Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of OSH
2.3. PA Adsorption of the OSH
2.4. Zeta-Potential Assessment and CLSM Characterization
2.5. ITC Analysis
2.6. In Vitro Digestion
2.7. SDS-PAGE and SPI Analysis
2.8. Half-Tongue Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
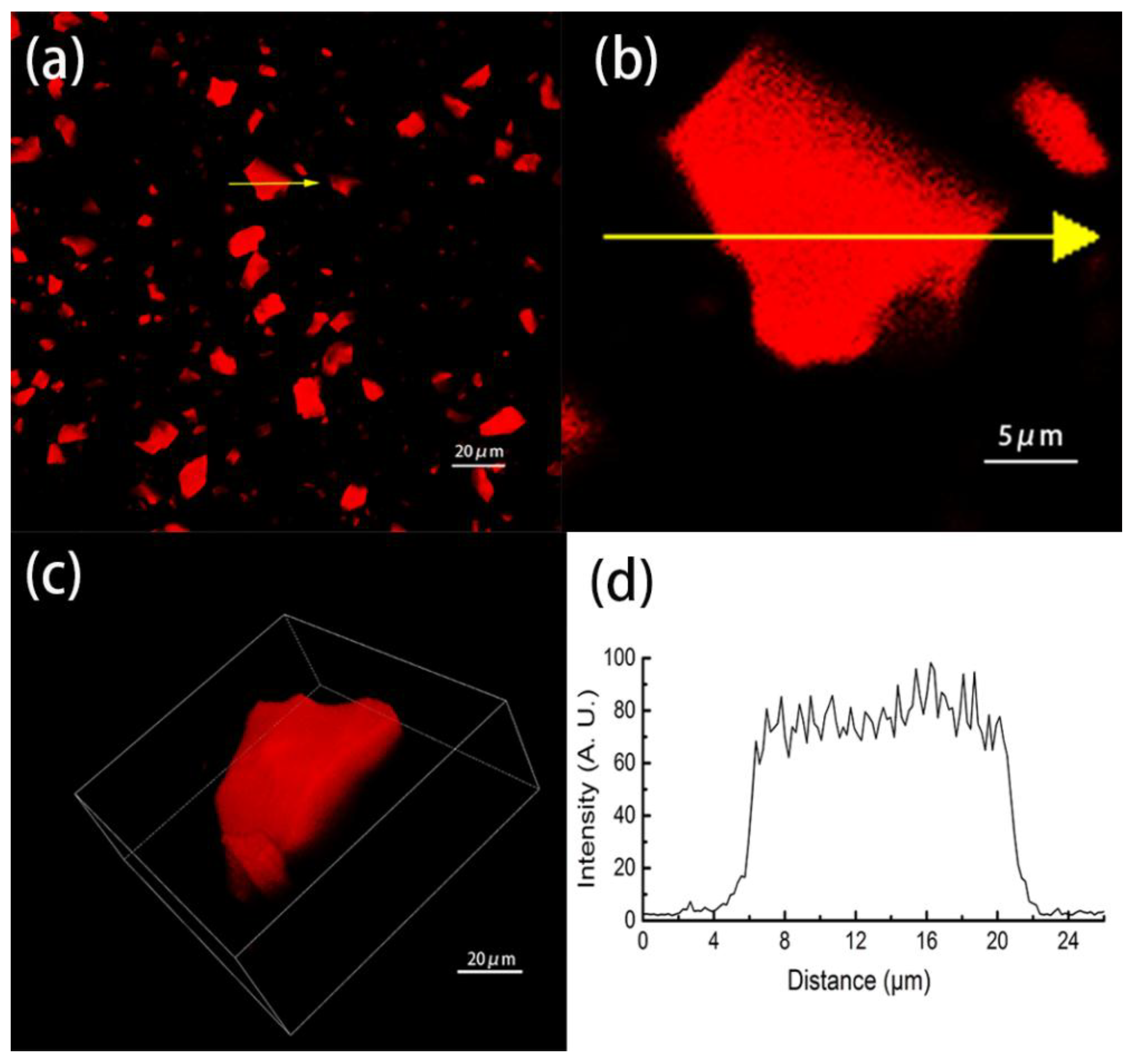
3.1. The Absorption of PA by OSH and Distribution
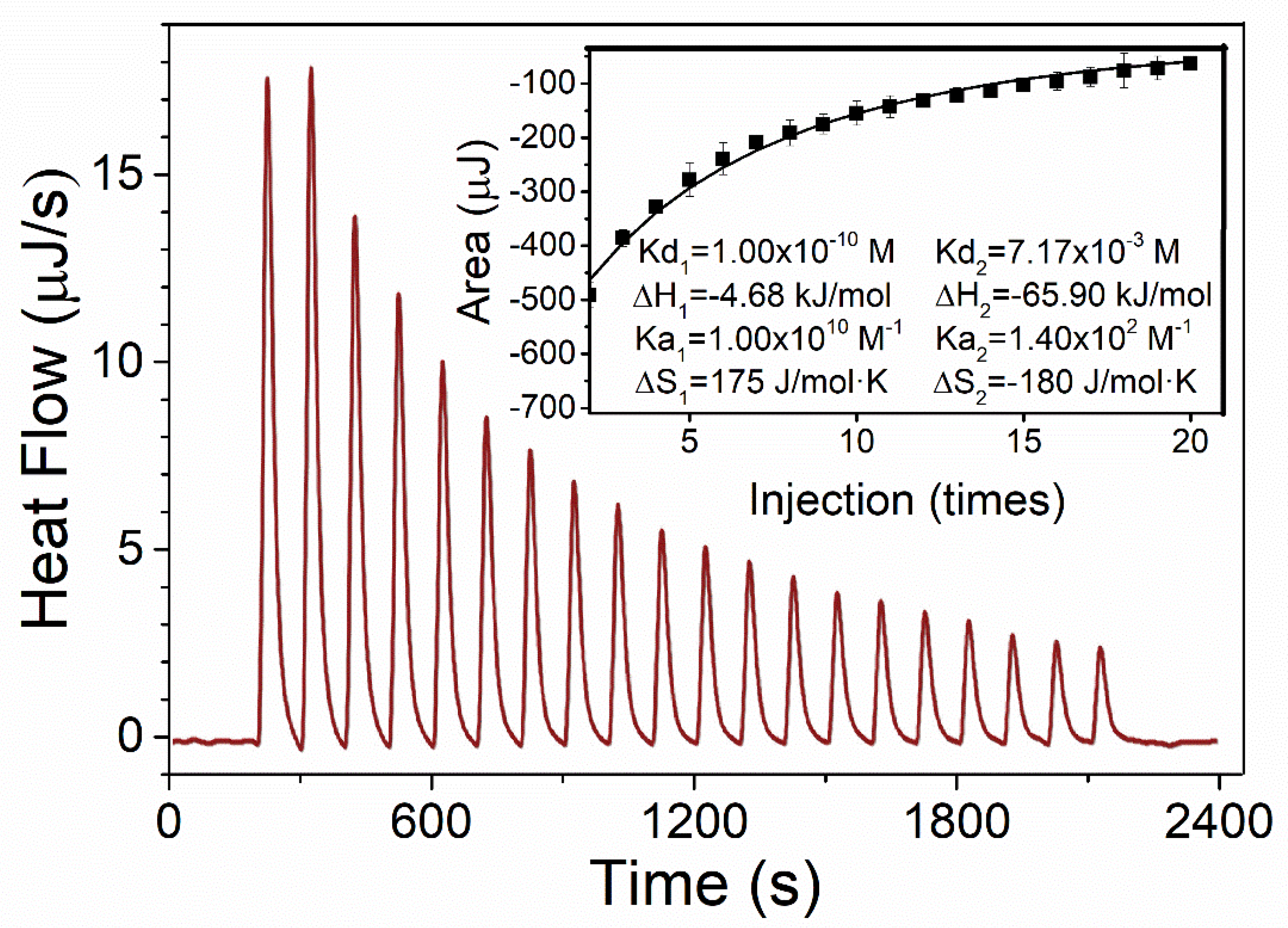
3.2. The Interaction between PA and OSH Studied by ITC
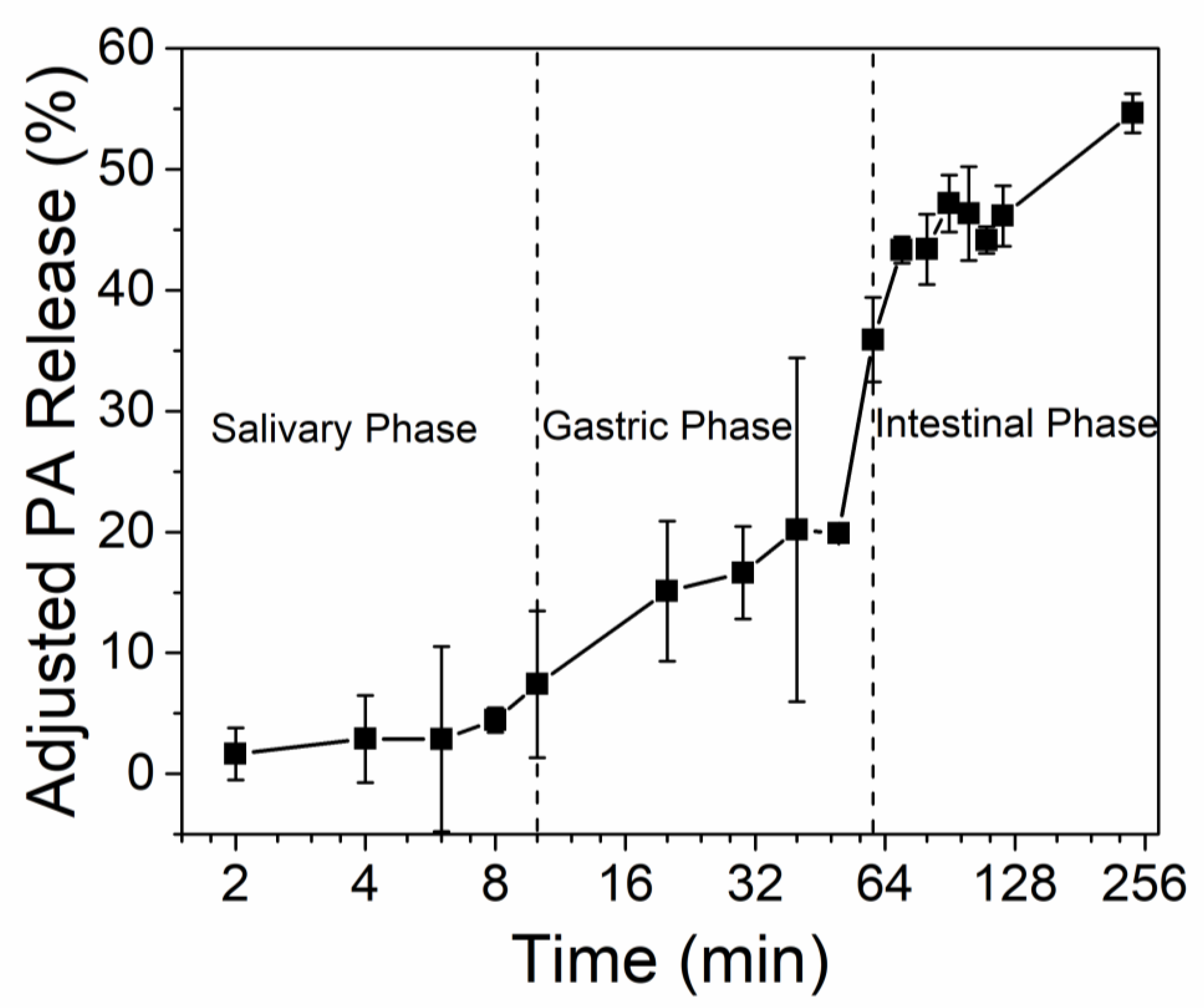
3.3. PA Release under In Vitro Digestion
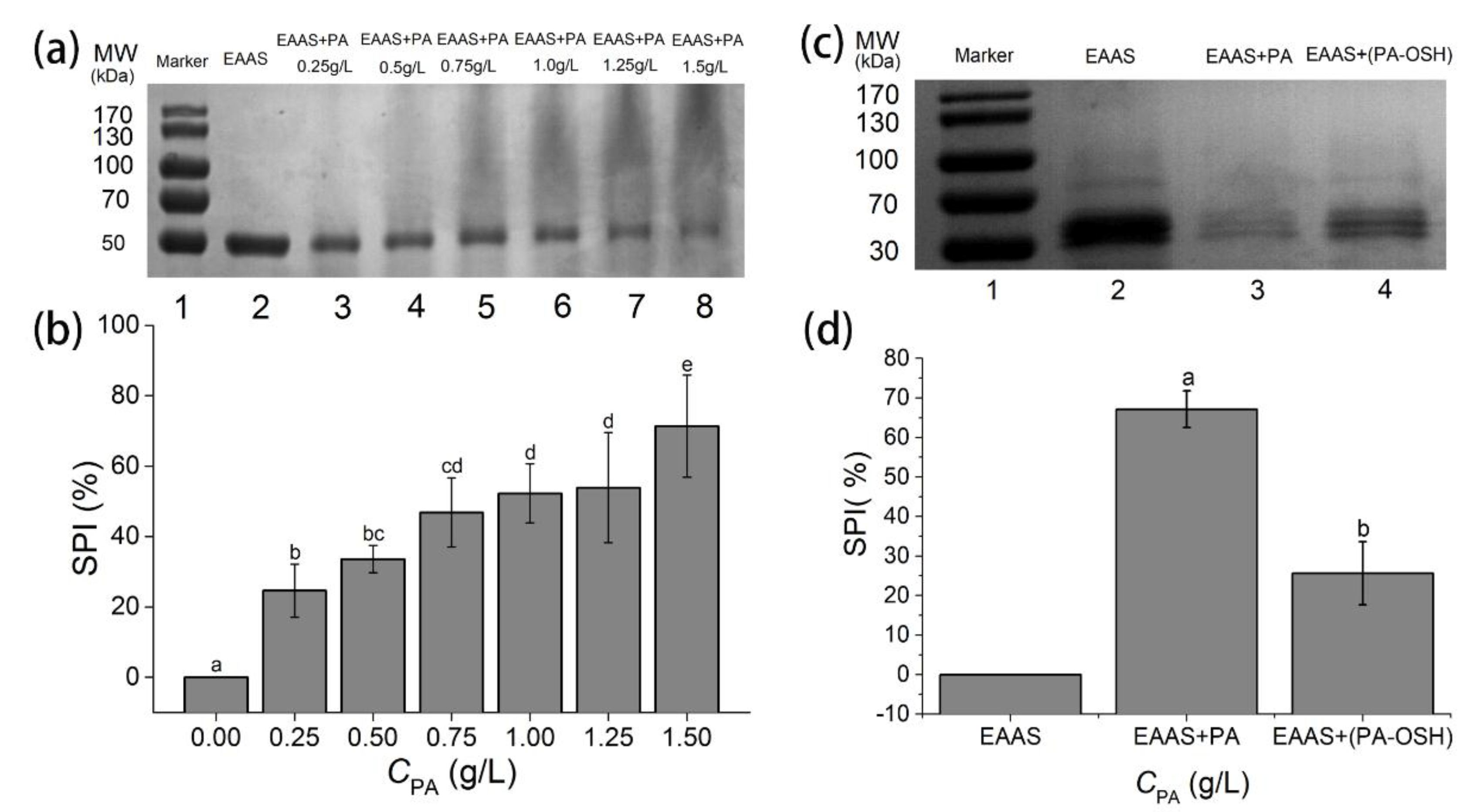
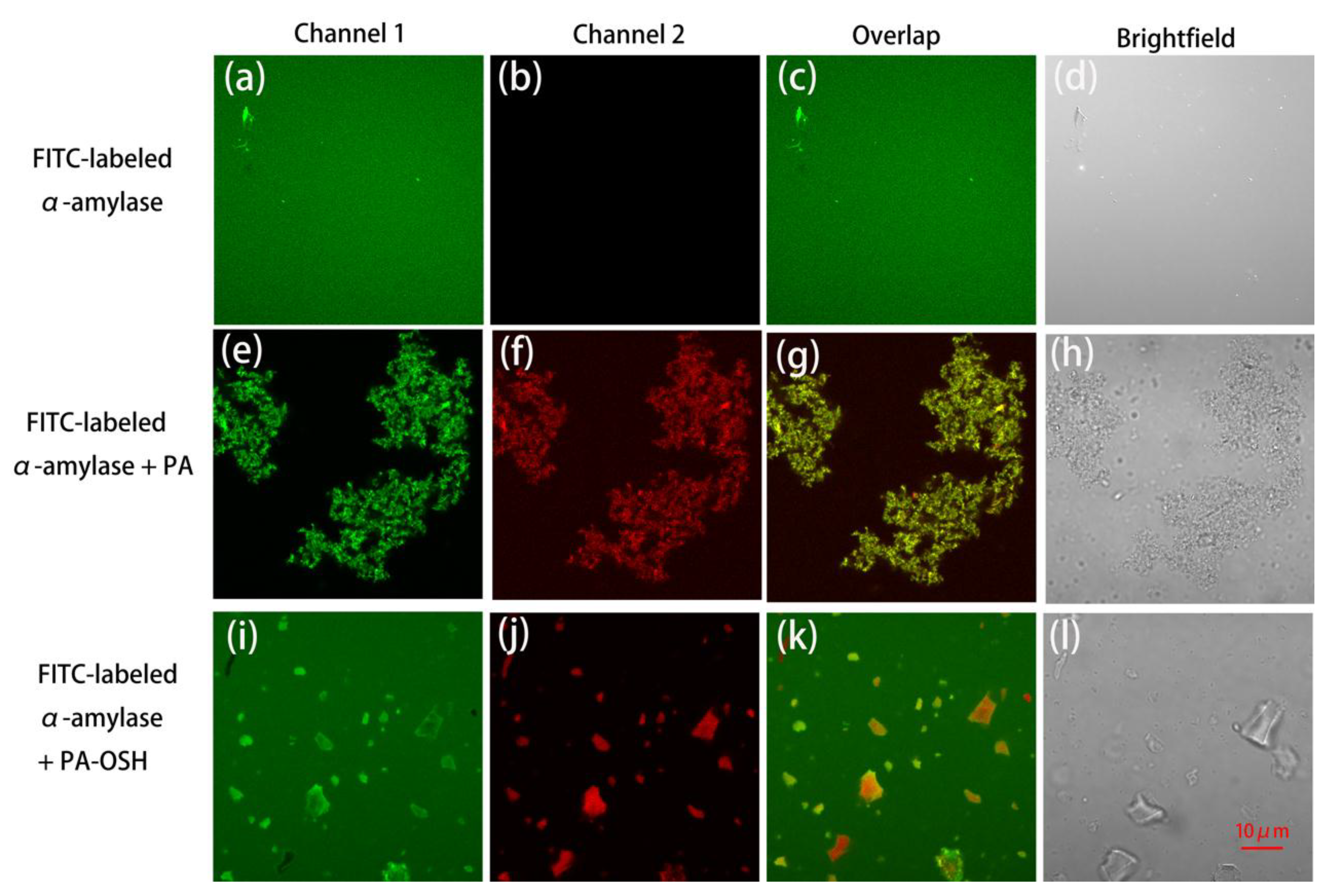
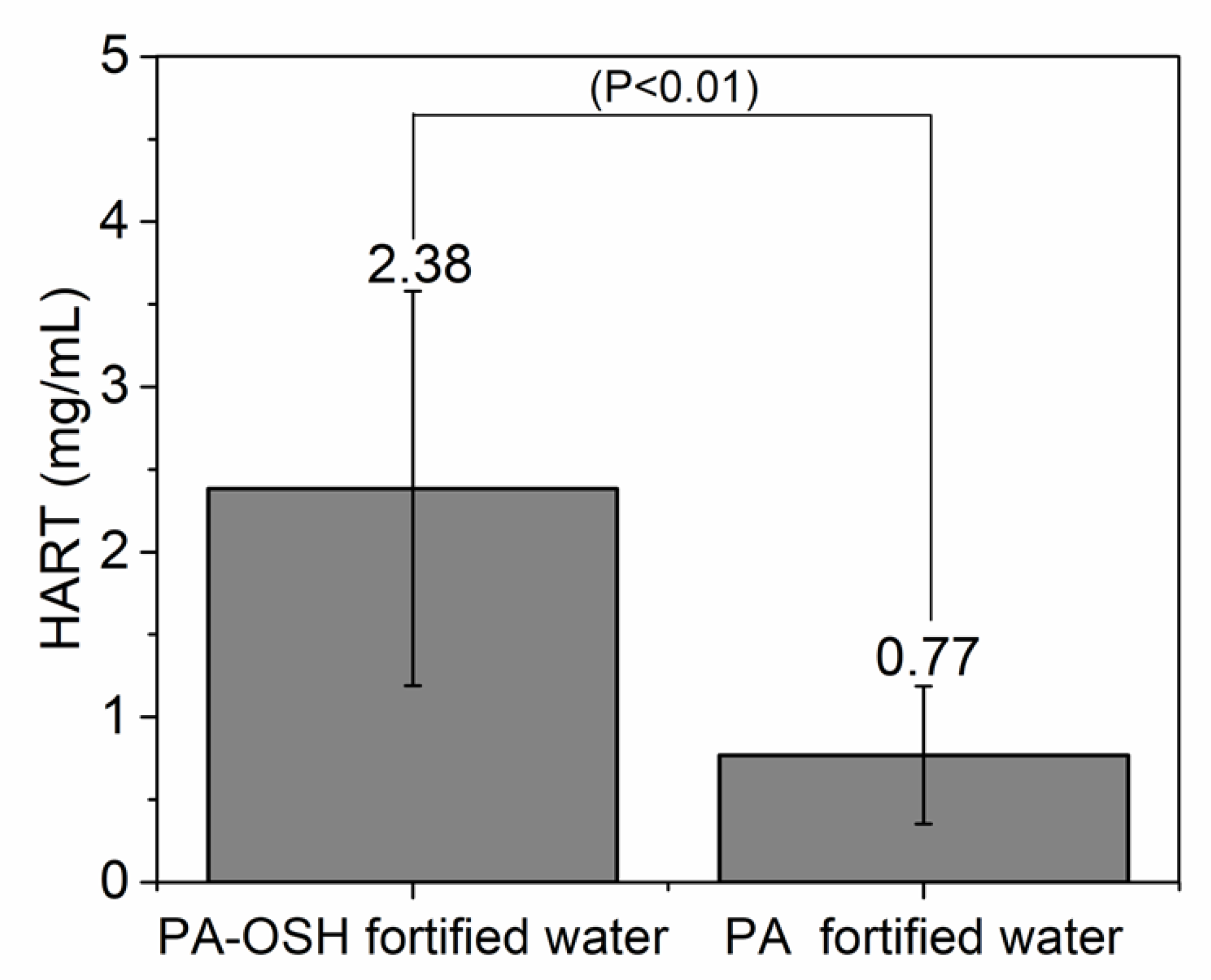
3.4. Interaction between PA/PA-OSH and Salivary Proteins
3.5. Sensory Evaluation for Decreasing the Astringent Threshold by OSH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yearbook, F.S. FAO Statistical Yearbook. World Food Agric. 2013. Available online: http://www.fao.org/3/i3107e/i3107e00.htm (accessed on 29 May 2020).
- Donado-Pestana, C.M.; Dos Santos-Donado, P.R.; Daza, L.D.; Belchior, T.; Festuccia, W.T.; Genovese, M.I. Cagaita fruit (Eugenia dysenterica DC.) and obesity: Role of polyphenols on already established obesity. Food Res. Int. 2018, 103, 40–47. [Google Scholar] [CrossRef]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.-C.; Gao, W.; Wang, J.-S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet–induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Llaudy, M.C.; Canals, R.; Canals, J.-M.; Rozés, N.; Arola, L.; Zamora, F. New method for evaluating astringency in red wine. J. Agric. Food Chem. 2004, 52, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Rawal, S.; Park, J.; Brand, M.H.; Sharafi, M.; Bolling, B.W.J.A. Characterizing and improving the sensory and hedonic responses to polyphenol-rich aronia berry juice. Appetite 2016, 107, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Moio, L. Effect of enological tannin addition on astringency subqualities and phenolic content of red wines. J. Sens. Stud. 2018, 33, e12325. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Pessina, R.; Moio, L. Evaluation of aglianico grape skin and seed polyphenol astringency by SDS–PAGE electrophoresis of salivary proteins after the binding reaction. Food Chem. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- Dinu, V.; Liu, C.; Ali, J.; Ayed, C.; Gershkovich, P.; Adams, G.G.; Harding, S.E.; Fisk, I.D. Analytical ultracentrifugation in saliva research: Impact of green tea astringency and its significance on the in-vivo aroma release. Sci. Rep. 2018, 8, 13350. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.-L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.; Hofmann, T. Identification of the Key Astringent Compounds in Spinach (Spinacia oleracea) by Means of the Taste Dilution Analysis. Chemosens. Percept. 2008, 1, 268–281. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C. Effect of Polyphenol Concentrations on Astringency Perception and Its Correlation with Gelatin Index of Red Wine. J. Sens. Stud. 2010, 25, 761–777. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Ekici, L.; Poyrazoglu, E.S. Phenolic composition of European cranberrybush (Viburnum opulus L.) berries and astringency removal of its commercial juice. Int. J. Food Sci. Technol. 2010, 41, 1011–1015. [Google Scholar] [CrossRef]
- Kang, W.; Niimi, J.; Bastian, S.E.P. Reduction of Red Wine Astringency Perception Using Vegetable Protein Fining Agents. Am. J. Enol. Vitic. 2018, 69, 22–31. [Google Scholar] [CrossRef]
- Laaksonen, O.A.; Salminen, J.-P.; Mäkilä, L.; Kallio, H.P.; Yang, B. Proanthocyanidins and their contribution to sensory attributes of black currant juices. J. Agric. Food Chem. 2015, 63, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, E.; Fathi, M.; Bassiri, A.; Javanmard, M.; Abbaszadeh, R. Novel starch based nanocarrier for vitamin D fortification of milk: Production and characterization. Food Bioprod. Process. 2015, 96, 264–277. [Google Scholar] [CrossRef]
- Tesch, S.; Gerhards, C.; Schubert, H. Stabilization of emulsions by OSA starches. J. Food Eng. 2002, 54, 167–174. [Google Scholar] [CrossRef]
- Ou, S.; Li, A.; Yang, A. A study on synthesis of starch ferulate and its biological properties. Food Chem. 2001, 74, 91–95. [Google Scholar] [CrossRef]
- Rajan, A.; Sudha, J.D.; Abraham, T.E. Enzymatic modification of cassava starch by fungal lipase. Ind. Crop. Prod. 2008, 27, 50–59. [Google Scholar] [CrossRef]
- Bragd, P.L.; Besemer, A.C.; Bekkum, H.V. TEMPO-derivatives as catalysts in the oxidation of primary alcohol groups in carbohydrates. J. Mol. Catal. Chem. 2001, 170, 35–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, T.; Lu, M.; Li, D.; Ren, F.; Zhao, H.; Li, Y. TEMPO-oxidized starch nanoassemblies of negligible toxicity compared with polyacrylic acids for high performance anti-cancer therapy. Int. J. Pharm. 2018, 547, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; de Vries, R.; Slaghek, T.; Timmermans, J.; Cohen Stuart, M.A.; Norde, W. Preparation and characterization of oxidized starch polymer microgels for encapsulation and controlled release of functional ingredients. Biomacromolecules 2009, 10, 1931–1938. [Google Scholar] [CrossRef]
- Li, D.; Liu, A.; Liu, M.; Li, X.; Guo, H.; Zuo, C.; Li, Y.J.F.H. The intestine-responsive lysozyme nanoparticles-in-oxidized starch microgels with mucoadhesive and penetrating properties for improved epithelium absorption of quercetin. Food Hydrocoll. 2020, 99, 105309. [Google Scholar] [CrossRef]
- Li, Y.; Vries, R.d.; Kleijn, M.; Slaghek, T.; Timmermans, J.; Stuart, M.C.; Norde, W. Lysozyme uptake by oxidized starch polymer microgels. Biomacromolecules 2010, 11, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Feng, Z.; Shan, L.; Yang, T.; Qin, M.; Tang, J.; Zhang, W. Preparation, characterization, and antioxidative activity of Bletilla striata polysaccharide/chitosan microspheres for oligomeric proanthocyanidins. Dry. Technol. 2017, 35, 1629–1643. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A study of controlled uptake and release of anthocyanins by oxidized starch microgels. J. Agric. Food Chem. 2013, 61, 5880–5887. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Soldevilla, A.; Sáenz-Navajas, M.-P.; Ayestarán, B. Analysis of polymeric phenolics in red wines using different techniques combined with gel permeation chromatography fractionation. J. Chromatogr. A 2006, 1112, 112–120. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Shobsngob, S.; Oonkhanond, B.; Varavinit, S. Zeta potential (ζ) and pasting properties of phosphorylated or crosslinked rice starches. Starch-Stärke 2005, 57, 32–37. [Google Scholar] [CrossRef]
- Leavitt, S.; Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 2001, 11, 560–566. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. Influence of nanocellulose on in vitro digestion of whey protein isolate. Carbohydr. Polym. 2019, 210, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Smits, M.; Tomassen, M.; De Jong, G.A.; Baumert, J.; Taylor, S.L.; Witkamp, R.; Veldman, R.J.; Pieters, R.; Wichers, H. Release of major peanut allergens from their matrix under various pH and simulated saliva conditions—Ara h2 and ara h6 are readily bio-accessible. Nutrients 2018, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Gambuti, A.; Moio, L. Application of the SPI (Saliva Precipitation Index) to the evaluation of red wine astringency. Food Chem. 2012, 135, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yu, Z.; Hu, H.; Lv, J.; Wang, H.; Jiang, S. Adsorption of procyanidins onto chitosan-modified porous rice starch. LWT 2017, 84, 10–17. [Google Scholar] [CrossRef]
- Shi, X.; Guo, S. Effect of diluent type on analysis of zeta potential of colloid particles of soymilk protein. Trans. Chin. Soc. Agric. Eng. 2016, 32, 270–275. [Google Scholar]
- Kosmulski, M. Zeta potentials in nonaqueous media: How to measure and control them. Colloid Surf. A-Physicochem. Eng. Asp. 1999, 159, 277–281. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Liang, H.; Chen, Y.; Shi, M.; Wu, J.; Liu, X.; Li, Z.; Liu, B.; Yuan, Q. Intestine-specific delivery of hydrophobic bioactives from oxidized starch microspheres with an enhanced stability. J. Agric. Food Chem. 2015, 63, 8669–8675. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, F.; Li, B.; Wei, T.; Guan, H.; Ren, S. Antioxidant And Fluorescence Properties Of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/Zro2 Solid Superacids Catalyst. Molecules 2018, 23, 2445. [Google Scholar] [CrossRef]
- Ragone, R.; Colonna, G.; Ambrosone, L. Reliability of the van′t Hoff Plots. J. Phys. Chem. 1995, 99, 13050. [Google Scholar] [CrossRef]
- Mateus, N.; Carvalho, E.; Luís, C.; de Freitas, V. Influence of the tannin structure on the disruption effect of carbohydrates on protein–tannin aggregates. Anal. Chim. Acta 2004, 513, 135–140. [Google Scholar] [CrossRef]
- Hoebler, C.; Karinthi, A.; Devaux, M.-F.; Guillon, F.; Gallant, D.; Bouchet, B.; Melegari, C.; Barry, J.-L. Physical and chemical transformations of cereal food during oral digestion in human subjects. Br. J. Nutr. 1998, 80, 429–436. [Google Scholar] [CrossRef]
- Wolf, B.W.; Bauer, L.L.; Fahey, G.C. Effects of chemical modification on in vitro rate and extent of food starch digestion: An attempt to discover a slowly digested starch. J. Agric. Food Chem. 1999, 47, 4178–4183. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Hara, K.; Ohara, M.; Hayashi, I.; Hino, T.; Nishimura, R.; Iwasaki, Y.; Ogawa, T.; Ohyama, Y.; Sugiyama, M.; Amano, H. The green tea polyphenol (−)-epigallocatechin gallate precipitates salivary proteins including alpha-amylase: Biochemical implications for oral health. Eur. J. Oral Sci. 2012, 120, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Gambuti, A.; Moine-Ledoux, V.; Moio, L. Evaluation of the astringency of commercial tannins by means of the SDS–PAGE-based method. Food Chem. 2010, 122, 951–956. [Google Scholar] [CrossRef]
- Carvalho, E.; Póvoas, M.J.; Mateus, N.; De Freitas, V. Application of flow nephelometry to the analysis of the influence of carbohydrates on protein–tannin interactions. J. Sci. Food Agric. 2006, 86, 891–896. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of patatin, a protein extracted from potato, as alternative to animal proteins in fining of red wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- de Souza, V.B.; Thomazini, M.; Chaves, I.E.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Microencapsulation by complex coacervation as a tool to protect bioactive compounds and to reduce astringency and strong flavor of vegetable extracts. Food Hydrocoll. 2020, 98, 105244. [Google Scholar] [CrossRef]
- Ostroschi, L.C.; de Souza, V.B.; Echalar-Barrientos, M.A.; Tulini, F.L.; Comunian, T.A.; Thomazini, M.; Baliero, J.C.C.; Roudaut, G.; Genovese, M.I.; Favaro-Trindade, C.S. Production of spray-dried proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract as a potential functional ingredient: Improvement of stability, sensory aspects and technological properties. Food Hydrocoll. 2018, 79, 343–351. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Ai, Y.; Hu, Y.; Wang, Y.; Zhao, L.; Yang, D.; Chen, F.; Wu, X.; Li, Y.; Liao, X. Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation. Foods 2020, 9, 756. https://doi.org/10.3390/foods9060756
Zhao X, Ai Y, Hu Y, Wang Y, Zhao L, Yang D, Chen F, Wu X, Li Y, Liao X. Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation. Foods. 2020; 9(6):756. https://doi.org/10.3390/foods9060756
Chicago/Turabian StyleZhao, Xiaodan, Yingchao Ai, Yulin Hu, Yongtao Wang, Liang Zhao, Dong Yang, Fang Chen, Xiaomeng Wu, Yuan Li, and Xiaojun Liao. 2020. "Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation" Foods 9, no. 6: 756. https://doi.org/10.3390/foods9060756
APA StyleZhao, X., Ai, Y., Hu, Y., Wang, Y., Zhao, L., Yang, D., Chen, F., Wu, X., Li, Y., & Liao, X. (2020). Masking the Perceived Astringency of Proanthocyanidins in Beverages Using Oxidized Starch Hydrogel Microencapsulation. Foods, 9(6), 756. https://doi.org/10.3390/foods9060756










