Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities
Abstract
:1. Introduction
2. Bioactive Flavonoids and Stilbenoids from the Genera Dracaena and Sansevieria
2.1. Dracaena Species
2.1.1. Dracaena angustifolia Medik, (Roxb.)
2.1.2. Dracaena cambodiana Pierre ex Gagnep
2.1.3. Dracaena cinnabari Balf. f.
2.1.4. Dracaena cochinchinensis (Lour.) S.C. Chen
2.1.5. Dracaena draco L.
2.1.6. Dracaena loureiri Gagnep
2.1.7. Dracaena usambarensis Engl. (synonym for Dracaena mannii Baker)
2.1.8. Other Dracaena spp.
2.1.9. Colorants of Dragon’s Blood Resins
2.2. Sansevieria Species
2.2.1. Sansevieria cylindrica Bojer ex Hook. (Dracaena angolensis (Welw. ex Carrière) Byng & Christenh)
2.2.2. Sansevieria roxburghiana Schult. & Schult. f.
2.2.3. Sansevieria trifasciata Prain (Dracaena trifasciata (Prain) Mabb)
3. Chemical Structures and Chemotaxonomic Considerations
4. Biological and Pharmacological Properties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.-L.; Morden, C.W. Phylogenetic relationships among dracaenoid genera (Asparagaceae: Nolinoideae) inferred from chloroplast DNAloci. Syst. Bot. 2014, 39, 90–104. [Google Scholar] [CrossRef]
- Takawira-Nyenya, R.; Mucina, L.; Cardinal-Mcteague, W.M.; Thiele, K.R. Sansevieria (Asparagaceae, nolinoideae) is a herbaceous clade within Dracaena: Inference from non-coding plastid and nuclear DNA sequence data. Phytotaxa 2018, 376, 254–276. [Google Scholar] [CrossRef]
- Maděra, P.; Forrest, A.; Hanáček, P.; Vahalík, P.; Gebauer, R.; Plichta, R.; Jupa, R.; Van Rensburg, J.J.; Morris, M.; Nadezhdina, N.; et al. What we know and what we do not know about dragon trees? Forests 2020, 11, 236. [Google Scholar] [CrossRef] [Green Version]
- WFO. World Flora Online. Published on the Internet. 2020. Available online: http://www.worldfloraonline.org (accessed on 26 April 2020).
- Zhang, Z.; Zhang, Y.; Song, M.; Guan, Y.; Ma, X. Species identification of Dracaena using the complete chloroplast genome as a super-barcode. Front. Pharmacol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Wu, C.; Cai, X.-Q.; Chang, Y.; Chen, C.-H.; Ho, T.-J.; Lai, S.-C.; Chen, H.-P. Rapid identification of dragon blood samples from Daemonorops draco, Dracaena cinnabari and Dracaena cochinchinensis by MALDI-TOF mass spectrometry. Phytochem. Anal. 2019, 30, 720–726. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.-N.; Fan, B.; Chen, X.-N.; Pang, D.-R.; Zheng, J.; Zhang, Q.; Zhao, Y.-F.; Xiao, W.; Tu, P.-F.; et al. Phenolic constituents, pharmacological activities, quality control, and metabolism od Dracaena species: A review. J. Ethnopharmacol. 2019, 244, 439–444. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Cai, X.T.; Xu, Z.F. A study on the resource of Chinese dragon’s blood. Yunnan Zhi Wu Yan Jiu 1979, 1, 1–10. [Google Scholar]
- Fan, J.-Y.; Yi, T.; Sze-To, C.-M.; Zhu, L.; Peng, W.-L.; Zhang, Y.-Z.; Zhao, Z.-Z.; Chen, H.-B. A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon’s blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machala, M.; Kubínová, R.; Hořavová, P.; Suchý, V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother. Res. 2001, 15, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Santos, R.P.; Mendes, L.S.; de Pinho, P.G.; Valentão, P.; Andrade, P.B.; Pereira, J.A.; Carvalho, M. Dracaena draco L. fruit: Phytochemical and antioxidant activity assessment. Food. Res. Int. 2011, 44, 2182–2189. [Google Scholar] [CrossRef]
- Mbugua, P.K.; Moore, D.M. Taxonomic studies of the genus Sansevieria (Dracaenaceae). In The Biodiversity of African Plants; Van der Maesen, L.J.G., Van der Burgt, M., Van Medenbach de Rooy, J.M., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 489–492. [Google Scholar]
- Takawira, R.; Nordal, I. The genus of Sansevieria (family Dracaenaceae) in Zimbabwe. Acta Hortic. 2002, 572, 189–198. [Google Scholar] [CrossRef]
- Menale, B.; De Luca, P.; Del Guacchio, E. A plea to restore Petagna’s authorship for the genus Sansevieria, nom. cons. (Liliaceae). Taxon 2013, 62, 387–390. [Google Scholar] [CrossRef]
- Andhare, R.N.; Raut, M.K.; Naik, S.R. Evaluation of antiallergic and anti-anaphylactic activity of ethanolic extract of Sanseveiria trifasciata leaves (EEST) in rodents. J. Ethnopharmacol. 2012, 142, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Bero, J.; Ganfon, H.; Jonville, M.-C.; Frédérich, M.; Gbaguidi, F.; DeMol, P.; Moudachirou, M.; Quetin-Leclercq, J. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J. Ethnopharmacol. 2009, 122, 439–444. [Google Scholar] [CrossRef]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.-J.R. Medicinal plants used to treat snakebite in Central America: Review and assessment of scientific evidence. J. Ethnopharmacol. 2017, 199, 240–256. [Google Scholar] [CrossRef]
- Lekawatana, S.; Suwannamek, B. Ornamental plants in Thailand. Acta Hortic. 2017, 1167, 11–16. [Google Scholar] [CrossRef]
- Saxena, P.; Ghosh, C. Ornamental plants as sinks and bioindicators. Environ. Technol. 2013, 34, 3059–3067. [Google Scholar] [CrossRef]
- Zhao, T.; Nong, X.-H.; Zhang, B.; Tang, M.-M.; Huang, D.-Y.; Wang, J.-L.; Xiao, J.-L.; Chen, G.-Y. New flavones from the stems of Dracaena angustifolia. Phytochem. Lett. 2020, 36, 115–119. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Zhao, Y.-X.; Zeng, Y.-B.; Shen, H.-Y.; Dai, H.-F.; Mei, W.-L. Cytotoxic and antibacterial flavonoids from dragon’s blood of Dracaena cambodiana. Planta Med. 2011, 77, 2053–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-Q.; Zuo, W.-J.; Wang, H.; Shen, H.-Y.; Luo, Y.; Dai, H.-F.; Mei, W.-L. Two new antimicrobial flavanes from dragon’s blood of Dracaena cambodiana. J. Asian Nat. Prod. Res. 2012, 14, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.-L.; Luo, Y.; Wang, H.; Shen, H.-Y.; Zeng, Y.-B.; Dai, H.-F. Two new flavonoids from dragon’s blood of Dracaena cambodiana. Bull. Korean Chem. Soc. 2013, 34, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Dai, H.-F.; Wang, H.; Mei, W.-L. Chemical constituents from dragon’s blood of Dracaena cambodiana. Chin. J. Nat. Med. 2011, 9, 112–114. [Google Scholar] [CrossRef]
- Dai, H.-F.; Wang, H.; Liu, J.; Wu, J.; Mei, W.-L. Two new biflavonoids from the stem of Dracaena cambodiana. Chem. Nat. Compd. 2012, 48, 376–378. [Google Scholar] [CrossRef]
- Liu, J.; Dai, H.-F.; Wu, J.; Zeng, Y.-B.; Mei, W.-L. Flavanes from Dracaena cambodiana. Z. Naturforsch. B. 2008, 63, 1407–1410. [Google Scholar] [CrossRef]
- Liu, J.; Mei, W.-L.; Wu, J.; Zhao, Y.-X.; Peng, M.; Dai, H.-F. A new cytotoxic homoisoflavonoid from Dracaena cambodiana. J. Asian Nat. Prod. Res. 2009, 11, 192–195. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.-M.; Li, F.-X.; Chen, H.-Q.; Liu, W.-C.; Ren, S.-Z.; Mei, W.-L.; Dai, H.-F. Flavonoids from artificially induced dragon’s blood of Dracaena cambodiana. Fitoterapia 2017, 121, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wu, J.; Mei, W.-L.; Dai, H.-F. Flavonoids from Dracaena cambodiana. Chem. Nat. Compd. 2011, 47, 624–626. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Xu, X.; Mei, W.; Dai, H. Antioxidant phenolic compounds of Dracaena cambodiana. Molecules 2010, 15, 8904–8914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.-X.; Wang, H.; Gai, C.-J.; Chen, H.-Q.; Li, W.; Mei, W.-L.; Dai, H.-F. Three new flavanoids from artificially induced dragon’s blood of Dracaena cambodiana. J. Asian Nat. Prod. Res. 2017, 20, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Masaoud, M.; Ripperger, H.; Porzel, A.; Adam, G. Flavonoids of dragon’s blood from Dracaena cinnabari. Phytochemistry 1995, 38, 745–749. [Google Scholar] [CrossRef]
- Gupta, D.; Verma, N.; Das, H.R.; Gupta, R.K. Evaluation of anti-inflammatory activity of Dracaena cinnabari Balf. f. resin. Indian J. Nat. Prod. Resour. 2014, 5, 215–222. [Google Scholar]
- Helal, I.E.; Elsbaey, M.; Zaghloul, A.M.; Mansour, E.-S.S. A unique C-linked chalcone-dihydrochalcone dimer from Dracaena cinnabari resin. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Himmelreich, U.; Masaoud, M.; Adam, G.; Ripperger, H. Damalachawin, a triflavonoid of a new structural type from dragon’s blood of Dracaena cinnabari. Phytochemistry 1995, 39, 949–951. [Google Scholar] [CrossRef]
- Masaoud, M.; Ripperger, H.; Himmelreich, U.; Adam, G. Cinnabarone, a biflavonoid from dragon’s blood of Dracaena cinnabari. Phytochemistry 1995, 38, 751–753. [Google Scholar] [CrossRef]
- Masaoud, M.; Himmelreich, U.; Ripperger, H.; Adam, G.J.P.M. New biflavonoids from dragon’s blood of Dracaena cinnabari. Planta Med. 1995, 61, 341–344. [Google Scholar] [CrossRef]
- Veselá, D.; Marek, R.; Ubik, K.; Lunerová, K.; Sklenář, V.R.; Suchý, V. Dracophane, a metacyclophane derivative from the resin of Dracaena cinnabari Balf. Phytochemistry 2002, 61, 967–970. [Google Scholar] [CrossRef]
- Tang, Y.; Su, G.; Li, N.; Li, W.; Chen, G.; Chen, R.; Zhou, D.; Hou, Y. Preventive agents for neurodegenerative diseases from resin of Dracaena cochinchinensis attenuate LPS-induced microglia over-activation. J. Nat. Med. 2019, 73, 318–330. [Google Scholar] [CrossRef]
- Li, N.; Ma, Z.; Li, M.; Xing, Y.; Hou, Y. Natural potential therapeutic agents of neurodegenerative diseases from the traditional herbal medicine Chinese dragon’s blood. J. Ethnopharmacol. 2014, 152, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Saito, Y.; Matsuo, Y.; Li, H.-Z.; Tanaka, T. Chalcane–stilbene conjugates and oligomeric flavonoids from Chinese dragon’s blood produced from Dracaena cochinchinensis. Phytochemistry 2015, 119, 76–82. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, Z.-H.; Liu, X.-H.; Fang, D.-C.; Li, H.-M. Cochinchin from Dracaena cochinchinensis. Chin. J. Chem. 2004, 22, 867–869. [Google Scholar] [CrossRef]
- Zheng, Q.-A.; Li, H.-Z.; Zhang, Y.-J.; Yang, C.-R. Flavonoids from the resin of Dracaena cochinchinensis. Helv. Chim. Acta 2004, 87, 1167–1171. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, P.; Yu, H.; Li, J.; Wang, M.-W.; Zhao, W. Anti-helicobacter pylori and thrombin inhibitory components from Chinese dragon’s blood, Dracaena cochinchinensis. J. Nat. Prod. 2007, 70, 1570–1577. [Google Scholar] [CrossRef]
- Su, X.-Q.; Song, Y.-L.; Zhang, J.; Huo, H.-X.; Huang, Z.; Zheng, J.; Zhang, Q.; Zhao, Y.-F.; Xiao, W.; Li, J.; et al. Dihydrochalcones and homoisoflavanes from the red resin of Dracaena cochinchinensis (Chinese dragon’s blood). Fitoterapia 2014, 99, 64–71. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, K.; Cheng, W.; Zhou, T.; Jiang, M.; Xu, J. Isolation and characterization of homoisoflavonoids from Dracaena cochinchinensis and their osteogenic activities in mouse mesenchymal stem cells. J. Pharm. Biomed. Anal. 2016, 129, 466–472. [Google Scholar] [CrossRef]
- Yong, K.-L.; Lv, J.-C.; Zhang, T.-B.; Xu, L.-R.; Chen, X. A new dihydrochalcone from dragon’s blood, red resin of Dracaena cochinchinensis. Nat. Prod. Res. 2008, 22, 1624–1626. [Google Scholar] [CrossRef]
- Lu, W.J.; Wang, X.F.; Chen, J.F.; Lv, Y.; Wu, N.; Kang, W.J.; Zheng, Q.T. Studies on the chemical constituents of chloroform extract of Dracaena cochinchinensis. Acta Pharm. Sin. 1998, 33, 755–758. [Google Scholar] [CrossRef]
- Pang, D.-R.; Pan, B.; Sun, J.; Sun, H.; Yao, H.-N.; Song, Y.-L.; Zhao, Y.-F.; Tu, P.-F.; Huang, W.-Z.; Zheng, J.; et al. Homoisoflavonoid derivatives from the red resin of Dracaena cochinchinensis. Fitoterapia 2018, 131, 105–111. [Google Scholar] [CrossRef]
- Lang, G.-Z.; Li, C.-J.; Gaohu, T.-Y.; Li, C.; Ma, J.; Yang, J.-Z.; Zhou, T.-T.; Yuan, Y.-H.; Ye, F.; Wei, J.-H.; et al. Bioactive flavonoid dimers from Chinese dragon’s blood, the red resin of Dracaena cochinchinensis. Bioorg. Chem. 2020, 97, 103659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xu, M.; Yang, C.; Wang, D.; Li, H.; Zhu, H.; Zhang, Y. A new red pigment from Chinese dragon’s blood, the red resin of Dracaena cochinchinensis. Bull. Korean Chem. Soc. 2012, 33, 4204–4206. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.-A.; Xu, M.; Yang, C.-R.; Wang, D.; Li, H.-Z.; Zhu, H.-T.; Zhang, Y.-J. Flavonoid oligomers from Chinese dragon’s blood, the red resin of Dracaena cochinchinensis. Nat. Prod. Bioprospect. 2012, 2, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Pang, D.-R.; Su, X.-Q.; Zhu, Z.-X.; Sun, J.; Li, Y.-T.; Song, Y.-L.; Zhao, Y.-F.; Tu, P.-F.; Zheng, J.; Li, J. Flavonoid dimers from the total phenolic extract of Chinese dragon’s blood, the red resin of Dracaena cochinchinensis. Fitoterapia 2016, 115, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Guo, S.-X. Three new biflavonoids from chinese dragon’s blood, Dracaena cochinchinensis. Nat. Prod. Commun. 2012, 7, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Liu, T.; Deng, Y.; Wang, W.; Zhang, Y.; Hong, W.; Zhang, D.; Hua, J.; Luo, S. Production and evaluation of antifungal stilbenoids in Dracaena cochinchinensis elicited by fungal inoculation. Ind. Crops Prod. 2020, 145, 112148. [Google Scholar] [CrossRef]
- Hu, L.; Wang, F.-F.; Wang, X.-H.; Yang, Q.-S.; Xiong, Y.; Liu, W.-X. Phytoconstituents from the leaves of Dracaena cochinchinensis (Lour.) S. C. Chen. Biochem. Syst. Ecol. 2015, 63, 1–5. [Google Scholar] [CrossRef]
- Zheng, Q.A.; Zhang, Y.J.; Yang, C.R. A new meta-homoisoflavane from the fresh stems of Dracaena cochinchinensis. J. Asian Nat. Prod. Res. 2006, 8, 571–577. [Google Scholar] [CrossRef]
- Camarda, L.; Merlini, L.; Nasini, G. Dragon’s blood from Dracaena draco, structure of novel homoisoflavanoids. Heterocycles 1983, 20, 39–43. [Google Scholar] [CrossRef]
- González, A.G.; León, F.; Sánchez-Pinto, L.; Padrón, J.I.; Bermejo, J. Phenolic compounds of dragon’s blood from Dracaena draco. J. Nat. Prod. 2000, 63, 1297–1299. [Google Scholar] [CrossRef]
- González, A.G.; León, F.; Hernández, J.C.; Padrón, J.I.; Sánchez-Pinto, L.; Barrera, J.B. Flavans of dragon’s blood from Dracaena draco and Dracaena tamaranae. Biochem. Syst. Ecol. 2004, 32, 179–184. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Schillaci, D. Phytochemical and anti-staphylococcal biofilm assessment of Dracaena draco L. spp. Draco Resin. Pharmacogn. Mag. 2014, 10, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.C.; León, F.; Estévez, F.; Quintana, J.; Bermejo, J. A homo-isoflavonoid and a cytotoxic saponin from Dracaena draco. Chem. Biodivers. 2006, 3, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.C.; León, F.; Quintana, J.; Estévez, F.; Bermejo, J. Icogenin, a new cytotoxic steroidal saponin isolated from Dracaena draco. Bioorg. Med. Chem. 2004, 12, 4423–4429. [Google Scholar] [CrossRef]
- González, A.G.; Hernández, J.C.; León, F.; Padrón, J.I.; Estévez, F.; Quintana, J.; Bermejo, J. Steroidal saponins from the bark of Dracaena draco and their cytotoxic activities. J. Nat. Prod. 2003, 66, 793–798. [Google Scholar] [CrossRef]
- Roskov, Y.; Ower, G.; Orrell, T.; Nicolson, D.; Bailly, N.; Kirk, P.M.; Bourgoin, T.; DeWalt, R.E.; Decock, W.; Nieukerken, E.; et al. Species 2000 & ITIS Catalogue of Life; Naturalis: Leiden, The Netherlands, 2020; ISSN 2405-8858. Available online: www.catalogueoflife.org/col (accessed on 24 February 2020).
- Meksuriyen, D.; Cordell, G.A. Retrodihydrochalcones from Dracaena loureiri. J. Nat. Prod. 1988, 51, 1129–1135. [Google Scholar] [CrossRef]
- Sun, X.; Wen, K.; Xu, Z.; He, Z.; Wu, B.; Yang, X.; Wang, X. Effect of loureirin B on Crohn’s disease rat model induced by TNBS via IL-6/STAT3/NF-κB signaling pathway. Chin. Med. 2020, 15, 2. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, K.; Kitaoka, M.; Taki, M.; Takaishi, S.; Boriboon, M.; Akiyama, T. Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity. Planta Med. 1997, 63, 540–543. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Sawasdee, K.; Kirtikara, K. Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri. Planta Med. 2002, 68, 841–843. [Google Scholar] [CrossRef]
- Cheng, S.; Rong, Y.; Ma, M.; Lin, X.; Liu, X.; Li, C.; Yang, X.; Chen, S. Modulation on tetrodotoxin-resistant sodium current of loureirin B in rat dorsal root ganglion neurons via cyclic AMP–dependent protein kinase A. J. Cell. Biochem. 2020, 121, 1790–1800. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Y.; Cao, J.; Qian, K.; Niu, B.; Chen, Q. Loureirin B promotes insulin secretion through inhibition of KATP channel and influx of intracellular calcium. J. Cell. Biochem. 2018, 119, 2012–2021. [Google Scholar] [CrossRef]
- Meksuriyen, D.; Cordell, G.A.; Ruangrungsi, N.; Tantivatana, P. Traditional medicinal plants of Thailand, IX. 10-Hydroxy-11-methoxydracaenone and 7,10-dihydroxy-11-methoxydracaenone from Dracaena loureiri. J. Nat. Prod. 1987, 50, 1118–1125. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.-A.; Omosa, L.K.; Derese, S.; Tane, P.; Heydenreich, M.; Spiteller, M.; Seo, E.-J.; Efferth, T. Two new flavonoids from Dracaena usambarensis Engl. Phytochem. Lett. 2020, 36, 80–85. [Google Scholar] [CrossRef]
- Moharram, F.A.; EI-Shenawy, S.M. Antinociceptive and anti-inflammatory steroidal saponins from Dracaena ombet. Planta Med. 2007, 73, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Yokosuka, A.; Sekiguchi, A.; Mimaki, Y. Chemical constituents of the leaves of Dracaena thalioides. Nat. Prod. Commun. 2013, 8, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.J.; Sousa, M.; Parola, A.J.; de Melo, J.S.S.; Catarino, F.; Marçalo, J.; Pina, F. Identification of 7,4′-dihydroxy-5-methoxyflavylium in “dragon’s blood”: To be or not to be an anthocyanin. Chem. Eur. J. 2007, 13, 1417–1422. [Google Scholar] [CrossRef] [Green Version]
- Raslan, M.A.; Melek, F.R.; Said, A.A.; Elshamy, A.I.; Umeyama, A.; Mounier, M.M. New cytotoxic dihydrochalcone and steroidal saponins from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem. Lett. 2017, 22, 39–43. [Google Scholar] [CrossRef]
- Said, A.; Aboutabl, E.A.; Melek, F.R.; Abdel Jaleel, G.A.R.; Raslan, M. Steroidal saponins and homoisoflavanone from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem. Lett. 2015, 12, 113–118. [Google Scholar] [CrossRef]
- Aye, M.M.; Aung, H.T.; Thu, Z.M.; Sein, M.M.; Takaya, Y.; Komori, Y.; Clericuzio, M.; Vidari, G. Constituents of the rhizomes of Sansevieria cylindrica. Nat. Prod. Commun. 2018, 13, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Aung, H.T.; Aye, M.M.; Thu, Z.M.; Komori, Y.; Sein, M.M.; Vidari, G.; Takaya, Y. Bioactive constituents from the rhizomes of Sansevieria cylindrica. Rec. Nat. Prod. 2020, 14, 269–275. [Google Scholar] [CrossRef]
- Sharma, R.; Williamd, I.S.; Gatchie, L.; Sonawane, V.R.; Chaudhuri, B.; Bharate, S.B. Furanoflavones pongapin and lanceolatin B block the cell cycle and induce senescence in CYP1A1-overexpressing breast cancer cells. Biorg. Med. Chem. 2018, 26, 6076–6086. [Google Scholar] [CrossRef] [Green Version]
- Xuo, X.; Zhang, L.; Gao, L.; Guo, Y.; Zhang, L.; Li, L.; Si, J.; Cao, L. Antiinflammatory and analgesic activities of ethanol extract and isolated compounds from Millettia pulchra. Biol. Pharm. Bull. 2015, 38, 1328–1336. [Google Scholar]
- Roy, J.; Aktar, F.; Haq, M.R.; Begum, B.; Hasan, C.M. A rare homoisoflavonoid cambodianol from Sansevieria roxburghiana. Asian J. Chem. 2013, 25, 5113–5114. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Tanaka, C.; Gabriel, A.F.; Tapondjou, L.A.; Miyamoto, T. Sappanin-type homoisoflavonoids from Sansevieria trifasciata Prain. Phytochem. Lett. 2015, 12, 262–266. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Jenett-Siems, K.; Melzig, M.F.; Miyamoto, T.; Tapondjou, L.A. A dihydrochalcone derivative and further steroidal saponins from Sansevieria trifasciata Prain. Z. Naturforsch. C J. Biosci. 2017, 72, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.V.; López, S.N. Homoisoflavonoids: Occurrence, biosynthesis, and biological activity. In Studies in Natural Products Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 315–354. [Google Scholar]











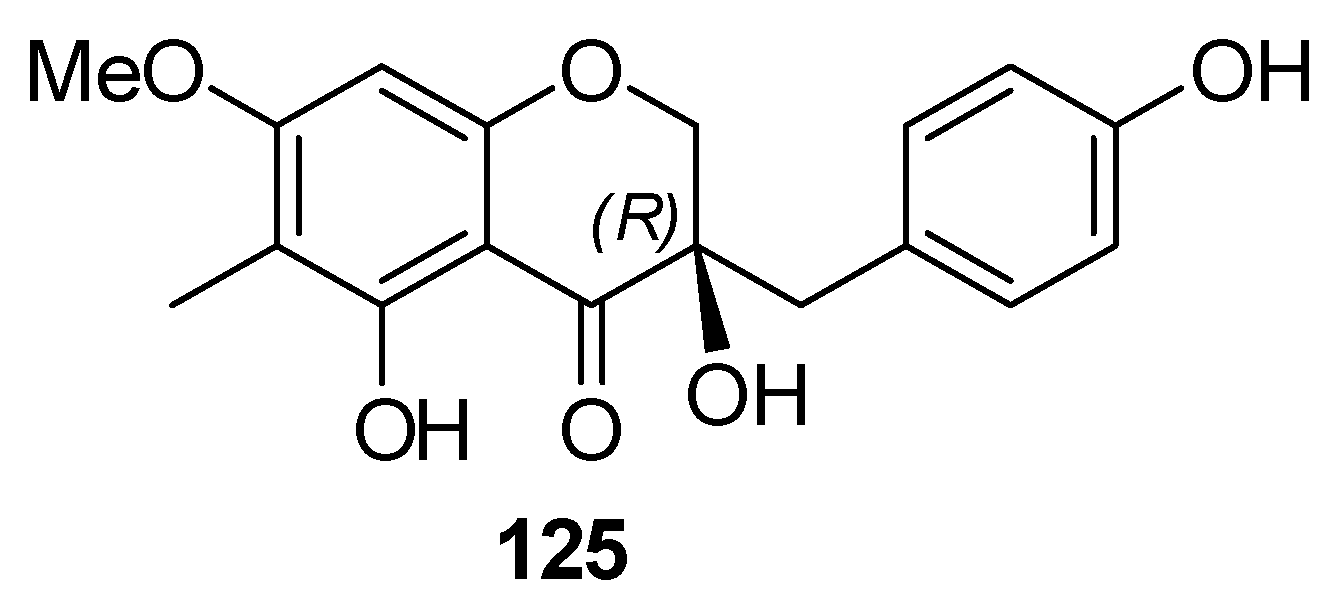

| Source(s) and Compound(s) | Biological/pharmacological Activities | References |
|---|---|---|
| Dracaena angustifolia | ||
| (2R)-3′,7-dihydroxy-5′,5-dimethoxy-8-methylflavone (2) | Anti-inflammatory activity: 2 (IC50 = 45 µM); 3 (IC50 = 33 µM); 4 (IC50 = 61 µM) | [23] |
| desmethylisoophiopogonone B (3) | ||
| 5,7,4′-trihydroxyhomoisoflavanone (4) | ||
| Dracaena cambodiana | ||
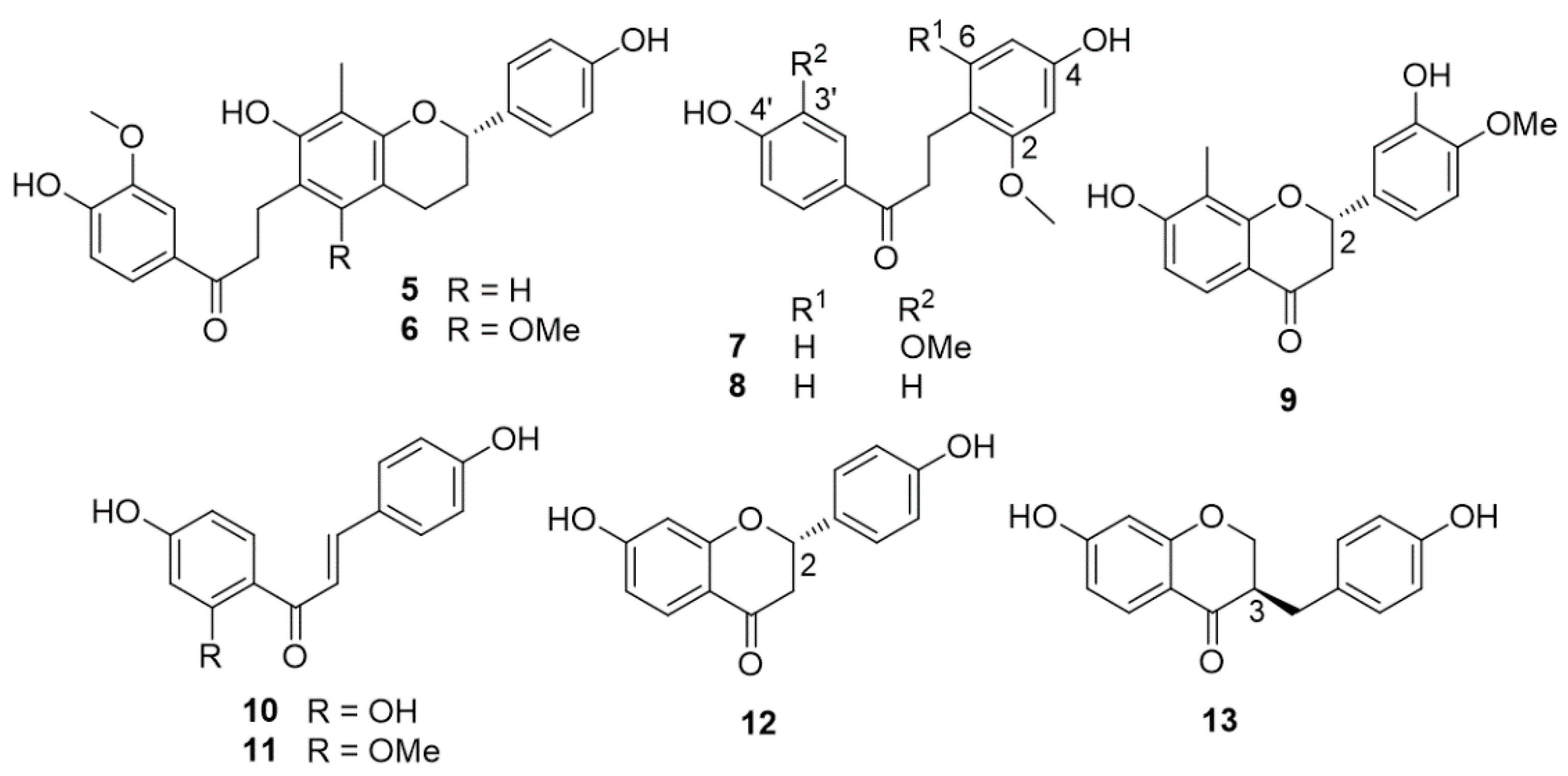
| cambodianins A (5) and B (6); 4,4′-dihydroxy-2′-methoxychalcone (11) (3R)-7,4′-dihydrohomoisoflavanone (13) | Cytotoxic effects against chronic myelogenous leukemia K-562 (5, IC50 = 13.300 ± 0.064 µg/mL; 6, IC50 = 14.100 ± 0.042 µg/mL; 11, IC50 = 32.500 ± 0.082 µg/mL; 13, IC50 = 15.600 ± 0.040 µg/mL), human hepatocarcinoma SMMC-7721 (6, IC50 = 25.000 ± 0.025 µg/mL; 11, IC50 = 12.500 ± 0.025 µg/mL; 13, IC50 = 30.200 ± 0.022 µg/mL), and human gastric tumor SGC-7901 (6, IC50 = 68.000 ± 0.226 µg/mL; 11, IC50 = 11.000 ± 0.018 µg/mL; 13, IC50 = 20.500 ± 0.082 µg/mL) cell lines. Antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) | [24] |
| (±)-7,4′-dihydroxyhomoisoflavanone | Moderate cytotoxic effects against human myeloid leukemia (K562) and human gastric tumor (SGC-7901) cell lines (IC50 = 16–29 μg/mL) | [31] |
| 4,4′-dihydroxy-2,3′-dimethoxydihydrochalcone (7) | Antibacterial activity against S. aureus and MRSA | [24,25] |
| 4,4′-dihydroxy-2-methoxydihydrochalcone (8) | ||
| cambodianins D (14) and E (15) | ||
| (2S)-7,4′-dihydroxy-6,8-dimethylflavan (16) | ||
| cambodianin G (18) | Cytotoxic effects against K-562 (IC50 = 9.5 µg/mL) and SGC-7901 (IC50 = 16.2 µg/mL) cell lines; antibacterial activity against S. aureus | [26] |
| cambodianin H (19) | Antibacterial activity against S. aureus | |
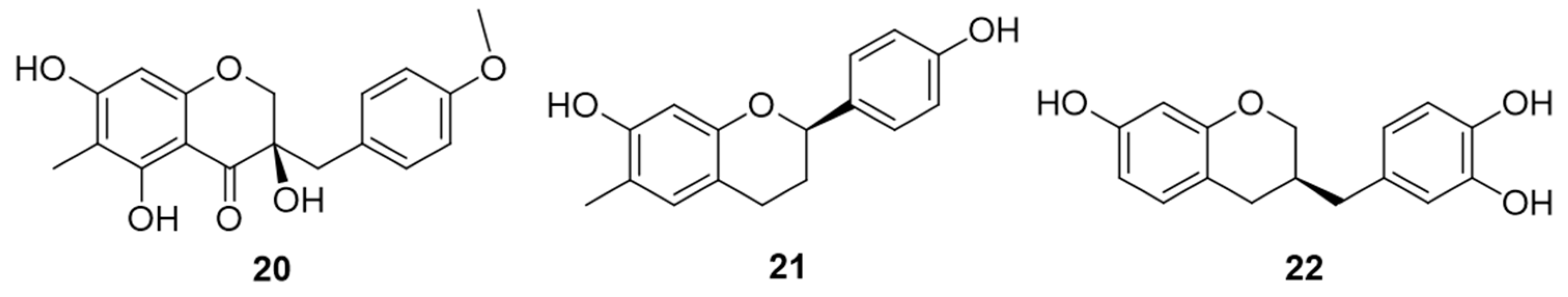
| cambodianol (20) | Cytotoxic effects against K562 (IC50 = 1.4 µg/mL), SGC-7901 (IC50 = 2.9 µg/mL) and SMMC-7721 (IC50 = 5.0 µg/mL) cell lines, comparable with the values of paclitaxel | [30,31] |
| 8-methylsocotrin-4′-ol | Moderate cytotoxic effects against K562 and SGC-7901 cell lines | [31] |
| 4,4′-dihydroxy-3,2′-dimethoxychalcone | Cytotoxic effects against K562, SMMC-7721, and SGC-7901 cell lines, with IC50 values of 2.5, 4.3, and 4.4 mg/mL, respectively, comparable with the values of mitomycin C | [32] |
| (2R)-7,4′-dihydroxy-6-methylflavan (21) | Weak cytotoxic effects (IC50 = 39.22 μM) against human hepatocellular carcinoma BEL-7402 cell line | [34] |
| (3R)-7,3′,4′-trihydroxyhomoisoflavan (22) | Acetylcholinesterase (AChE) inhibitory activity | [34] |
| Dracaena cinnabari | ||
| 4′-hydroxy-7,8-methylenedioxyhomoisoflavan (23) | Inhibition of NO (nitric oxide), TNF-α, and IL-6 production in lipopolysaccharide stimulated mouse macrophage RAW 264.7 cells | [36] |
| dracidione (24) | α-Glucosidase inhibitory activity (IC50 = 40.27 µg/mL) | [37] |
| Dracaena cochinchinensis | ||
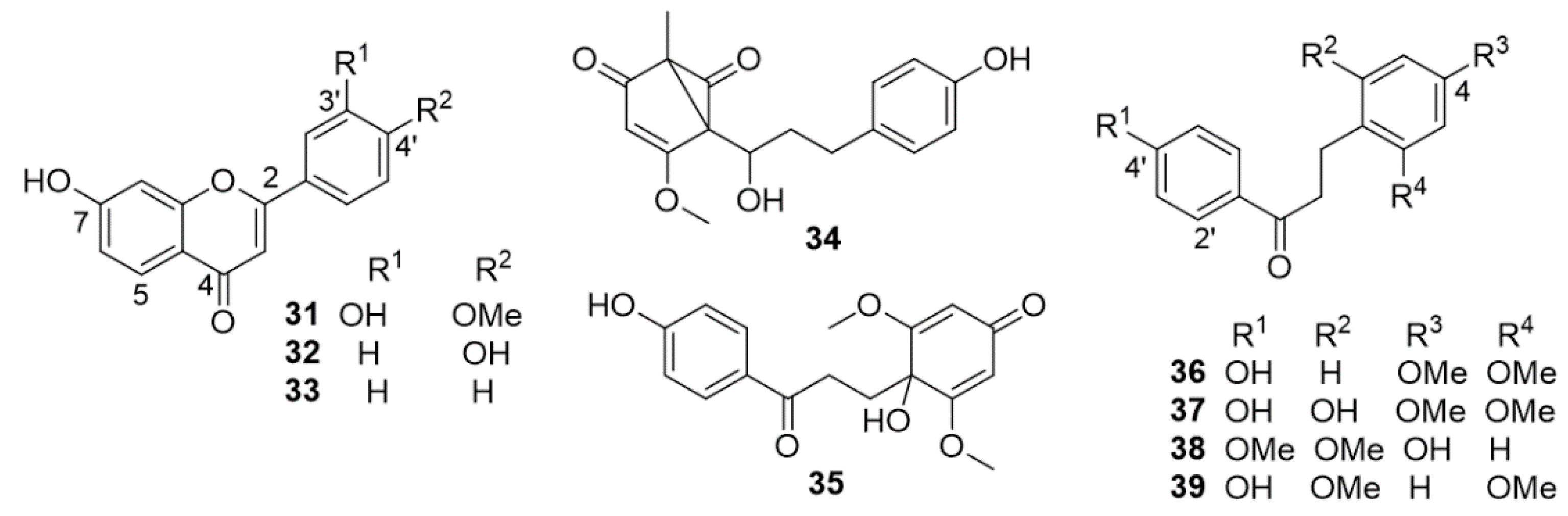
| 4,4′-dihydroxy-2-methoxydihydrochalcone (8) | Anti-neuroinflammatory effects (IC50 = 10.29 ± 1.05 µM) | [42] |
| apigenin | Remarkable anti-neuroinflammatory activity (inhibition of NO production): apigenin (IC50 = 3.12 ± 1.27 µM); 7-hydroxy-3-(4-hydroxybenzylidene) chroman-4-one (IC50 = 4.46 ± 0.95 μM); 31 (IC50 = 4.31 ± 1.79 µM); 32 (IC50 = 8.00 ± 2.04 µM); 33 (IC50 = 3.36 ± 1.67 µM) | [42] |
| 7-hydroxy-3-(4-hydroxybenzylidene)chroman-4-one | ||
| 7,3′-dihydroxy-4′-methoxyflavone (31) | ||
| 7,4′-dihydroxyflavone (32) | ||
| 7-hydroxyflavone (33) | ||
| 4′-hydroxy-2,4-dimethoxydihydrochalcone (36) | Anti-inflammatory activity | [43] |
| 4′-hydroxy-2,4,6-trimethoxydihydrochalcone (37) | ||
| (3S)-3,7,4′-trihydroxy-5-methoxyhomoisoflavanone (41) | Inhibitory activity against nitric oxide (NO) production: 41 (IC50 = 75.6 ± 1.2 µM); 42 (IC50 = 60.4 µM) | [52] |
| 7,4′-dihydroxyhomoisoflavanone (42) | ||
| pterostilbene (43) | NQO1 [NAD(P)H Quinone Dehydrogenase 1] inducing activity and anti-inflammatory effects | [43] |
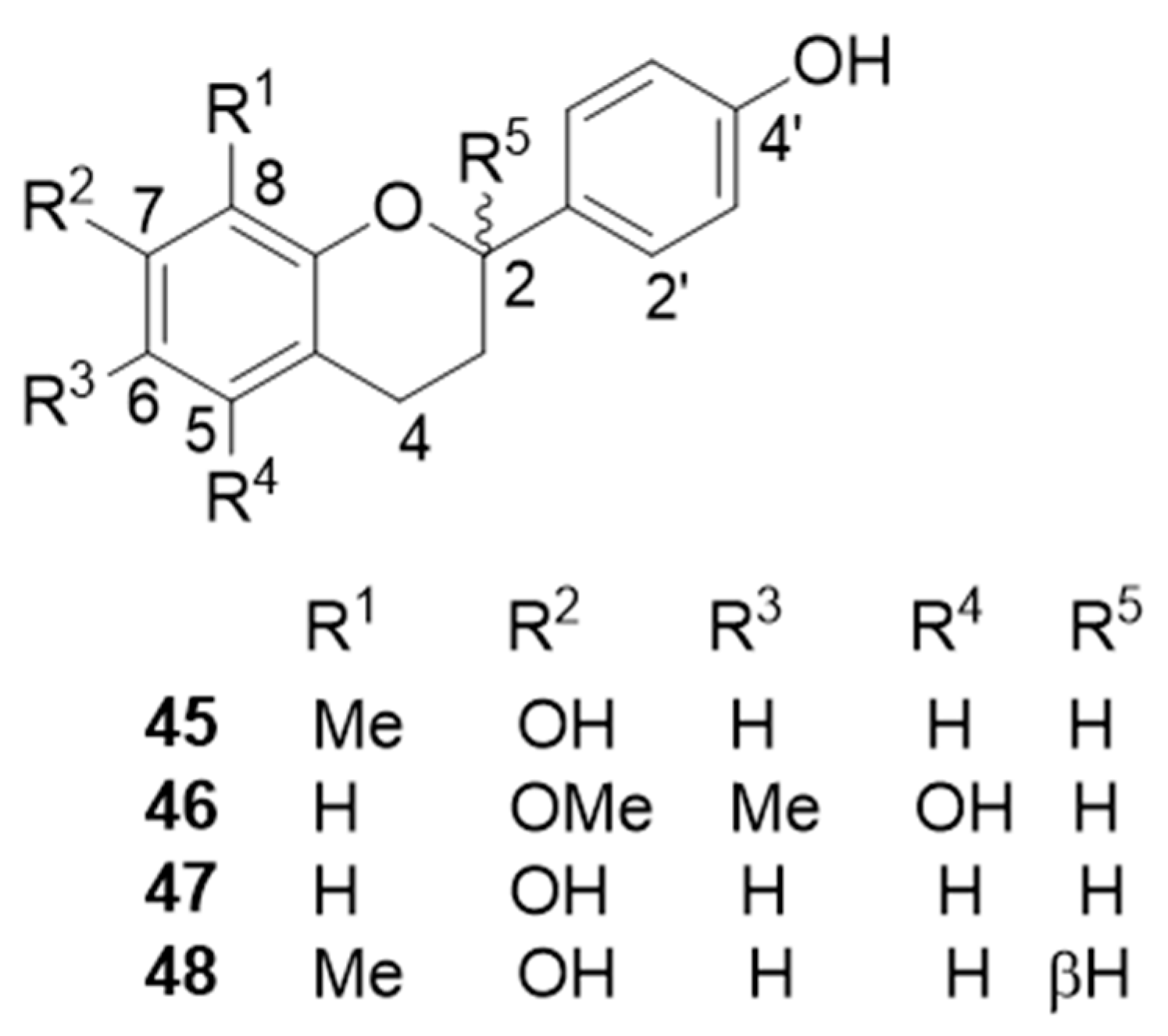
| 7,4′-dihydroxy-8-methylflavan (45) | Osteogenic effects on mesenchymal stem cells (MSCs) | [49] |
| 5,4′-dihydroxy-7-methoxy-6-methylflavan (46) | Osteogenic effects on mesenchymal stem cells (MSCs) and anti-inflammatory activity | [43,49] |
| 7,4′-dihydroxyflavan (47) | Osteogenic effects on mesenchymal stem cells (MSCs) | [49] |
| (2S)-7,4′-dihydroxy-8-methylflavan (48) | Antibacterial activities against Helicobacter pylori (MIC = 31.3 µM) | [47] |
| dracaeconolide B (49) | Osteogenic effects on mesenchymal stem cells (MSCs) | [49] |
| (3R)-7,4′-dihydroxy-8-methoxyhomoisoflavan (50) | ||
| (3R)-7,4′-dihydroxyhomoisoflavan (51) | ||
| (3R)-7,4′-dihydroxy-5-methoxyhomoisoflavan (52) | Anti-neuroinflammatory activity (IC50 = 8.50 ± 1.28 µM) | [42] |
| 7,4′-dihydroxyhomoisoflavan (53) | Anti-inflammatory activity | [43] |
| 6,4′-dihydroxy-7-methoxyhomoisoflavan (54) | ||
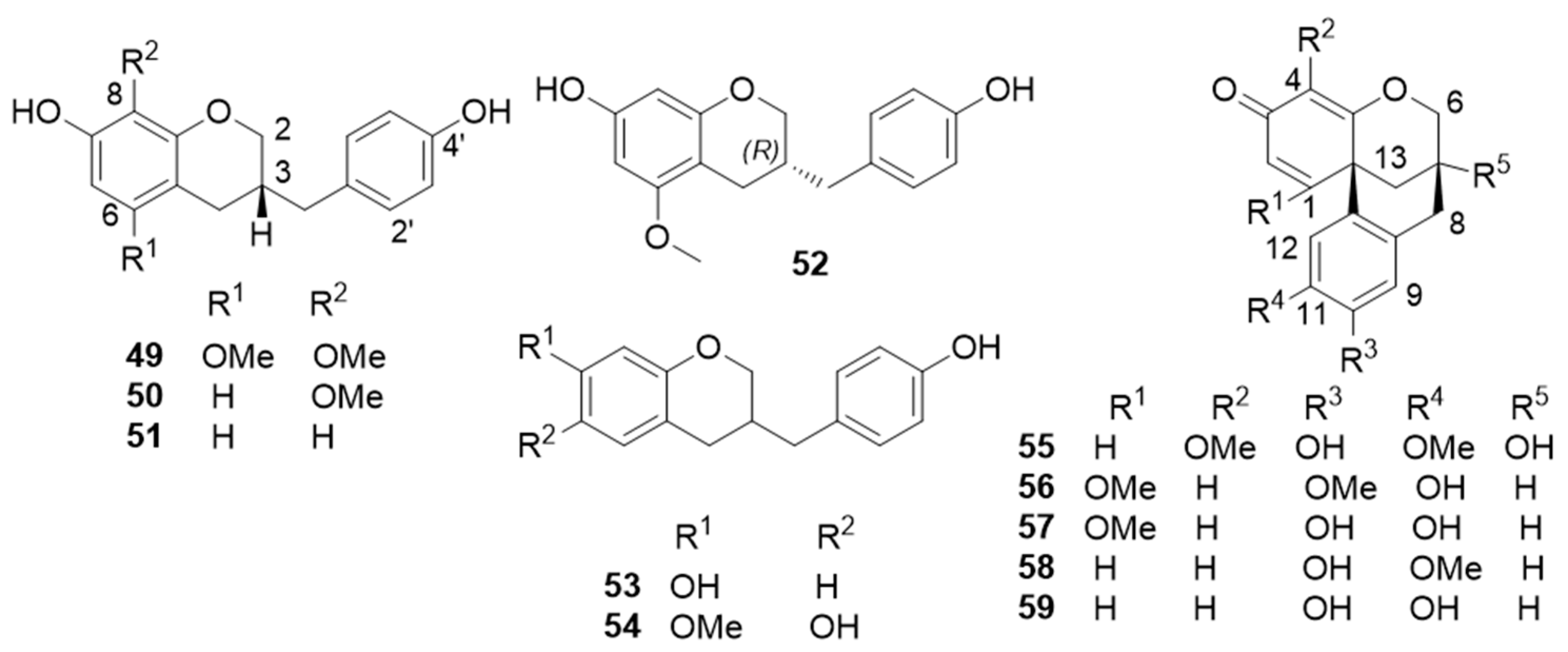
| 10-hydroxy-11-methoxydracaenone (58) | Inhibitory activity against NO production (IC50 = 62.4 ± 3.5 µM) and NQO1 inducing activity | [43,52] |
| cochinchinenene F (61) | NQO1 inducing activity | [43] |
| cochinchinenenes A (60), B (63), C (64), and D (65) | Thrombin inhibitory activity: 60 (IC50 > 9.5 µM); 63 (IC50 = 17.8 µM); 64 (IC50 = 26.7 µM); 65 (IC50 > 41.3 µM) | [47] |
| cochinchinenene E (66) | NQO1 inducing activity | [43] |
| “cochinchinenene G” (68) | Inhibitory activity against NO production (IC50 = 2.18 ± 1.43 µM) | [52] |
| (2R)-8-methylsocotrin-4′-ol (69) | Thrombin inhibitory activity (IC50 = 21.5 µM) | [47] |
| cochinchinenins L (72) and M (73) | Inhibitory effects on NO production in lipopolysaccharide (LPS)-stimulated BV-2 microglial cells: 72 (IC50 = 4.9 ± 0.4 µM); 73 (IC50 = 5.4 ± 0.6 µM) | [56] |
| cochinchinenin C (76) | Thrombin inhibitory activity (IC50 > 9.2 µM); antibacterial activity against Helicobacter pylori (MIC = 29.5 µM) | [47] |
| 1-[5-(2-methoxy-4,4′- dihydroxydihydrochalconyl)]-1-(4-hydroxyphenyl)-3-(2-methoxy-4-hydroxyphenyl)propane | Thrombin inhibitory activity (IC50 = 26.3 µM) | [47] |
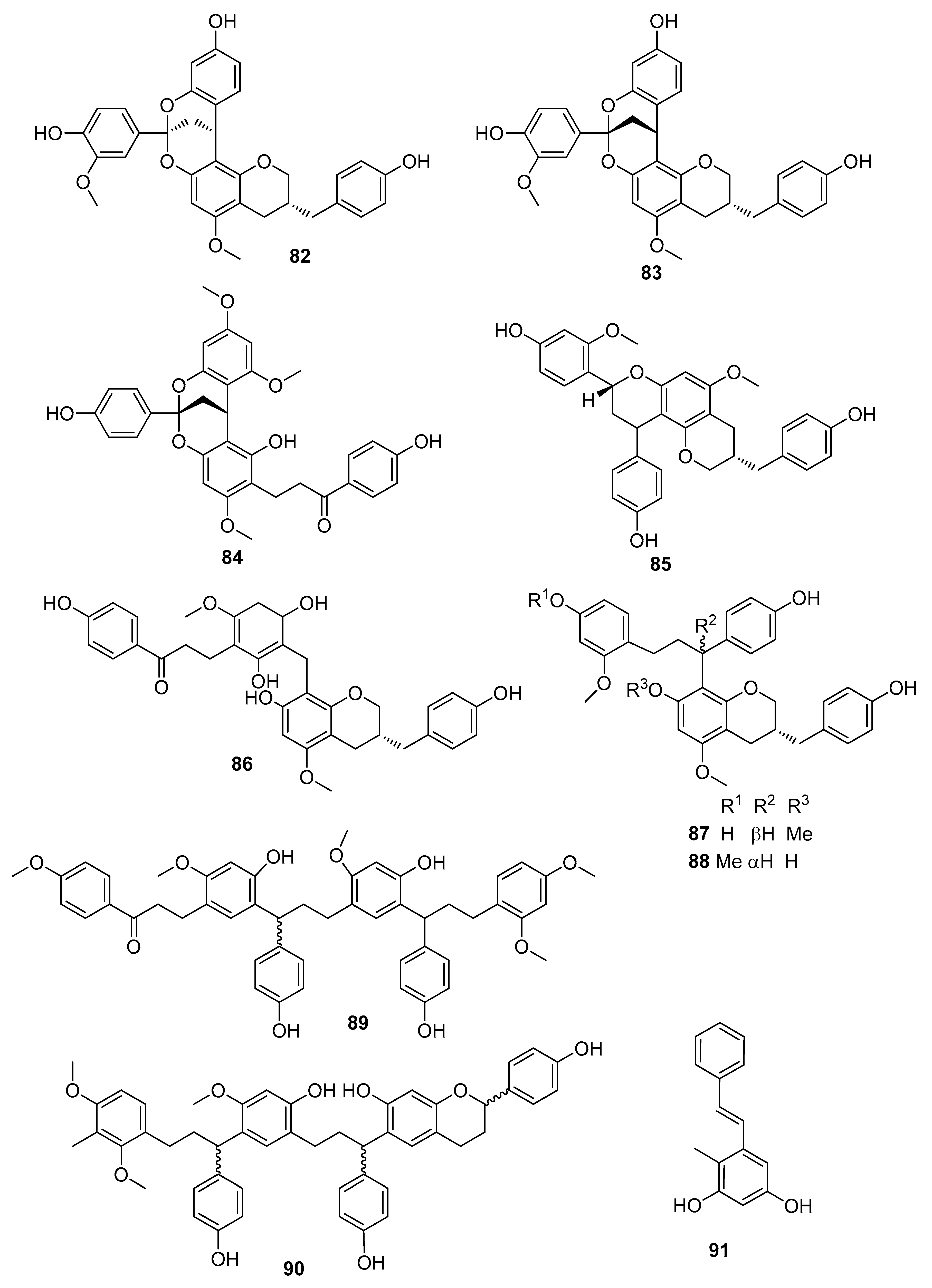
| biflavocochin A (82) | Neuroprotective effect on serum deficiency-induced cellular damage in neuroendocrine PC12 cells (pheochromocytoma cells derived from the adrenal gland of Rattus norvegicus) | [53] |
| biflavocochins B (83), F (87), and G (88) | Protein-tyrosine phosphatase 1B (PTP1B) inhibitory activity | [53] |
| (7E)-2,4-dihydroxy-1-methylstilbene (91) | Antioxidant activity and antifungal activity against Exserohilum turcicum, Bipolaris maydis, Curvularia lunata, and Fusarium graminearum | [58] |
| Dracaena loureiri | ||
| loureirin B (94) | Modulatory activity on the TTX-R sodium channel in dorsal root ganglion (DRG) neurons; effect on insulin secretion of pancreatic β-cells, increase of the mRNA level of Pdx-1, MafA, and intracellular ATP level; suppression of inflammatory cytokines and inhibition of apoptosis through regulation of IL-6/STAT3/NF-κB signalling pathway | [70,73,74] |
| loureirin D (96) | Inhibition of NO production stimulated by LPS-activated RAW 264.7 murine macrophages (IC50 = 50.3 µM) | [48,70] |
| 4,4′-dihydroxy-2,6-dimethoxydihydrochalcone (97) (also isolated from D. cambodiana) | Cytotoxic effects against K-562 (IC50 = 12.800 ± 0.015 µg/mL), SMMC-7721 (IC50 = 16.200 ± 0.040 µg/mL), and SGC-7901 (IC50 = 10.000 ± 0.060 µg/mL) cell lines; antibacterial activities against S. aureus; stimulation of the hormone-dependent MCF-7 cell growth in a concentration dependent manner between 10−8 and 10−5 M | [24,71] |
| 4′,2-dihydroxy-4,6-dimethoxydihydrochalcone (98) | Stimulation of the MCF-7 cell proliferation in a concentration dependent manner between 10−8 and 10−5 M | [71] |
| 4,3′,5′-trihydroxystilbene (100) | Inhibitory activities of the enzymes COX-1 and COX-2: 100 (IC50 = 2.61 and 2.16 µM, respectively); 101 (IC50 = 4.92 and 2.21 µM, respectively); 102 (IC50 = 4.84 and 1.29 µM, respectively) | [72] |
| 4,3′-dihydroxy-5′-methoxystilbene (101) | ||
| 4-hydroxy-3′,5′-dimethoxystilbene (102) | ||
| 5,7,4′-trihydroxyhomoisoflavanone (4) | Binding affinity for the bovine uterine estrogen receptor (IC50 = 375 nM) | [71] |
| Dracaena usambarensis | ||
| (3S)-3,4ʹ,5,6-tetrahydroxy-7-methoxyhomoisoflavanone (104) | Moderate cytotoxicity against drug sensitive human lymphoblastic leukemia CCRF-CEM cells | [76] |
| Sansevieria cylindrica | ||
| (+)-trifasciatine C (114) | Moderate cytotoxicity against MCF7 cells (IC50 = 34.1 µg/mL) | [80] |
| (+)-trifasciatine B (116) | Weak DPPH radical scavenging activity (IC50 = 35.2 µg/mL) | [81] |
| lanceolatin B (121) | High cancer chemopreventive potential, anti-neuroinflammatory and analgesic properties | [84,85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities. Molecules 2020, 25, 2608. https://doi.org/10.3390/molecules25112608
Thu ZM, Myo KK, Aung HT, Armijos C, Vidari G. Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities. Molecules. 2020; 25(11):2608. https://doi.org/10.3390/molecules25112608
Chicago/Turabian StyleThu, Zaw Min, Ko Ko Myo, Hnin Thanda Aung, Chabaco Armijos, and Giovanni Vidari. 2020. "Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities" Molecules 25, no. 11: 2608. https://doi.org/10.3390/molecules25112608





