Neuroprotection or Neurotoxicity of Illicit Drugs on Parkinson’s Disease
Abstract
:1. Introduction
2. Methodology
3. Phytocannabinoids and Parkinson’s Disease
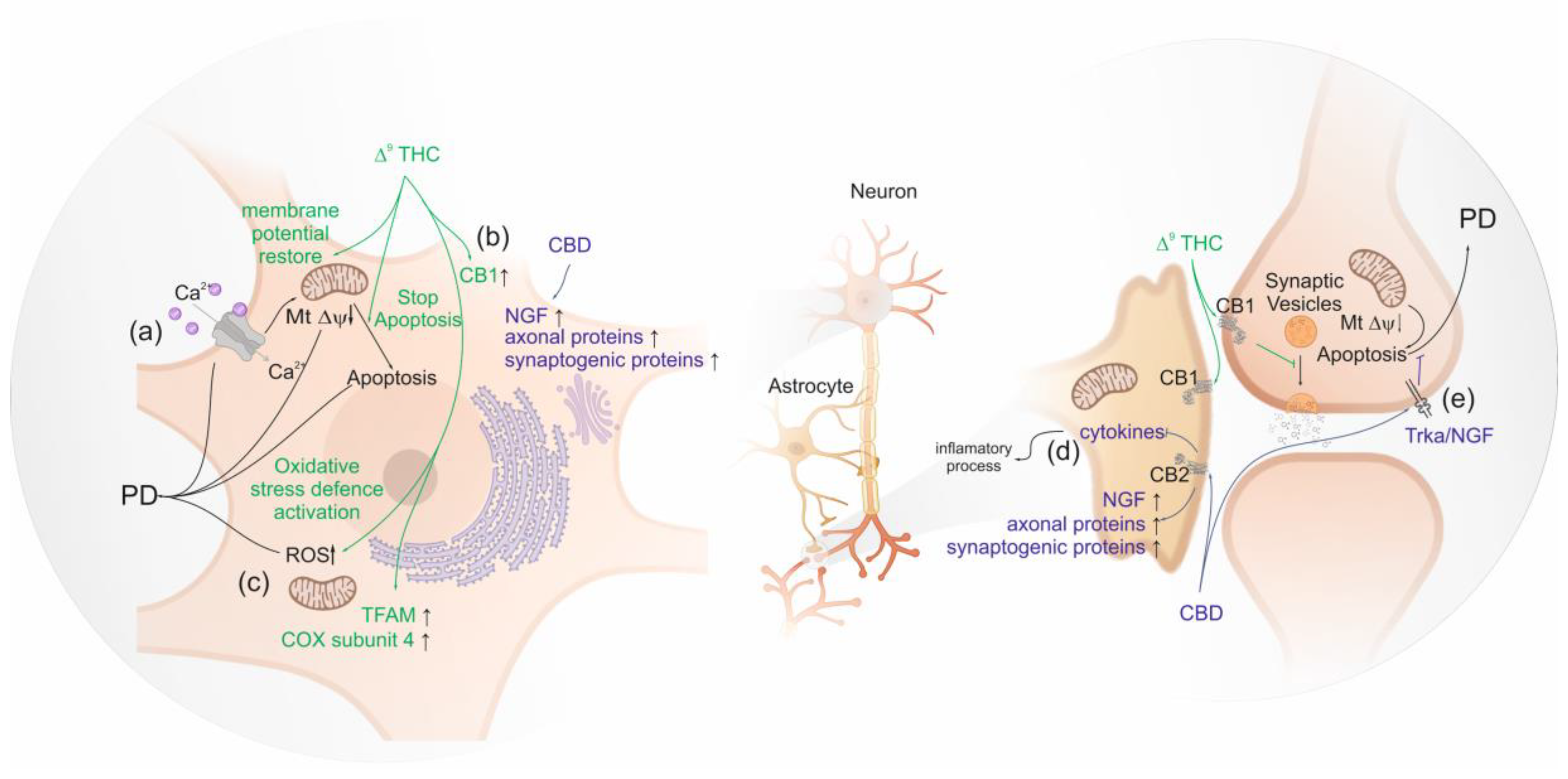
3.1. Endocannabinoid System and Parkinson’s Disease
3.2. Clinical Observations on Phytocannabinoids Use in Parkinson’s Disease
3.3. Studies on the Molecular and Cellular Mechanisms Underlying Clinical Observations
3.4. Is There Enough Data Supporting Protective or Therapeutic Role of Cannabinoids on PD?
4. Amphetamine-Type Stimulants and Parkinson’s Disease
4.1. Clinical Observations of Amphetamine-Type Stimulants Use in Parkinson’s Disease
4.2. Studies on the Molecular and Cellular Mechanisms Underlying Clinical Observations
4.3. Is There Enough Data Supporting a Neurotoxic Role of Amphetamine-Type Stimulants on PD?
5. Cocaine
5.1. Clinical Observations of Cocaine Use in Parkinson’s Disease
5.2. Studies on the Molecular and Cellular Mechanisms
5.3. Is There Enough Data Supporting a Neurotoxic role of Cocaine on PD?
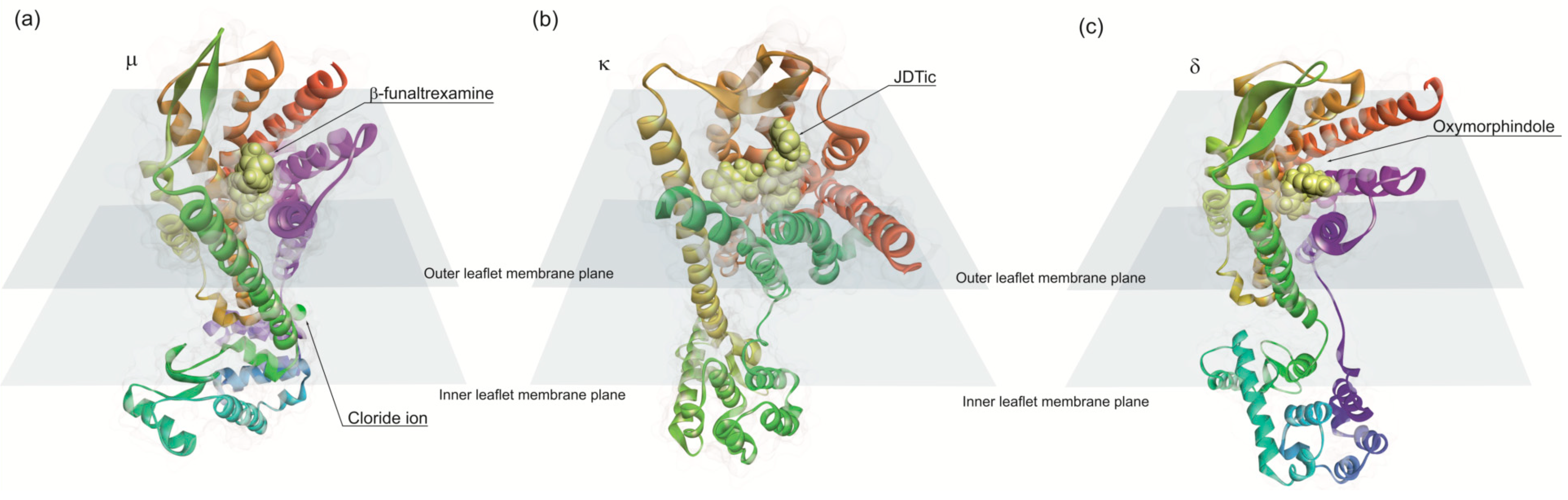
6. Opiates and Parkinson’s Disease
6.1. Morphine and Parkinson’s Disease
6.2. Heroin
7. Future Issues: Novel Psychoactive Substances-Protective or Neurotoxic?
7.1. Synthetic Cannabinoids
7.2. Synthetic Cathinones
8. Synthesis of the Available Data on Illicit Drugs and Parkinson’s Disease
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bondy, S.C. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology 2016, 341–343, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Krishnamurthi, R.V.; Theadom, A.M.; Abajobir, A.A.; Mishra, S.R.; Ahmed, M.B.; Abate, K.H.; Mengistie, M.A.; Wakayo, T.; Abd-Allah, F.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Castillo, X.; Castro-Obregón, S.; Gutiérrez-Becker, B.; Gutiérrez-Ospina, G.; Karalis, N.; Khalil, A.A.; Lopez-Noguerola, J.S.; Rodríguez, L.L.; Martínez-Martínez, E.; Perez-Cruz, C.; et al. Re-thinking the Etiological Framework of Neurodegeneration. Front. Neurosci. 2019, 13, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Soto, C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 2001, 498, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-J.; Yu, M.-H. Protein Folding and Diseases. BMB Rep. 2005, 38, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Quintas, A.; Vaz, D.C.; Cardoso, I.; Saraiva, M.J.M.; Brito, R.M.M. Tetramer Dissociation and Monomer Partial Unfolding Precedes Protofibril Formation in Amyloidogenic Transthyretin Variants. J. Biol. Chem. 2001, 276, 27207–27213. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.J.; Qu, B.-H.; Pedersen, P.L. Defective protein folding as a basis of human disease. Trends Biochem. Sci. 1995, 20, 456–459. [Google Scholar] [CrossRef]
- Eva, Ž. Amyloid-fibril formation. Eur. J. Biochem. 2002, 269, 3362–3371. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, I.; Isooka, N.; Imafuku, F.; Sun, J.; Kikuoka, R.; Furukawa, C.; Asanuma, M. Chronic Systemic Exposure to Low-Dose Rotenone Induced Central and Peripheral Neuropathology and Motor Deficits in Mice: Reproducible Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.; Ballard, P.; Tetrud, J.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan-Montojo, F.; Reichmann, H. Considerations on the role of environmental toxins in idiopathic Parkinson’s disease pathophysiology. Transl. Neurodegener. 2014, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Ferrara, N.; Brooks, D.J.; Pavese, N. Age at onset and Parkinson disease phenotype. Neurology 2016, 86, 1400–1407. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Khan, A.U.; Akram, M.; Daniyal, M.; Zainab, R. Awareness and current knowledge of Parkinson’s disease: A neurodegenerative disorder. Int. J. Neurosci. 2019, 129, 55–93. [Google Scholar] [CrossRef]
- Thacker, E.L.; Ascherio, A. Familial aggregation of Parkinson’s disease: A meta-analysis. Mov. Disord. 2008, 23, 1174–1183. [Google Scholar] [CrossRef]
- Billingsley, K.J.; Bandres-Ciga, S.; Saez-Atienzar, S.; Singleton, A.B. Genetic risk factors in Parkinson’s disease. Cell Tissue Res. 2018, 373, 9–20. [Google Scholar] [CrossRef]
- Barrett, M.J.; Hac, N.E.; Yan, G.; Harrison, M.B.; Wooten, G.F. Relationship of age of onset and family history in Parkinson disease. Mov. Disord. 2015, 30, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Sellbach, A.N.; Boyle, R.S.; Silburn, P.A.; Mellick, G.D. Parkinson’s disease and family history. Parkinsonism Relat. Disord. 2006, 12, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Fahn, S. Description of Parkinson’s disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 2003, 991, 1–14. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Anwarullah; Aslam, M.; Badshah, M.; Abbasi, R.; Sultan, A.; Khan, K.; Ahmad, N.; von Engelhardt, J. Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson’s disease: A case control study. Genes Environ. 2017, 39, 18. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Cawthon, D.; McCastlain, K.A.; Slikker, W.; Ali, S.F. Selective alterations of gene expression in mice induced by MPTP. Synapse 2005, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Miner, L.L.; Sora, I.; Ujike, H.; Revay, R.S.; Kostic, V.; Jackson-Lewis, V.; Przedborski, S.; Uhl, G.R. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl. Acad. Sci. USA 1997, 94, 9938–9943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakye, G.F.; McMinimy, R.A.; Aschner, M. Disease-Toxicant Interactions in Parkinson’s Disease Neuropathology. Neurochem. Res. 2017, 42, 1772–1786. [Google Scholar] [CrossRef]
- Ylikotila, P.; Tiirikka, T.; Moilanen, J.S.; Kääriäinen, H.; Marttila, R.; Majamaa, K. Epidemiology of early-onset Parkinson’s disease in Finland. Parkinsonism Relat. Disord. 2015, 21, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016, 73, 981. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegrzynowicz, M.; Bar-On, D.; Calo’, L.; Anichtchik, O.; Iovino, M.; Xia, J.; Ryazanov, S.; Leonov, A.; Giese, A.; Dalley, J.W.; et al. Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 2019, 138, 575–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cookson, M.R. α-Synuclein and neuronal cell death. Mol. Neurodegener. 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, T.; Nakata, Y.; Mochizuki, H. α-Synuclein and Neuronal Cell Death. Mol. Neurobiol. 2013, 47, 466–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.L.; Nutt, J.G. Treatment of Parkinson’s disease with trophic factors. Neurotherapeutics 2008, 5, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Dzamko, N.; Zhou, J.; Huang, Y.; Halliday, G.M. Parkinson’s disease-implicated kinases in the brain; insights into disease pathogenesis. Front. Mol. Neurosci. 2014, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Model. Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, C.; Stetler, C.; Petrucelli, L. Disruption of Protein Quality Control in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gramage, E.; Herradón, G. Connecting Parkinson’s disease and drug addiction: Common players reveal unexpected disease connections and novel therapeutic approaches. Curr. Pharm. Des. 2011, 17, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Ribaric, S. The pharmacological properties and therapeutic use of apomorphine. Molecules 2012, 17, 5289–5309. [Google Scholar] [CrossRef] [Green Version]
- Heales, S.; Crawley, F.; Rudge, P. Reversible parkinsonism following heroin pyrolysate inhalation is associated with tetrahydrobiopterin deficiency. Mov. Disord. 2004, 19, 1248–1251. [Google Scholar] [CrossRef]
- Balash, Y.; Bar-Lev Schleider, L.; Korczyn, A.D.; Shabtai, H.; Knaani, J.; Rosenberg, A.; Baruch, Y.; Djaldetti, R.; Giladi, N.; Gurevich, T. Medical Cannabis in Parkinson Disease. Clin. Neuropharmacol. 2017, 40, 268–272. [Google Scholar] [CrossRef]
- Koppel, B.S.; Brust, J.C.M.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewski, K.; Barbano, R.; Mink, J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Kluger, B.; Triolo, P.; Jones, W.; Jankovic, J. The therapeutic potential of cannabinoids for movement disorders. Mov. Disord. 2015, 30, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.B.; Zeissler, M.-L.; Hanemann, C.O.; Zajicek, J.P. Δ9 -tetrahydrocannabinol (Δ9 -THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2012, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, D.E.; de la Monte, S.M. Adverse Structural and Functional Effects of Marijuana on the Brain: Evidence Reviewed. Pediatr. Neurol. 2017, 66, 12–20. [Google Scholar] [CrossRef]
- United Nations. World Drug Report 2019—Cannabis and Hallucinogens; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagan, S.G.; Campbell, V.A. The influence of cannabinoids on generic traits of neurodegeneration. Br. J. Pharmacol. 2014, 171, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, S.P.H. Therapeutic potential of cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Leweke, F.M.; Mueller, J.K.; Lange, B.; Rohleder, C. Therapeutic Potential of Cannabinoids in Psychosis. Biol. Psychiatry 2016, 79, 604–612. [Google Scholar] [CrossRef]
- Gérard, C.M.; Mollereau, C.; Vassart, G.; Parmentier, M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 1991, 279, 129–134. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Rossi, A.; Calabresi, P. The Endocannabinoid System in Parkinsons Disease. Curr. Pharm. Des. 2008, 14, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; García-Gutiérrez, M.S.; Aracil-Fernández, A.; Lanciego, J.L.; Manzanares, J. Cannabinoid CB1 and CB2 Receptors, and Monoacylglycerol Lipase Gene Expression Alterations in the Basal Ganglia of Patients with Parkinson’s Disease. Neurotherapeutics 2018, 15, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo-Bustamante, E.; Abellanas, M.A.; Clavero, P.; Thiolat, M.-L.; Li, Q.; Luquin, M.R.; Bezard, E.; Aymerich, M.S. The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol. Dis. 2018, 118, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Katz, I.; Katz, D.; Shoenfeld, Y.; Porat-Katz, B.S. Clinical Evidence for Utilizing Cannabinoids in the Elderly. Isr. Med. Assoc. J. 2017, 19, 71–75. [Google Scholar] [PubMed]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Doyle, M.E.; Liu, Q.-R.; Cimbro, R.; Santa-Cruz Calvo, S.; Ghosh, S.; Cieśla, Ł.; Moaddel, R.; Carlson, O.D.; et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016, 6, 33302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.-R.; Pan, C.-H.; Hishimoto, A.; Li, C.-Y.; Xi, Z.-X.; Llorente-Berzal, A.; Viveros, M.-P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species differences in cannabinoid receptor 2 ( CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Dhopeshwarkar, A.; Mackie, K. CB 2 Cannabinoid Receptors as a Therapeutic Target—What Does the Future Hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- den Boon, F.S.; Chameau, P.; Schaafsma-Zhao, Q.; van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkman, T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 3534–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, A.R.; Pisanu, A.; Carboni, E. Do PPAR-Gamma Agonists Have a Future in Parkinson’s Disease Therapy? Parkinsons. Dis. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanveer, R.; McGuinness, N.; Daniel, S.; Gowran, A.; Campbell, V.A. Cannabinoid receptors and neurodegenerative diseases. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 633–639. [Google Scholar] [CrossRef]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef] [Green Version]
- Celorrio, M.; Rojo-Bustamante, E.; Fernández-Suárez, D.; Sáez, E.; Estella-Hermoso de Mendoza, A.; Müller, C.E.; Ramírez, M.J.; Oyarzábal, J.; Franco, R.; Aymerich, M.S. GPR55: A therapeutic target for Parkinson’s disease? Neuropharmacology 2017, 125, 319–332. [Google Scholar] [CrossRef]
- Katz, D.; Katz, I.; Porat-Katz, B.S.; Shoenfeld, Y. Medical cannabis: Another piece in the mosaic of autoimmunity? Clin. Pharmacol. Ther. 2017, 101, 230–238. [Google Scholar] [CrossRef]
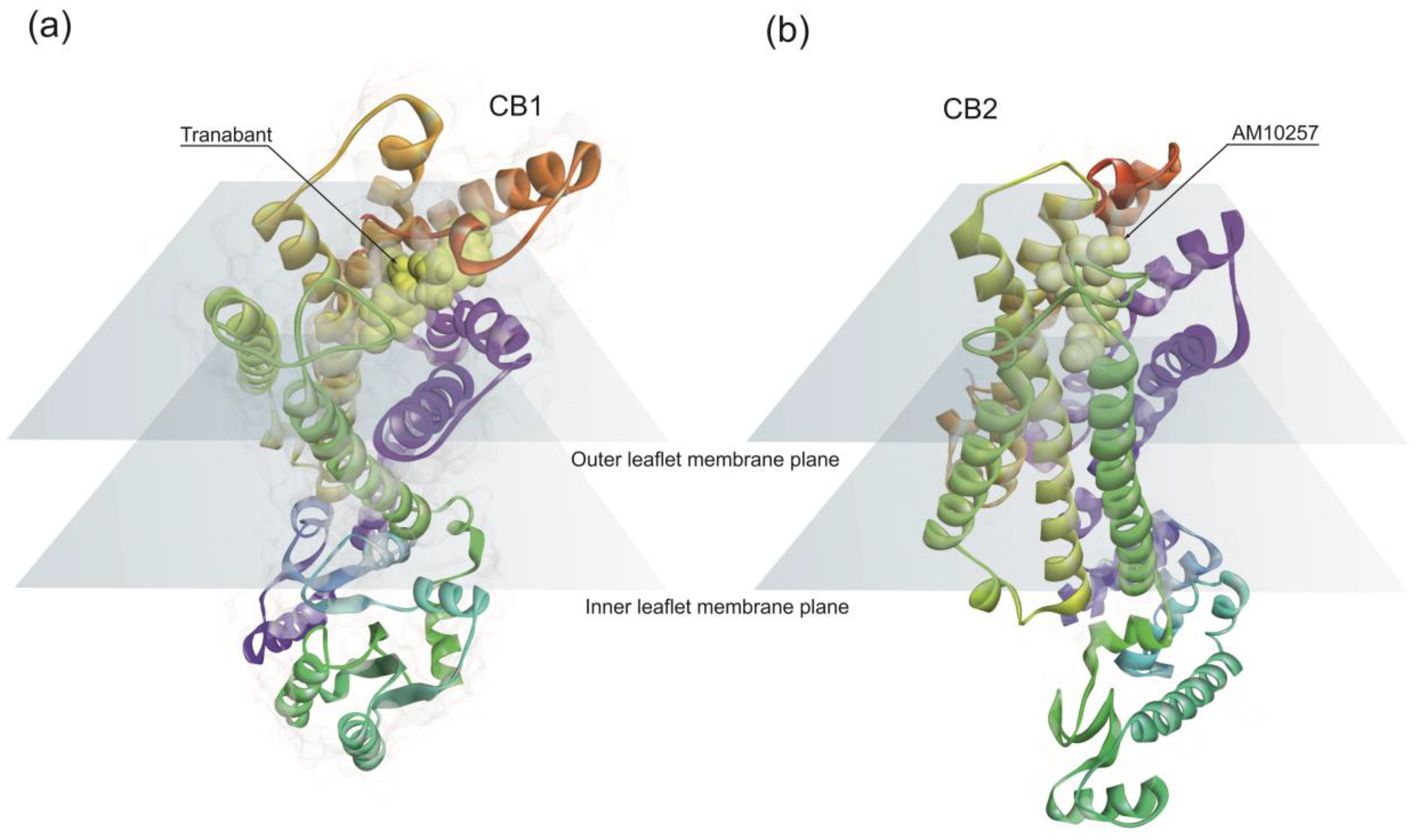
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef] [Green Version]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devane, W.; Hanus, L.; Breuer, A.; Pertwee, R.; Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Hanus, L.; Gopher, A.; Almog, S.; Mechoulam, R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J. Med. Chem. 1993, 36, 3032–3034. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 231, ISBN 978-3-319-20824-4. [Google Scholar]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G. Cannabinoid Receptors and Their Ligands: Beyond CB 1 and CB 2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, F.; Larsen, B.; Gao, M.; Luo, Z.; Chen, D.; Ma, X.; Qiu, S.; Zhou, Y.; Xie, J.; et al. Mechanisms of cannabinoid CB2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine 2019, 42, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, H.; Cordova, M.; Chen, C.-S.J.; Rajadhyaksha, M. Confocal Imaging–Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puente, N.; Cui, Y.; Lassalle, O.; Lafourcade, M.; Georges, F.; Venance, L.; Grandes, P.; Manzoni, O.J. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 2011, 14, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Fezza, F.; Musella, A.; Gasperi, V.; Prosperetti, C.; Bernardi, G.; Finazzi-Agrò, A.; et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 2008, 11, 152–159. [Google Scholar] [CrossRef]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agrò, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef]
- Oz, M.; Jaligam, V.; Galadari, S.; Petroianu, G.; Shuba, Y.M.; Shippenberg, T.S. The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor-independent mechanism. J. Neurochem. 2010, 112, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelt, M.; Fox, S.H.; Hill, M.; Crossman, A.R.; Petrosino, S.; Di Marzo, V.; Brotchie, J.M. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005, 19, 1140–1142. [Google Scholar] [CrossRef]
- Mnich, K.; Finn, D.P.; Dowd, E.; Gorman, A.M. Inhibition by Anandamide of 6-Hydroxydopamine-Induced Cell Death in PC12 Cells. Int. J. Cell Biol. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Matas, D.; Juknat, A.; Pietr, M.; Klin, Y.; Vogel, Z. Anandamide Protects from Low Serum-induced Apoptosis via Its Degradation to Ethanolamine. J. Biol. Chem. 2007, 282, 7885–7892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shouman, B.; Fontaine, R.H.; Baud, O.; Schwendimann, L.; Keller, M.; Spedding, M.; Lelièvre, V.; Gressens, P. Endocannabinoids potently protect the newborn brain against AMPA-kainate receptor-mediated excitotoxic damage. Br. J. Pharmacol. 2009, 148, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Sinor, A.D.; Irvin, S.M.; Greenberg, D.A. Endocannabinoids protect cerebral cortical neurons from in vitro ischemia in rats. Neurosci. Lett. 2000, 278, 157–160. [Google Scholar] [CrossRef]
- Contassot, E.; Wilmotte, R.; Tenan, M.; Belkouch, M.-C.; Schnüriger, V.; de Tribolet, N.; Bourkhardt, K.; Dietrich, P.-Y. Arachidonylethanolamide Induces Apoptosis of Human Glioma Cells through Vanilloid Receptor-1. J. Neuropathol. Exp. Neurol. 2004, 63, 956–963. [Google Scholar] [CrossRef]
- Babayeva, M.; Assefa, H.; Basu, P.; Chumki, S.; Loewy, Z. Marijuana Compounds: A Nonconventional Approach to Parkinson’s Disease Therapy. Parkinsons. Dis. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Romero, J.; Garcia, L.; Cebeira, M.; Zadrozny, D.; Fernández-Ruiz, J.J.; Ramos, J.A. The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: Role of nigrostriatal dopaminergic neurons. Life Sci. 1995, 56, 2033–2040. [Google Scholar] [CrossRef]
- De Lago, E.; Fernández-Ruiz, J.; Ortega-Gutiérrez, S.; Viso, A.; López-Rodríguez, M.L.; Ramos, J.A. UCM707, a potent and selective inhibitor of endocannabinoid uptake, potentiates hypokinetic and antinociceptive effects of anandamide. Eur. J. Pharmacol. 2002, 449, 99–103. [Google Scholar] [CrossRef]
- Solinas, M.; Justinova, Z.; Goldberg, S.R.; Tanda, G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J. Neurochem. 2006, 98, 408–419. [Google Scholar] [CrossRef]
- Gubellini, P.; Picconi, B.; Bari, M.; Battista, N.; Calabresi, P.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Maccarrone, M. Experimental Parkinsonism Alters Endocannabinoid Degradation: Implications for Striatal Glutamatergic Transmission. J. Neurosci. 2002, 22, 6900–6907. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, D.G.; Ueda, N.; Yamamoto, S. The fatty acid amide hydrolase (FAAH). Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 201–210. [Google Scholar] [CrossRef]
- Di Marzo, V.; Maccarrone, M. FAAH and anandamide: Is 2-AG really the odd one out? Trends Pharmacol. Sci. 2008, 29, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espejo, E.; Caraballo, I.; Rodriguez de Fonseca, F.; Ferrer, B.; El Banoua, F.; Flores, J.A.; Galan-Rodriguez, B. Experimental Parkinsonism Alters Anandamide Precursor Synthesis, and Functional Deficits are Improved by AM404: A Modulator of Endocannabinoid Function. Neuropsychopharmacology 2004, 29, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Aparicio, R.; Moratalla, R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2014, 62, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavón, N.; Martín, A.B.; Mendialdua, A.; Moratalla, R. ERK Phosphorylation and FosB Expression Are Associated with L-DOPA-Induced Dyskinesia in Hemiparkinsonian Mice. Biol. Psychiatry 2006, 59, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflamm. 2018, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and Neurodegenerative Disorders: Parkinson’s Disease, Huntington’s Chorea, Alzheimer’s Disease, and Others. Handb. Exp. Pharmacol. 2015, 231, 233–259. [Google Scholar] [PubMed]
- Little, J.P.; Villanueva, E.B.; Klegeris, A. Therapeutic potential of cannabinoids in the treatment of neuroinflammation associated with Parkinson’s disease. Mini Rev. Med. Chem. 2011, 11, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Gowran, A.; Noonan, J.; Campbell, V.A. The Multiplicity of Action of Cannabinoids: Implications for Treating Neurodegeneration. CNS Neurosci. Ther. 2011, 17, 637–644. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Differential upregulation of the cannabinoid CB2 receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2015, 269, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, K.; Casteels, C.; Lunskens, S.; Goffin, K.; Grachev, I.D.; Bormans, G.; Vandenberghe, W. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging 2012, 33, 620.e1–620.e8. [Google Scholar] [CrossRef]
- Farkas, S.; Nagy, K.; Jia, Z.; Harkany, T.; Palkovits, M.; Donohou, S.R.; Pike, V.W.; Halldin, C.; Máthé, D.; Csiba, L.; et al. The decrease of dopamine D2/D3 receptor densities in the putamen and nucleus caudatus goes parallel with maintained levels of CB1 cannabinoid receptors in Parkinson’s disease: A preliminary autoradiographic study with the selective dopamine D2/D3 antagoni. Brain Res. Bull. 2012, 87, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Song, L.; Yang, X.; Ma, Y.; Wu, N. The CB1 cannabinoid receptor agonist reduces L-DOPA-induced motor fluctuation and ERK1/2 phosphorylation in 6-OHDA-lesioned rats. Drug Des. Devel. Ther. 2014, 8, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- García, M.C.; Cinquina, V.; Palomo-Garo, C.; Rábano, A.; Fernández-Ruiz, J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci. Lett. 2015, 587, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gálvez, Y.; Palomo-Garo, C.; Fernández-Ruiz, J.; García, C. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 200–208. [Google Scholar]
- Chung, Y.C.; Shin, W.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.; Jin, B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Levandis, G.; Ambrosi, G.; Montepeloso, E.; Antoninetti, G.F.; Franco, R.; Lanciego, J.L.; Baqi, Y.; Müller, C.E.; Pinna, A.; et al. Neuroprotective Potential of Adenosine A 2A and Cannabinoid CB 1 Receptor Antagonists in an Animal Model of Parkinson Disease. J. Neuropathol. Exp. Neurol. 2014, 73, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Suryadevara, U.; Bruijnzeel, D.M.; Nuthi, M.; Jagnarine, D.A.; Tandon, R.; Bruijnzeel, A.W. Pros and Cons of Medical Cannabis use by People with Chronic Brain Disorders. Curr. Neuropharmacol. 2017, 15, 800–814. [Google Scholar] [CrossRef] [Green Version]
- Kindred, J.H.; Li, K.; Ketelhut, N.B.; Proessl, F.; Fling, B.W.; Honce, J.M.; Shaffer, W.R.; Rudroff, T. Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complement. Ther. Med. 2017, 33, 99–104. [Google Scholar] [CrossRef]
- Patel, R.S.; Kamil, S.; Shah, M.R.; Bhimanadham, N.N.; Imran, S. Pros and Cons of Marijuana in Treatment of Parkinson’s Disease. Cureus 2019, 11, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Venderová, K.; Růžička, E.; Voříšek, V.; Višňovský, P. Survey on cannabis use in Parkinson’s disease: Subjective improvement of motor symptoms. Mov. Disord. 2004, 19, 1102–1106. [Google Scholar]
- Arjmand, S.; Vaziri, Z.; Behzadi, M.; Abbassian, H.; Stephens, G.J.; Shabani, M. Cannabinoids and Tremor Induced by Motor-related Disorders: Friend or Foe? Neurotherapeutics 2015, 12, 778–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotan, I.; Treves, T.A.; Roditi, Y.; Djaldetti, R. Cannabis (Medical Marijuana) Treatment for Motor and Non–Motor Symptoms of Parkinson Disease. Clin. Neuropharmacol. 2014, 37, 41–44. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Kunz, M.; Valeriani, M. Therapeutic approach to pain in neurodegenerative diseases: Current evidence and perspectives. Expert Rev. Neurother. 2017, 17, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.B.; Bain, P.G.; Teare, L.; Liu, X.; Joint, C.; Wroath, C.; Parkin, S.G.; Fox, P.; Wright, D.; Hobart, J.; et al. Cannabis for dyskinesia in Parkinson disease: A randomized double-blind crossover study. Neurology 2004, 63, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Noel, C. Evidence for the use of “medical marijuana” in psychiatric and neurologic disorders. Ment. Heal. Clin. 2017, 7, 29–38. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [Green Version]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Crippa, J.A.S.; Hallak, J.E.C.; Zuardi, A.W.; Guimarães, F.S.; Tumas, V.; dos Santos, R.G. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms? Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 121–133. [Google Scholar] [CrossRef]
- Zuardi, A.; Crippa, J.; Hallak, J.; Pinto, J.; Chagas, M.; Rodrigues, G.; Dursun, S.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.N.; Eckeli, A.L.; Zuardi, A.W.; Pena-Pereira, M.A.; Sobreira-Neto, M.A.; Sobreira, E.T.; Camilo, M.R.; Bergamaschi, M.M.; Schenck, C.H.; Hallak, J.E.C.; et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: A case series. J. Clin. Pharm. Ther. 2014, 39, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.; Monaghan, M. Cannabis and Psychosis: Are We any Closer to Understanding the Relationship? Curr. Psychiatry Rep. 2019, 21, 48. [Google Scholar] [CrossRef] [Green Version]
- Udow, S.J.; Freitas, M.E.; Fox, S.H.; Lang, A.E. Exacerbation of psychosis triggered by a synthetic cannabinoid in a 70-year-old woman with Parkinson disease. Can. Med. Assoc. J. 2018, 190, E50–E52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mursaleen, L.R.; Stamford, J.A. Drugs of abuse and Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bega, D.; Simuni, T.; Okun, M.S.; Chen, X.; Schmidt, P. Medicinal Cannabis for Parkinson’s Disease: Practices, Beliefs, and Attitudes Among Providers at National Parkinson Foundation Centers of Excellence. Mov. Disord. Clin. Pract. 2017, 4, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, P.; Yu, Y.; Bao, J. Association of Dopamine Beta-Hydroxylase (DBH) Polymorphisms with Susceptibility to Parkinson’s Disease. Med. Sci. Monit. 2016, 22, 1617–1622. [Google Scholar] [CrossRef] [Green Version]
- Busquets-Garcia, A.; Gomis-González, M.; Srivastava, R.K.; Cutando, L.; Ortega-Alvaro, A.; Ruehle, S.; Remmers, F.; Bindila, L.; Bellocchio, L.; Marsicano, G.; et al. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 9904–9909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeissler, M.; Eastwood, J.; McCorry, K.; Hanemann, C.O.; Zajicek, J.P.; Carroll, C.B. Delta-9-tetrahydrocannabinol protects against MPP+ toxicity in SH-SY5Y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget 2016, 7, 46603–46614. [Google Scholar] [CrossRef] [Green Version]
- Fabregat-Andres, O.; Paredes, F.; Monsalve, M.; Milara, J.; Ridocci-Soriano, F.; Gonzalez-Hervas, S.; Mena, A.; Facila, L.; Hornero, F.; Morell, S.; et al. mRNA PGC-1α levels in blood samples reliably correlates with its myocardial expression: Study in patients undergoing cardiac surgery. Anatol. J. Cardiol. 2015, 16, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Kadenbach, B. Mitochondrial Oxidative Phosphorylation; Kadenbach, B., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 748, ISBN 978-1-4614-3572-3. [Google Scholar]
- Nguyen, C.H.; Krewenka, C.; Radad, K.; Kranner, B.; Huber, A.; Duvigneau, J.C.; Miller, I.; Moldzio, R. THC (Δ9-Tetrahydrocannabinol) Exerts Neuroprotective Effect in Glutamate-affected Murine Primary Mesencephalic Cultures Through Restoring Mitochondrial Membrane Potential and Anti-apoptosis Involving CB 1 Receptor-dependent Mechanism. Phyther. Res. 2016, 30, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.G.; Martins, N.M.; Sisti, F.M.; Fernandes, L.S.; Ferreira, R.S.; Queiroz, R.H.C.; Santos, A.C. The neuroprotection of cannabidiol against MPP + -induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol. Vitr. 2015, 30, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, C.; Palomo-Garo, C.; García-Arencibia, M.; Ramos, J.; Pertwee, R.; Fernández-Ruiz, J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ 9 -THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Lippmann, S. Marijuana for Parkinson’s Disease? Innov. Clin. Neurosci. 2019, 16, 33–34. [Google Scholar]
- Turner, H.; Chueh, D.; Ortiz, T.; Stokes, A.J.; Small-Howard, A.L. Cannabinoid Therapeutics in Parkinson’s Disease: Promise and Paradox. J. Herbs. Spices Med. Plants 2017, 23, 231–248. [Google Scholar] [CrossRef]
- Brucki, S.M.D.; Frota, N.A.; Schestatsky, P.; Souza, A.H.; Carvalho, V.N.; Manreza, M.L.G.; Mendes, M.F.; Comini-Frota, E.; Vasconcelos, C.; Tumas, V.; et al. Cannabinoids in neurology—Brazilian Academy of Neurology. Arq. Neuropsiquiatr. 2015, 73, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aymerich, M.S.; Aso, E.; Abellanas, M.A.; Tolon, R.M.; Ramos, J.A.; Ferrer, I.; Romero, J.; Fernández-Ruiz, J. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem. Pharmacol. 2018, 157, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.C.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front. Pharmacol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Office on Drugs and Crime. World Drug Report—2009; United Nations Office on Drugs and Crime: Vienna, Austria, 2009. [Google Scholar]
- Edeleano, L. Ueber einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure. Berichte der Dtsch. Chem. Gesellschaft 1887, 20, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N. Studies on the components of Ephedraceaein herb medicine. Yakugaku Zasshi 1893, 139, 901–933. [Google Scholar]
- Chen, K.K.; Kao, C.H. Ephedrine and Pseudoephedrine, their Isolation, Constitution, Isomerism, Properties, Derivatives and Synthesis. (with a Bibliography) **The expense of this work has been defrayed by a part of a grant from the Committee on Therapeutic Research, Council on. J. Am. Pharm. Assoc. 1926, 15, 625–639. [Google Scholar]
- Rasmussen, N. Medical Science and the Military: The Allies’ Use of Amphetamine during World War II. J. Interdiscip. Hist. 2011, 42, 205–233. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report—Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2019.
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Teng, L.; Crooks, P.A.; Dwoskin, L.P. Lobeline Displaces [3H]Dihydrotetrabenazine Binding and Releases [3H]Dopamine from Rat Striatal Synaptic Vesicles: Comparison with d-Amphetamine. J. Neurochem. 2002, 71, 258–265. [Google Scholar] [CrossRef] [Green Version]
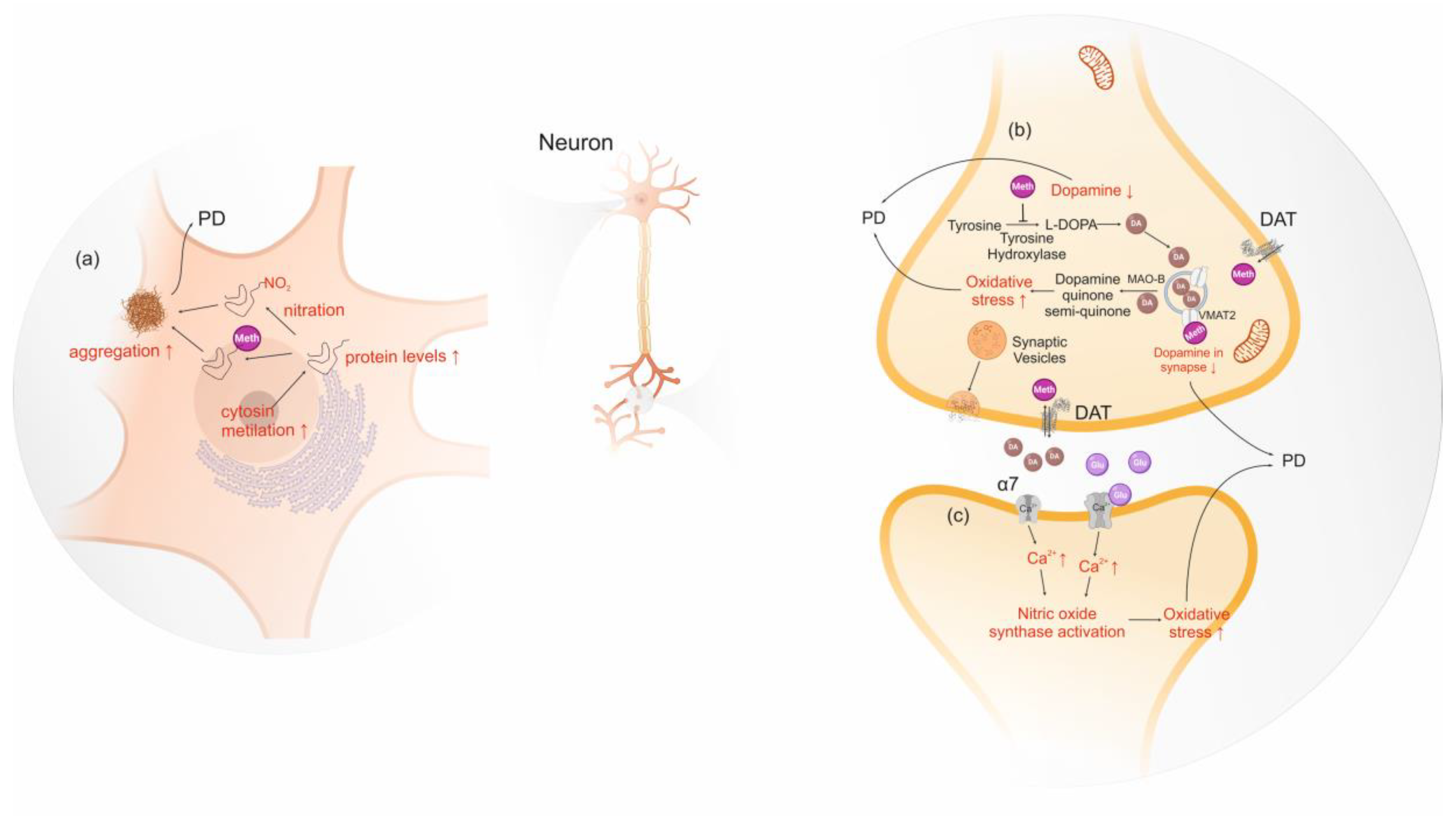
- Calipari, E.S.; Ferris, M.J. Amphetamine Mechanisms and Actions at the Dopamine Terminal Revisited. J. Neurosci. 2013, 33, 8923–8925. [Google Scholar] [CrossRef] [Green Version]
- Perfeito, R.; Cunha-Oliveira, T.; Rego, A.C. Reprint of: Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease—resemblance to the effect of amphetamine drugs of abuse. Free Radic. Biol. Med. 2013, 62, 186–201. [Google Scholar] [CrossRef]
- Melega, W.P.; Williams, A.E.; Schmitz, D.A.; DiStefano, E.W.; Cho, A.K. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J. Pharmacol. Exp. Ther. 1995, 274, 90–96. [Google Scholar]
- Franssen, M.; Winward, C.; Collett, J.; Wade, D.; Dawes, H. Interventions for fatigue in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- De Wit, H. Acute Administration of d-Amphetamine Decreases Impulsivity in Healthy Volunteers. Neuropsychopharmacology 2002, 27, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Johanson, C.E.; Uhlenhuth, E.H. Drug preference and mood in humans: Repeated assessment of d-amphetamine. Pharmacol. Biochem. Behav. 1981, 14, 159–163. [Google Scholar] [CrossRef]
- Chapotot, F.; Pigeau, R.; Canini, F.; Bourdon, L.; Buguet, A. Distinctive effects of modafinil and d-amphetamine on the homeostatic and circadian modulation of the human waking EEG. Psychopharmacology (Berl). 2003, 166, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef]
- Kousik, S.M.; Graves, S.M.; Napier, T.C.; Zhao, C.; Carvey, P.M. Methamphetamine-induced vascular changes lead to striatal hypoxia and dopamine reduction. Neuroreport 2011, 22, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Rusyniak, D.E. Neurologic Manifestations of Chronic Methamphetamine Abuse. Psychiatr. Clin. North Am. 2013, 36, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garwood, E.R.; Bekele, W.; McCulloch, C.E.; Christine, C.W. Amphetamine exposure is elevated in Parkinson’s disease. Neurotoxicology 2006, 27, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Albers, J.; Fricke, C.; Mueller, W.; Classen, J. Structural abnormality of substantia nigra induced by methamphetamine abuse. Mov. Disord. 2017, 32, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.D.; Shin, E.; Nguyen, X.T.; Jin, C.; Bach, J.; Park, S.J.; Nah, S.; Wie, M.; Bing, G.; Kim, H. Potentiation of methamphetamine neurotoxicity by intrastriatal lipopolysaccharide administration. Neurochem. Int. 2010, 56, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Frau, L.; Wardas, J.; Pinna, A.; Plumitallo, A.; Morelli, M. MPTP-induced dopamine neuron degeneration and glia activation is potentiated in MDMA-pretreated mice. Mov. Disord. 2013, 28, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Moszczynska, A.; Fitzmaurice, P.; Ang, L.; Kalasinsky, K.S.; Schmunk, G.A.; Peretti, F.J.; Aiken, S.S.; Wickham, D.J.; Kish, S.J. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004, 127, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kousik, S.M.; Carvey, P.M.; Napier, T.C. Methamphetamine self-administration results in persistent dopaminergic pathology: Implications for Parkinson’s disease risk and reward-seeking. Eur. J. Neurosci. 2014, 40, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Kakish, J.; Lee, D.; Lee, J.S. Drugs That Bind to α-Synuclein: Neuroprotective or Neurotoxic? ACS Chem. Neurosci. 2015, 6, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Tavassoly, O.; Lee, J.S. Methamphetamine binds to α-synuclein and causes a conformational change which can be detected by nanopore analysis. FEBS Lett. 2012, 586, 3222–3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Witt, S.N. The Parkinson’s Disease-Associated Protein α-Synuclein Disrupts Stress Signaling—A Possible Implication for Methamphetamine Use? Microb. Cell (Graz, Austria) 2014, 1, 131–132. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Zhu, L.; Wang, Y.; Hui, J.; Xie, W.; Liu, C.; Chen, L.; Qiu, P. Implications of alpha-synuclein nitration at tyrosine 39 in methamphetamine-induced neurotoxicity in vitro and in vivo. Neural Regen. Res. 2019, 14, 319. [Google Scholar]
- Tenreiro, S.; Reimão-Pinto, M.M.; Antas, P.; Rino, J.; Wawrzycka, D.; Macedo, D.; Rosado-Ramos, R.; Amen, T.; Waiss, M.; Magalhães, F.; et al. Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson’s Disease. PLoS Genet. 2014, 10, e1004302. [Google Scholar] [CrossRef] [Green Version]
- Watson, M.D.; Lee, J.C. N-Terminal Acetylation Affects α-Synuclein Fibril Polymorphism. Biochemistry 2019, 58, 3630–3633. [Google Scholar] [CrossRef]
- Rott, R.; Szargel, R.; Haskin, J.; Shani, V.; Shainskaya, A.; Manov, I.; Liani, E.; Avraham, E.; Engelender, S. Monoubiquitylation of α-Synuclein by Seven in Absentia Homolog (SIAH) Promotes Its Aggregation in Dopaminergic Cells. J. Biol. Chem. 2008, 283, 3316–3328. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.L.; Brannock, C.; Krasnova, I.N.; Ladenheim, B.; McCoy, M.T.; Chou, J.; Lehrmann, E.; Wood, W.H.; Becker, K.G.; Wang, Y. Methamphetamine-Induced Dopamine-Independent Alterations in Striatal Gene Expression in the 6-Hydroxydopamine Hemiparkinsonian Rats. PLoS ONE 2010, 5, e15643. [Google Scholar] [CrossRef] [PubMed]
- Berke, J.D.; Paletzki, R.F.; Aronson, G.J.; Hyman, S.E.; Gerfen, C.R. A Complex Program of Striatal Gene Expression Induced by Dopaminergic Stimulation. J. Neurosci. 1998, 18, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, D.; You, Z.-B.; Castel, M.-N.; Johansson, S.; Goiny, M.; Terenius, L.; Hökfelt, T.; Herrera-Marschitz, M. Effect of single and repeated methamphetamine treatment on neurotransmitter release in substantia nigra and neostriatum of the rat. J. Neurochem. 2002, 83, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, J.; Zhang, Z.; Wang, H.; Wang, Z. Epigenetic upregulation of alpha-synuclein in the rats exposed to methamphetamine. Eur. J. Pharmacol. 2014, 745, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; Itzhak, Y. Effects of 7-Nitroindazole, an NOS Inhibitor on Methamphetamine-Induced Dopaminergic and Serotonergic Neurotoxicity in Micea. Ann. N. Y. Acad. Sci. 1998, 844, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.P.; Yadav, S.; Singhal, N.K.; Tiwari, M.N.; Mishra, S.K.; Singh, M.P. Does Restraining Nitric Oxide Biosynthesis Rescue from Toxins-Induced Parkinsonism and Sporadic Parkinson’s Disease? Mol. Neurobiol. 2014, 49, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, A.-F.; Chen, L.; Huang, E.-P.; Xie, W.; Liu, C.; Huang, W.; Chen, C.; Qiu, P.; Wang, H. S-nitrosylating protein disulphide isomerase mediates α-synuclein aggregation caused by methamphetamine exposure in PC12 cells. Toxicol. Lett. 2014, 230, 19–27. [Google Scholar] [CrossRef]
- Chen, L.; Huang, E.; Wang, H.; Qiu, P.; Liu, C. RNA interference targeting α-synuclein attenuates methamphetamine-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2013, 1521, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Morrow, B.A.; Roth, R.H.; Redmond, D.E.; Elsworth, J.D. Impact of methamphetamine on dopamine neurons in primates is dependent on age: Implications for development of Parkinson’s disease. Neuroscience 2011, 189, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betarbet, R.; Sherer, T.B.; Greenamyre, J.T. Animal models of Parkinson’s disease. BioEssays 2002, 24, 308–318. [Google Scholar] [CrossRef]
- Kuehn, B.M. Meth use linked to risk of Parkinson disease. JAMA 2011, 306, 814. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S. Mechanisms of neurotoxicity and implications for neurological disorders. J. Intern. Med. 2013, 273, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Cunningham, J.K.; Sajeev, G.; Kish, S.J. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov. Disord. 2010, 25, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Curtin, K.; Fleckenstein, A.E.; Robison, R.J.; Crookston, M.J.; Smith, K.R.; Hanson, G.R. Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: A population-based assessment. Drug Alcohol Depend. 2015, 146, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kish, S.J.; Boileau, I.; Callaghan, R.C.; Tong, J. Brain dopamine neurone ‘damage’: Methamphetamine users vs. Parkinson’s disease—A critical assessment of the evidence. Eur. J. Neurosci. 2017, 45, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.; Smith, L.; Fowler, J.S.; Telang, F.; Logan, J.; Tomasi, D. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. Neuroimage 2015, 121, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lappin, J.M.; Darke, S.; Farrell, M. Methamphetamine use and future risk for Parkinson’s disease: Evidence and clinical implications. Drug Alcohol Depend. 2018, 187, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T. The identification of coca (Erythroxylum species): 1860-1910. Bot. J. Linn. Soc. 1982, 84, 329–353. [Google Scholar] [CrossRef]
- EMCDDA. EU Drug Markets Report 2019; EMCDDA: Lisbon, Portugal, 2019; ISBN 9789294974211.
- Ciccarone, D. Stimulant Abuse: Pharmacology, Cocaine, Methamphetamine, Treatment, Attempts at Pharmacotherapy. Prim. Care Clin. Off. Pract. 2011, 38, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.H.; Block, E.; Hu, F.; Cobanoglu, M.C.; Sorkin, A.; Bahar, I. Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding. Front. Neurol. 2015, 6, 8966–8975. [Google Scholar] [CrossRef] [Green Version]
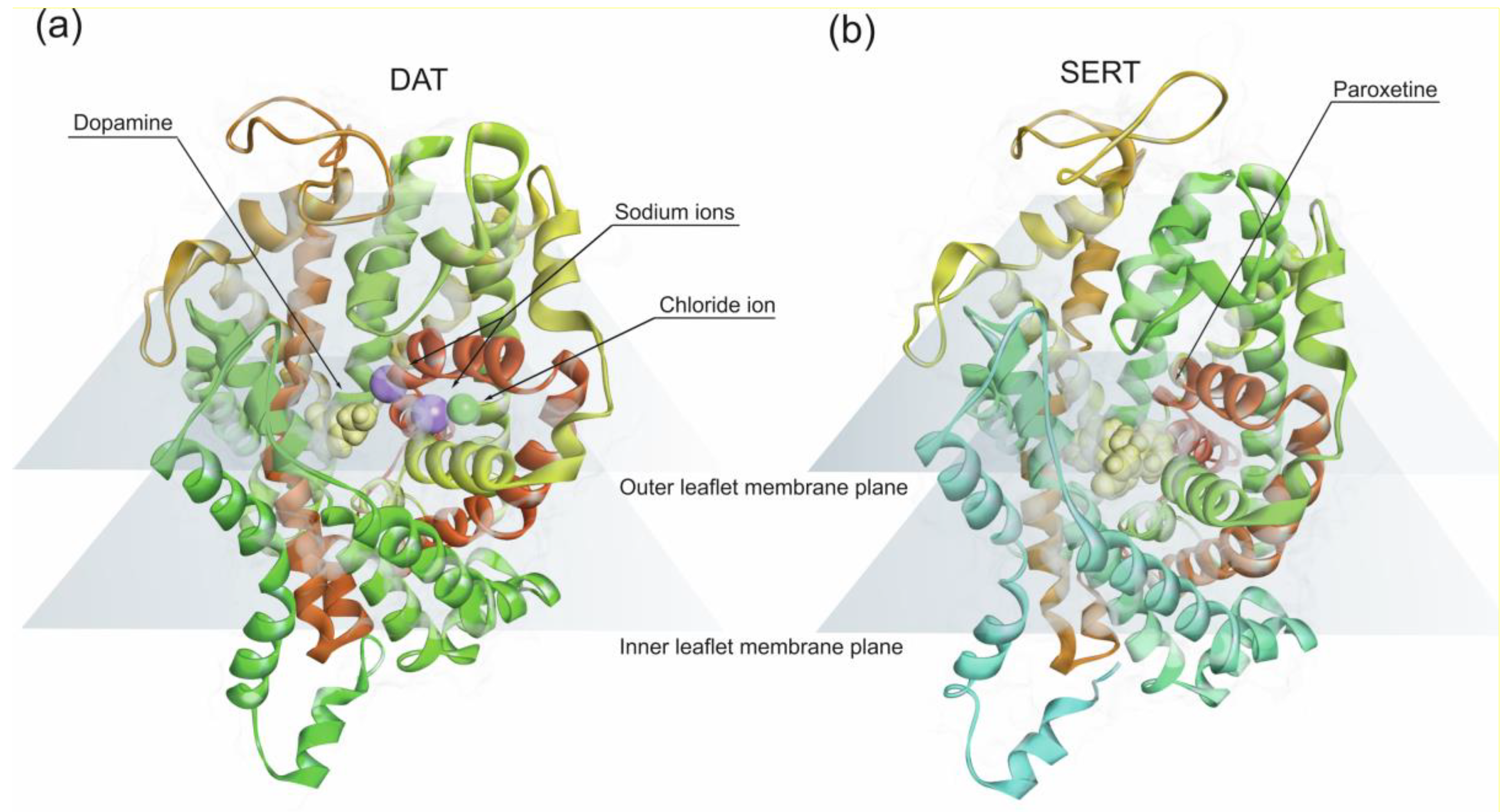
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhar, M.J.; Ritz, M.C.; Boja, J.W. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991, 14, 299–302. [Google Scholar] [CrossRef]
- Little, K.Y.; Ramssen, E.; Welchko, R.; Volberg, V.; Roland, C.J.; Cassin, B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009, 168, 173–180. [Google Scholar] [CrossRef]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Volkow, N.D.; Tomasi, D.; Wang, G.-J.; Logan, J.; Alexoff, D.L.; Jayne, M.; Fowler, J.S.; Wong, C.; Yin, P.; Du, C. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol. Psychiatry 2014, 19, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitcher, T.L.; Melzer, T.R.; MacAskill, M.R.; Graham, C.F.; Livingston, L.; Keenan, R.J.; Watts, R.; Dalrymple-Alford, J.C.; Anderson, T.J. Reduced striatal volumes in Parkinson’s disease: A magnetic resonance imaging study. Transl. Neurodegener. 2012, 1, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, J.-R.; Zang, Y.-F.; Wu, T. Consistent decreased activity in the putamen in Parkinson’s disease: A meta-analysis and an independent validation of resting-state fMRI. Gigascience 2018, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Illés, A.; Balicza, P.; Molnár, V.; Bencsik, R.; Szilvási, I.; Molnar, M.J. Dynamic interaction of genetic risk factors and cocaine abuse in the background of Parkinsonism—A case report. BMC Neurol. 2019, 19, 260. [Google Scholar] [CrossRef] [Green Version]
- Brenz Verca, M.S.; Bahi, A.; Boyer, F.; Wagner, G.C.; Dreyer, J.-L. Distribution of alpha- and gamma-synucleins in the adult rat brain and their modification by high-dose cocaine treatment. Eur. J. Neurosci. 2003, 18, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Mash, D.C.; Ouyang, Q.; Pablo, J.; Basile, M.; Izenwasser, S.; Lieberman, A.; Perrin, R.J. Cocaine Abusers Have an Overexpression of α-Synuclein in Dopamine Neurons. J. Neurosci. 2003, 23, 2564–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Ouyang, Q.; Pablo, J.; Mash, D.C. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Neuroreport 2005, 16, 1489–1493. [Google Scholar] [CrossRef]
- Pregeljc, D.; Teodorescu-Perijoc, D.; Vianello, R.; Umek, N.; Mavri, J. How Important Is the Use of Cocaine and Amphetamines in the Development of Parkinson Disease? A Computational Study. Neurotox. Res. 2020, 37, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.G.; Davis, M.I.; Lovinger, D.M.; Mateo, Y. Dopamine dynamics and cocaine sensitivity differ between striosome and matrix compartments of the striatum. Neuropharmacology 2016, 108, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, L.J.; Martone, M.E.; Linder, J.C.; Groves, P.M. Cocaine, in contrast to D-amphetamine, does not cause axonal terminal degeneration in neostriatum and agranular frontal cortex of long-evans rats. Life Sci. 1988, 43, 1403–1409. [Google Scholar] [CrossRef]
- Madras, B.K.; Fahey, M.A.; Goulet, M.; Lin, Z.; Bendor, J.; Goodrich, C.; Meltzer, P.C.; Elmaleh, D.R.; Livni, E.; Bonab, A.A.; et al. Dopamine Transporter (DAT) Inhibitors Alleviate Specific Parkinsonian Deficits in Monkeys: Association with DAT Occupancy in Vivo. J. Pharmacol. Exp. Ther. 2006, 319, 570–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/31643176/ (accessed on 11 June 2020).
- UNODC. World Drug Report 2019—Depressants; UNODC: Vienna, Austria, 2019. [Google Scholar]
- Kieffer, B.L. Opioid Peptides and Receptors. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 235–240. [Google Scholar]
- Wei, L. Regulation of opioid receptor expression. Curr. Opin. Pharmacol. 2002, 2, 69–75. [Google Scholar] [CrossRef]
- Minami, M.; Satoh, M. Molecular biology of the opioid receptors: Structures, functions and distributions. Neurosci. Res. 1995, 23, 121–145. [Google Scholar] [CrossRef]
- Moles, A. Deficit in Attachment Behavior in Mice Lacking the -Opioid Receptor Gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef]
- Filliol, D.; Ghozland, S.; Chluba, J.; Martin, M.; Matthes, H.W.D.; Simonin, F.; Befort, K.; Gavériaux-Ruff, C.; Dierich, A.; LeMeur, M.; et al. Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000, 25, 195–200. [Google Scholar] [CrossRef]
- Lutz, P.-E.; Kieffer, B.L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013, 36, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Beckett, A.H.; Casy, A.F. Synthetic analgesics: Stereochemical considerations. J. Pharm. Pharmacol. 1954, 6, 986–1001. [Google Scholar] [CrossRef]
- Beckett, A.H.; Casy, A.F.; Harper, N.J. Analgesics and their antagonists: Some steric and chemical considerations: Part III. J. Pharm. Pharmacol. 1956, 8, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.H.; Casy, A.F. Analgesics and their Antagonists: Biochemical Aspects and Structure-Activity Relationships. In Progress in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 1965; Volume 4, pp. 171–218. [Google Scholar]
- Manglik, A. Molecular Basis of Opioid Action: From Structures to New Leads. Biol. Psychiatry 2020, 87, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeo, G.; Schirinzi, T.; Natoli, S.; Pierantozzi, M.; Stefani, A.; Dauri, M.; Pisani, A. Efficacy and safety profile of prolonged release oxycodone in combination with naloxone (OXN PR) in Parkinson’s disease patients with chronic pain. J. Neurol. 2015, 262, 2164–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlongan, C.V.; Su, T.-P.; Wang, Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport 2000, 11, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Billet, F.; Costentin, J.; Dourmap, N. Influence of corticostriatal δ-opioid receptors on abnormal involuntary movements induced by L-DOPA in hemiparkinsonian rats. Exp. Neurol. 2012, 236, 339–350. [Google Scholar] [CrossRef]
- Henry, B.; Fox, S.H.; Crossman, A.R.; Brotchie, J.M. μ- and δ-Opioid Receptor Antagonists Reduce Levodopa-Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Exp. Neurol. 2001, 171, 139–146. [Google Scholar] [CrossRef]
- Stefano, G.B.; Goumon, Y.; Casares, F.; Cadet, P.; Fricchione, G.L.; Rialas, C.; Peter, D.; Sonetti, D.; Guarna, M.; Welters, I.D.; et al. Endogenous morphine. Trends Neurosci. 2000, 23, 436–442. [Google Scholar] [CrossRef]
- Charron, G.; Doudnikoff, E.; Laux, A.; Berthet, A.; Porras, G.; Canron, M.H.; Barroso-Chinea, P.; Li, Q.; Qin, C.; Nosten-Bertrand, M.; et al. Endogenous morphine-like compound immunoreactivity increases in parkinsonism. Brain 2011, 134, 2321–2338. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Falk, T.; Zuniga, L.A.; Szabò, L.; Porreca, F.; Polt, R.; Sherman, S.J. Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson’s disease. Brain Res. 2011, 1413, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Begum M, E.T.; Sen, D. DOR agonist (SNC-80) exhibits anti-parkinsonian effect via downregulating UPR/oxidative stress signals and inflammatory response in vivo. Neurosci. Lett. 2018, 678, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Lang, A.E.; Brotchie, J.M. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: Keys to success and roads to failure. Mov. Disord. 2006, 21, 1578–1594. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P. Opioid antagonists increase the dyskinetic response to dopaminergic agents in parkinsonian monkeys: Interaction between dopamine and opioid systems. Neuropharmacology 2003, 45, 954–963. [Google Scholar] [CrossRef]
- Antonini, A.; Tinazzi, M. Targeting pain in Parkinson’s disease. Lancet Neurol. 2015, 14, 1144–1145. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Chao, D.; Sandhu, H.K.; Liao, X.; Zhao, J.; Wen, G.; Xia, Y. δ-Opioid receptor activation reduces α-synuclein overexpression and oligomer formation induced by MPP+ and/or hypoxia. Exp. Neurol. 2014, 255, 127–136. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Chao, D.; Xia, T.C.; Sheng, S.; Li, Z.R.; Zhao, J.N.; Wen, G.Q.; Ding, G.; Xia, Y. δ-Opioid Receptor Activation Attenuates the Oligomer Formation Induced by Hypoxia and/or α-Synuclein Overexpression/Mutation Through Dual Signaling Pathways. Mol. Neurobiol. 2019, 56, 3463–3475. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhi, F.; Shao, N.; Wang, R.; Yang, Y.; Xia, Y. Cytoprotection against hypoxic and/or MPP+ injury: Effect of δ-opioid receptor activation on caspase 3. Int. J. Mol. Sci. 2016, 17, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R. Tramadol-induced parkinsonism: A case report of a 75-year-old woman. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, A.; Brannan, T.; Prikhojan, A.; Yahr, M.D. Sertraline induced parkinsonim. A case report and an in-vivo study of the effect of sertraline on dopamine metabolism. J. Neural Transm. 1998, 105, 247–251. [Google Scholar] [CrossRef]
- Gonul, A.S.; Aksu, M. SSRI-Induced Parkinsonism May Be an Early Sign of Future Parkinson’s Disease. J. Clin. Psychiatry 1999, 60, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.; North, R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, R.C.; Kumaresan, V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006, 30, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Rizak, J.D.; Yang, S.C.; Li, H.; Huang, B.H.; Ma, Y.Y.; Hu, X.T. Acute morphine treatments alleviate tremor in 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-treated monkeys. PLoS ONE 2014, 9, e88404. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, B. Regulation of -Synuclein Expression in Limbic and Motor Brain Regions of Morphine-Treated Mice. J. Neurosci. 2005, 25, 4996–5003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Chen, Y.; Zhang, S.; Huang, M.; Wang, S.; Li, Y.; Bai, J. Morphine reverses the effects of 1-methyl-4-phenylpyridinium in PC12 cells through activating PI3K/Akt. Int. J. Neurosci. 2019, 129, 30–35. [Google Scholar] [CrossRef]
- Elyasi, L.; Eftekhar-Vaghefi, S.H.; Esmaeili-Mahani, S. Morphine protects SH-SY5Y human neuroblastoma cells against 6-hydroxydopamine-induced cell damage: Involvement of anti-oxidant, calcium blocking, and anti-apoptotic properties. Rejuvenation Res. 2014, 17, 255–263. [Google Scholar] [CrossRef]
- Elyasi, L.; Eftekhar-Vaghefi, S.H.; Asadi-Shekaari, M.; Esmaeili-Mahani, S. Induction of cross-tolerance between protective effect of morphine and nicotine in 6-hydroxydopamine-induce neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Int. J. Neurosci. 2019, 129, 131–140. [Google Scholar] [CrossRef]
- Mantione, K. The effects of morphine on Parkinson’s-related genes PINK1 and PARK2. Med. Sci. Monit. Basic Res. 2014, 20, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Spratt, D.E.; Julio Martinez-Torres, R.; Noh, Y.J.; Mercier, P.; Manczyk, N.; Barber, K.R.; Aguirre, J.D.; Burchell, L.; Purkiss, A.; Walden, H.; et al. A molecular explanation for the recessive nature of parkin-linked Parkinson’s disease. Nat. Commun. 2013, 4, 1983. [Google Scholar] [CrossRef] [Green Version]
- Shimura, H.; Hattori, N.; Kubo, S.; Mizuno, Y.; Asakawa, S.; Minoshima, S.; Shimizu, N.; Iwai, K.; Chiba, T.; Tanaka, K.; et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000, 25, 302–305. [Google Scholar] [CrossRef]
- Healy, D.G.; Abou-Sleiman, P.M.; Gibson, J.M.; Ross, O.A.; Jain, S.; Gandhi, S.; Gosal, D.; Muqit, M.M.K.; Wood, N.W.; Lynch, T. PINK1 (PARK6) associated Parkinson disease in Ireland. Neurology 2004, 63, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, N.; Morbin, M.; Ferrari, S.; Cavallaro, T.; Sparaco, M.; Boso, G.; Gaetti, L. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol. 1997, 94, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kass-Hout, T.; Kass-Hout, O.; Darkhabani, M.Z.; Mokin, M.; Mehta, B.; Radovic, V. “Chasing the Dragon”—Heroin-Associated Spongiform Leukoencephalopathy. J. Med. Toxicol. 2011, 7, 240–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villella, C.; Iorio, R.; Conte, G.; Batocchi, A.P.; Bria, P. Toxic leukoencephalopathy after intravenous heroin injection: A case with clinical and radiological reversibility. J. Neurol. 2010, 257, 1924–1926. [Google Scholar] [CrossRef]
- Cilia, R. Molecular Imaging of the Cannabinoid System in Idiopathic Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 305–345. ISBN 9780128154182. [Google Scholar]
- Weber, W.; Henkes, H.; Möller, P.; Bade, K.; Kühne, D. Toxic spongiform leucoencephalopathy after inhaling heroin vapour. Eur. Radiol. 1998, 8, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Halloran, O.; Ifthikharuddin, S.; Samkoff, L. Leukoencephalopathy from “chasing the dragon”. Neurology 2005, 64, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.-C.; Lo, C.-P.; Kao, H.-W.; Chen, C.-Y. MRI features of spongiform leukoencephalopathy following heroin inhalation. Neurology 2006, 67, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, J.A.; Sebastian, R.; Clearsky, L.; Angus, N.; Shah, L.; Lem, M.; Spacey, S.D. Chasing the dragon—characterizing cases of leukoencephalopathy associated with heroin inhalation in British Columbia. Harm Reduct. J. 2011, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Hedley-Whyte, E.T.; Kriegstein, A.R.; Shungu, D.C.; Millar, W.S.; Armitage, B.A.; Brust, J.C.; Chillrud, S.; Goldman, J.; Lynch, T. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation. Neurology 2000, 54, 2027–2028. [Google Scholar] [CrossRef]
- Matzler, W.; Nagele, T.; Gasser, T.; Kruger, R. Acute parkinsonism with corresponding lesions in the basal ganglia after heroin abuse. Neurology 2007, 68, 414. [Google Scholar] [CrossRef] [Green Version]
- Van Hout, M.C.; Bingham, T. “A Costly Turn On”: Patterns of use and perceived consequences of mephedrone based head shop products amongst Irish injectors. Int. J. Drug Policy 2012, 23, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Riederer, P. Understanding Parkinson’s Disease. Sci. Am. 1997, 276, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-β-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, H.; Iwata, J.; Sakamoto, K.; Furukawa, H.; Takada, M.; Iwamura, H.; Watanabe, T.; Kodama, Y. Specificity of Pyridinium Inhibitors of the Ubiquinone Reduction Sites in Mitochondrial Complex I. J. Biol. Chem. 1998, 273, 17368–17374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunnett, S.B.; Björklund, A. Prospects for new restorative and neuroprotective treatments in Parkinson’s disease. Nature 1999, 399, A32–A39. [Google Scholar] [CrossRef] [PubMed]
- Sablin, S.O.; Krueger, M.J.; Yankovskaya, V.L.; Tkachenko, S.E.; Razdolsky, A.N.; Bachurin, S.O.; Ramsay, R.R.; Singer, T.P. Inhibition of NADH oxidation by 1-methyl-4-phenylpyridinium analogs as the basis for the prediction of the inhibitory potency of novel compounds. J. Biochem. Toxicol. 1996, 11, 33–43. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. The Challenge of New Psychoactive Substances; United Nations Office on Drugs and Crime: Vienna, Austria, 2013. [Google Scholar]
- European Union. Council Decision 2005/387/JHA; European Union: Brussels, Belgium, 2005; Volume 2005, pp. 32–37.
- Ferreira, C.; Vaz, A.R.; Florindo, P.R.; Lopes, Á.; Brites, D.; Quintas, A. Development of a high throughput methodology to screen cathinones’ toxicological impact. Forensic Sci. Int. 2019, 298, 1–9. [Google Scholar] [CrossRef]
- Wiley, J.; Marusich, J.; Huffman, J.W.; Balster, R.L.; Thomas, B. Hijacking of Basic Research: The Case of Synthetic Cannabinoids; RTI Press publication No. OP-0007-1111; Research Triangle Park: Raleigh, NC, USA, 2011. [Google Scholar]
- De Luca, M.A.; Fattore, L. Therapeutic Use of Synthetic Cannabinoids: Still an Open Issue? Clin. Ther. 2018, 40, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Ossato, A.; Canazza, I.; Trapella, C.; Vincenzi, F.; De Luca, M.A.; Rimondo, C.; Varani, K.; Borea, P.A.; Serpelloni, G.; Marti, M. Effect of JWH-250, JWH-073 and their interaction on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 67, 31–50. [Google Scholar] [CrossRef]
- Ferreira, C.; Couceiro, J.; Família, C.; Jardim, C.; Antas, P.; Santos, C.N.; Outeiro, T.F.; Tenreiro, S.; Quintas, A. The synthetic cannabinoid JWH-018 modulates Saccharomyces cerevisiae energetic metabolism. FEMS Yeast Res. 2019, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress; Springer: Tokyo, Japan, 2015; ISBN 9784431556503. [Google Scholar]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Jerry, J.; Collins, G.; Streem, D. Synthetic legal intoxicating drugs: The emerging “incense” and “bath salt” phenomenon. Cleve. Clin. J. Med. 2012, 79, 258–264. [Google Scholar] [CrossRef]
- Omer, T.A.; Doherty, C. Posterior reversible encephalopathy syndrome (PRES) complicating the “legal high” mephedrone. Case Rep. 2011, 2011, bcr0220113904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Sykes, C.E.; Shah, M.M.; Thomas, D.M.; Kuhn, D.M. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J. Neurochem. 2013, 125, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. Neurotoxicology 2019, 75, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Sewalia, K.; Watterson, L.R.; Hryciw, A.; Belloc, A.; Ortiz, J.B.; Olive, M.F. Neurocognitive dysfunction following repeated binge-like self-administration of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 2018, 134, 36–45. [Google Scholar] [CrossRef]
- Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F. Cognitive deficits and neurotoxicity induced by synthetic cathinones: Is there a role for neuroinflammation? Psychopharmacology (Berl). 2019, 236, 1079–1095. [Google Scholar] [CrossRef]
| Class of Substance | Substance | Mechanism of Action Related to PD | Neurotoxic or Neuroprotector | Ref |
|---|---|---|---|---|
| Phytocannabinoids | Δ9-THC | upregulates the expression of gene encoding CB1 | Neuroprotector | [55] |
| induce the transcription of proteins involved in oxidative stress defense and mitochondrial biogenesis, promoting mitochondrial normal function | [151] | |||
| expresses mitochondria transcription factors (TFAM) and restore mitochondrial DNA levels leading to increased cytochrome c oxidase subunit 4 (COX4) | [151] | |||
| effective against glutamate-induced neurotoxicity restoring mitochondrial membrane potential which produces an anti-apoptotic effect. | [154] | |||
| cannabidiol | effective against MPP+ neurotoxin by the activation of NGF/TRKA receptors and the increment in expression of axonal and synaptogenic proteins | Neuroprotector | [155] | |
| β-caryophyllene | decreases oxidative/nitrosative stress, decrease pro-inflammatory cytokines release and to an inhibition of gliosis | Neuroprotector | [156,157] | |
| Δ9-THCV | acute administration changes glutamatergic transmission, and the chronic administration was shown to reduce the loss of tyrosine hydroxylase–positive neurons caused by 6-hydroxydopamine in the substantia nigra | Neuroprotector | [158] | |
| Stimulants | Amphetamine and methamphetamine | bind tightly to N-terminus of intrinsically unstructured α-syn adopting a folded conformation, increasing the likelihood of misfolding | Neurotoxic | [189,190] |
| Amphetamine and methamphetamine | involvement of tyrosine hydroxylase, dopamine transporter and vesicular monoamine transporter 2 in the decrease of dopamine levels | Neurotoxic | [203] | |
| methamphetamine | increments α-syn levels induced by excessive heat | Neurotoxic | [191] | |
| causes post-translational modification of α-syn by nitration increase expression of nT39 α-syn. | [192] | |||
| decreases cytosine methylation in SNCA promoter region, and consequently upregulates α-syn in the in substantia nigra | [199] | |||
| activates nicotinic alpha-7 receptors, which increase intra-synaptosomal calcium, nitric oxide synthase and protein kinase C, leading to the production of unjustified nitric oxide and dopamine oxidation | [200] | |||
| induces higher levels of oxidative stress as a consequence of dopamine autoxidation and increasing excitotoxicity as a result of perturbations in energy metabolism. | [205] | |||
| low doses induce the expression of a different set of genes in lesioned denervated striatum, completely lacking dopamine (i) decreases basal ERK 1/2 and kinase b levels, involved in multiple cellular processes such as apoptosis; (ii) reduces the activity of protein phosphatase 2, a protein phosphatase implicated in ERK1/2 dephosphorylation, inhibiting it; and (iii) upregulates the pro-survival protein BCL-2, which plays an anti-apoptotic role | Neuroprotector | [196] | ||
| Cocaine | binds tightly to N-terminus of intrinsically unstructured α-syn adopting a folded conformation, increasing the likelihood of misfolding | Neurotoxic | [189] | |
| increments α-syn levels | [225,226,227] | |||
| Opioids | Morphine | elevates brain dopamine levels by stimulating µ opioid receptor, which inhibits GABA release and consequently enhances dopamine release | Neuroprotector | [261,262] |
| reverses MPP+ toxicity through activating P13K/Akt pathway | [265] | |||
| stabilizes Ca2+ homeostasis and decreases ROS production and cytochrome c in 6-OHDA-treated cells. | [266,267] | |||
| alters PD-associated genes expression, whereas PARK2 is up-regulated and PINK1 is down-regulated. | [268] | |||
| Synthetic cannabinoid | JWH-018 | enhances glycolytic flux at expenses of a decrease in pentose phosphate pathway | Neurotoxic | [294] |
| JWH-133 | suppresses blood–brain barrier damage, astroglial myeloperoxidase expression, infiltration of peripheral immune cells and production of inducible nitric oxide synthase, proinflammatory cytokines and chemokines by activated microglia | Neuroprotector | [127] | |
| Synthetic cathinone | mephedrone | monoamine reuptake inhibitor, increasing serotonin, norepinephrine and dopamine levels at neuronal synapses | Neurotoxic | [298,299,300] |
| 3,4-DMMC, methcathinone and pentedrone | Increases the levels of reactive oxygen species | Neurotoxic | [302] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.; Almeida, C.; Tenreiro, S.; Quintas, A. Neuroprotection or Neurotoxicity of Illicit Drugs on Parkinson’s Disease. Life 2020, 10, 86. https://doi.org/10.3390/life10060086
Ferreira C, Almeida C, Tenreiro S, Quintas A. Neuroprotection or Neurotoxicity of Illicit Drugs on Parkinson’s Disease. Life. 2020; 10(6):86. https://doi.org/10.3390/life10060086
Chicago/Turabian StyleFerreira, Carla, Catarina Almeida, Sandra Tenreiro, and Alexandre Quintas. 2020. "Neuroprotection or Neurotoxicity of Illicit Drugs on Parkinson’s Disease" Life 10, no. 6: 86. https://doi.org/10.3390/life10060086






