA and POM analyses. Inorg. Chim. Acta 2016, 439, 145–158. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Bhushan, B.; Sharma, A.K. Bis N (chlorophenyl) dithiocarbamato Complexes of Cobalt (II), Nickel (II), Palladium (II) and Platinum (II). Transit. Met. Chem. 1985, 255, 250–255. [Google Scholar]
- Onwudiwe, D.C.; Arfin, T.; Strydom, C.A.; Kriek, R.J. A study of the thermal and AC impedance properties of N-phenyldithiocarbamate complexes of Zn (II). Electrochim. Acta 2013, 109, 809–817. [Google Scholar] [CrossRef]
- Affan, M.A.; Salam, M.A.; Ahmad, F.B.; White, F.; Ali, H.M. Organotin (IV) complexes of 2-hydroxyacetophenone-N(4)-cyclohexylthiosemicarbazone (H2dact): Synthesis, spectral characterization, crystal structure and biological studies. Inorganica Chim. Acta 2012, 387, 219–225. [Google Scholar] [CrossRef]
- Menezes, D.C.; De Lima, G.M.; Porto, A.O.; Donnici, C.L.; Ardisson, J.D.; Doriguetto, A.C.; Ellena, J. Synthesis, characterisation and thermal decomposition of tin(IV) dithiocarbamate derivatives—Single source precursors for tin sulfide powders. Polyhedron 2004, 23, 2103–2109. [Google Scholar] [CrossRef]
- Sathish, M.; Mitani, S.; Tomai, T.; Unemoto, A.; Honma, I. Nanocrystalline tin compounds/graphene nanocomposite electrodes as anode for lithium-ion battery. J. Solid State Electrochem. 2012, 16, 1767–1774. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Majeed Khan, M.A.; Alhadlaq, H.A. Oxidative stress mediated cytotoxicity of tin (IV) oxide (SnO2) nanoparticles in human breast cancer (MCF-7) cells. Colloids Surfaces B Biointerfaces 2018, 172, 152–160. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Yu, G.; Zhang, H.; Yao, K. A simple hydrothermal route for synthesizing SnO2 quantum dots. Nanotechnology 2006, 17, 2386–2389. [Google Scholar] [CrossRef]
- Yagi, I.; Kakizawa, K.; Murakami, K.; Kaneko, S. Preferred Orientation of SnO2 Thin Films Grown from Tri-n-Butyltin Acetate by Spray Pyrolysis Technique. J. Ceram. Soc. Jpn. 1994, 102, 296–298. [Google Scholar] [CrossRef]
- Mendes, P.G.; Moreira, M.L.; Tebcherani, S.M.; Orlandi, M.O.; Andrés, J.; Li, M.S.; Diaz-Mora, N.; Varela, J.A.; Longo, E. SnO2 nanocrystals synthesized by microwave-assisted hydrothermal method: Towards a relationship between structural and optical properties. J. Nanopart. Res. 2012, 14, 750. [Google Scholar] [CrossRef]
- Wang, G.; Peng, J.; Zhang, L.; Zhang, J.; Dai, B.; Zhu, M.; Xia, L.; Yu, F. Two-dimensional SnS2 @PANI nanoplates with high capacity and excellent stability for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 3659–3666. [Google Scholar] [CrossRef]
- Li, M.; Liu, E.; Hu, H.; Ouyang, S.; Xu, H.; Wang, D. Surfactant-Free Synthesis of Single Crystalline SnS2 and Effect of Surface Atomic Structure on the Photocatalytic Property. Int. J. Photoenergy 2014, 2014, 1–7. [Google Scholar]
- Mali, J.M.; Arbuj, S.S.; Ambekar, J.D.; Rane, S.B.; Mulik, U.P.; Amalnerkar, D.P. Hydrothermal synthesis of SnS2 faceted nano sheets and their visible light driven photocatalytic performance. Sci. Adv. Mater. 2013, 5, 1994–1998. [Google Scholar] [CrossRef]
- Hu, X.; Song, G.; Li, W.; Peng, Y.; Jiang, L.; Xue, Y.; Liu, Q.; Chen, Z.; Hu, J. Phase-controlled synthesis and photocatalytic properties of SnS, SnS2 and SnS/SnS2 heterostructure nanocrystals. Mater. Res. Bull. 2013, 48, 2325–2332. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Sagade, A.A.; Gudage, Y.G.; Lokhande, C.D.; Sharma, R. Growth and characterization of tin disulfide (SnS2) thin film deposited by successive ionic layer adsorption and reaction (SILAR) technique. J. Alloys Compd. 2007, 436, 421–426. [Google Scholar] [CrossRef]
- Mayandi, J.; Marikkannan, M.; Ragavendran, V.; Jayabal, P. Hydrothermally Synthesized Sb and Zn Doped SnO2. Nanoparticles 2014, 2, 707–710. [Google Scholar]
- Sinha, A.K.; Manna, P.K.; Pradhan, M.; Mondal, C.; Yusuf, S.M.; Pal, T. Tin oxide with a p-n heterojunction ensures both UV and visible light photocatalytic activity. RSC Adv. 2014, 4, 208–211. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yao, L.; Zhang, G.; Dionysiou, D.D.; Li, J.; Du, X. One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI). Appl. Catal. B Environ. 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Johnson Jeyakumar, S.; Praveen, P.; Joseph Panneerdoss, I. Photocatalytic activity of spray deposited ZrO2 nano-thin films on methylene blue decolouration. J. Mater. Sci. Mater. Electron. 2016, 27, 5851–5859. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Miao, S.; Liu, Z.; Ding, K.; Miao, Z.; An, G.; Yang, Z. One-pot synthesis of ZnS/polymer composites in supercritical CO2-ethanol solution and their applications in degradation of dyes. J. Colloid Interface Sci. 2008, 318, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Muhd Julkapli, N.; Bagheri, S.; Bee Abd Hamid, S. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalin. Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Lucena, R.; Fresno, F.; Conesa, J.C. Hydrothermally synthesized nanocrystalline tin disulphide as visible light-active photocatalyst: Spectral response and stability. Appl. Catal. A Gen. 2012, 415–416, 111–117. [Google Scholar] [CrossRef]
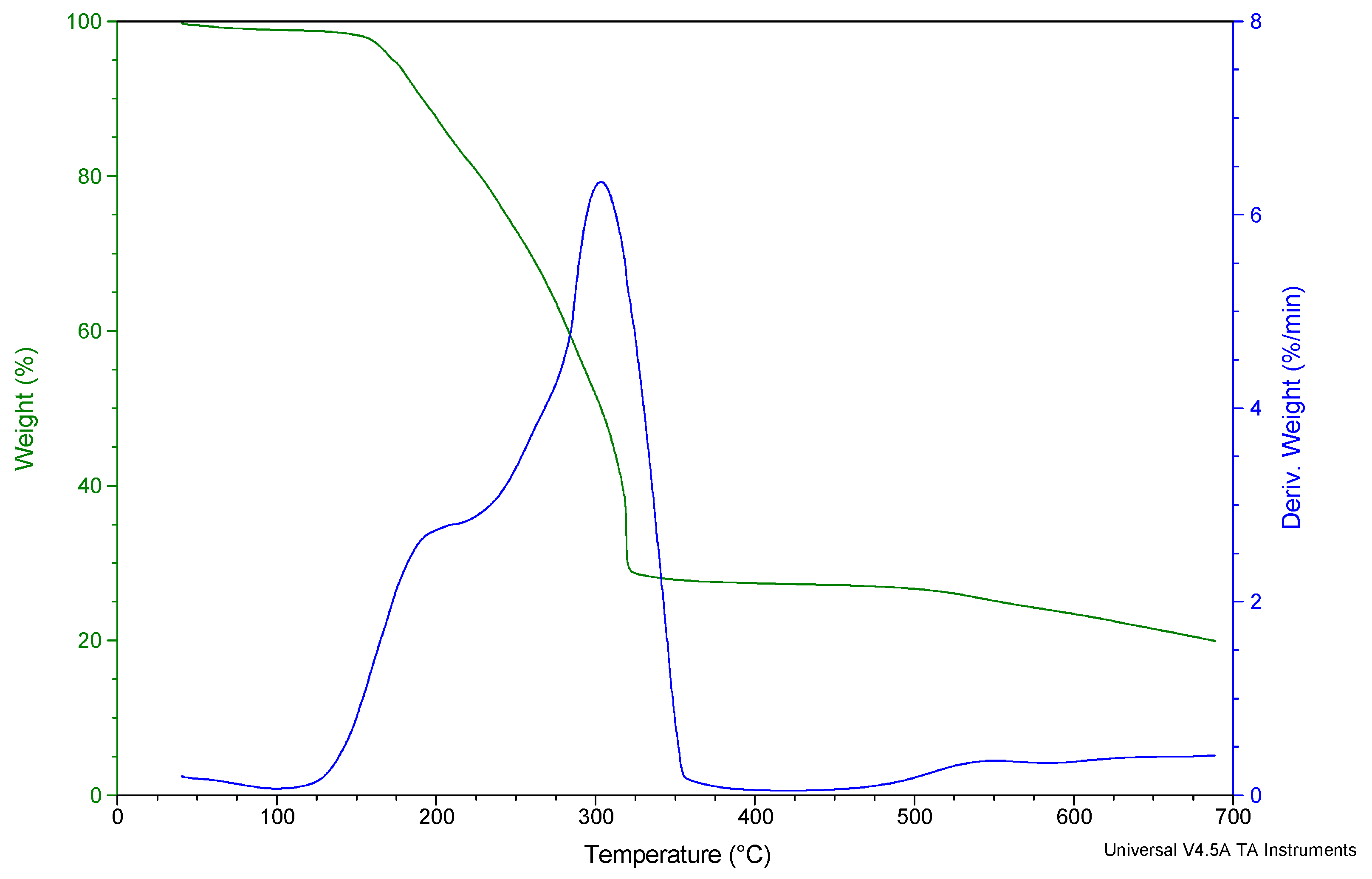
| Temperature Range of Decomposition (°C) | DTG Peak T (°C) | Product Obtained after Decomposition | Mass of Residue (mg) Found (Calc) | ||||
|---|---|---|---|---|---|---|---|
| 1st step | 2nd step | 1st step | 2nd step | 1st step | 2nd step | 1st step | 2nd step |
| 100–217 | 230–321 | 216 | 303 | (CH3-Ph) (HNCS2)2 Sn (Ph)2 | Sn2S3 | 11.90 (11.93) | 7.19 (7.47) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, J.O.; Onwudiwe, D.C. SnS2 and SnO2 Nanoparticles Obtained from Organotin(IV) Dithiocarbamate Complex and Their Photocatalytic Activities on Methylene Blue. Materials 2020, 13, 2766. https://doi.org/10.3390/ma13122766
Adeyemi JO, Onwudiwe DC. SnS2 and SnO2 Nanoparticles Obtained from Organotin(IV) Dithiocarbamate Complex and Their Photocatalytic Activities on Methylene Blue. Materials. 2020; 13(12):2766. https://doi.org/10.3390/ma13122766
Chicago/Turabian StyleAdeyemi, Jerry O., and Damian C. Onwudiwe. 2020. "SnS2 and SnO2 Nanoparticles Obtained from Organotin(IV) Dithiocarbamate Complex and Their Photocatalytic Activities on Methylene Blue" Materials 13, no. 12: 2766. https://doi.org/10.3390/ma13122766
APA StyleAdeyemi, J. O., & Onwudiwe, D. C. (2020). SnS2 and SnO2 Nanoparticles Obtained from Organotin(IV) Dithiocarbamate Complex and Their Photocatalytic Activities on Methylene Blue. Materials, 13(12), 2766. https://doi.org/10.3390/ma13122766






