Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Nonalcoholic Fatty Liver Disease (NAFLD) Diagnosis
2.3. Clinical, Anthropometric, and Biochemical Procedures
2.4. Obesity Definition
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Characteristics of Participants
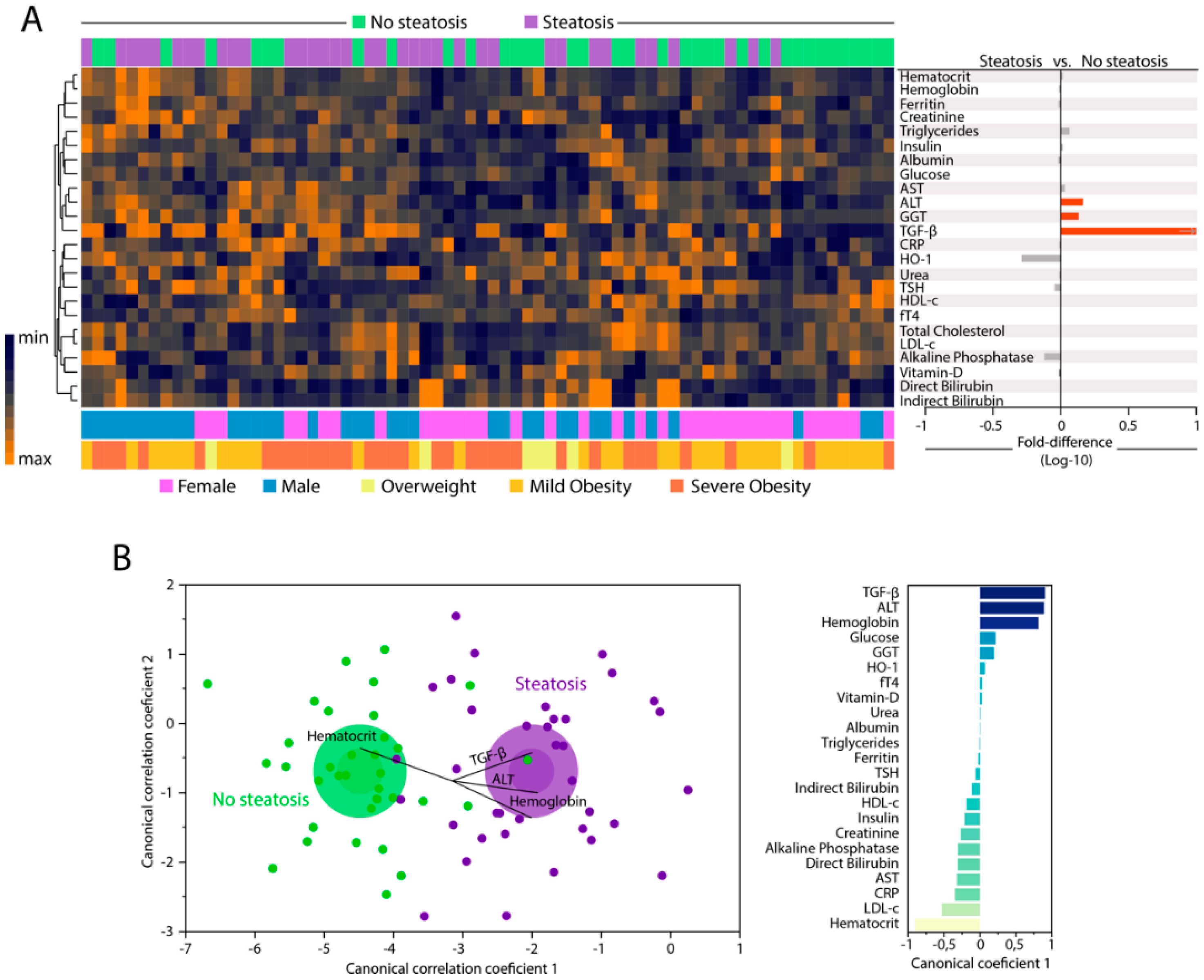
3.2. Changes in Biochemical Parameters in Peripheral Blood Can Distinguish Patients with and without Hepatic Steatosis
3.3. Hepatic Steatosis Leads to Consistent Changes in the Profile of Correlations between Blood Biochemical Parameters
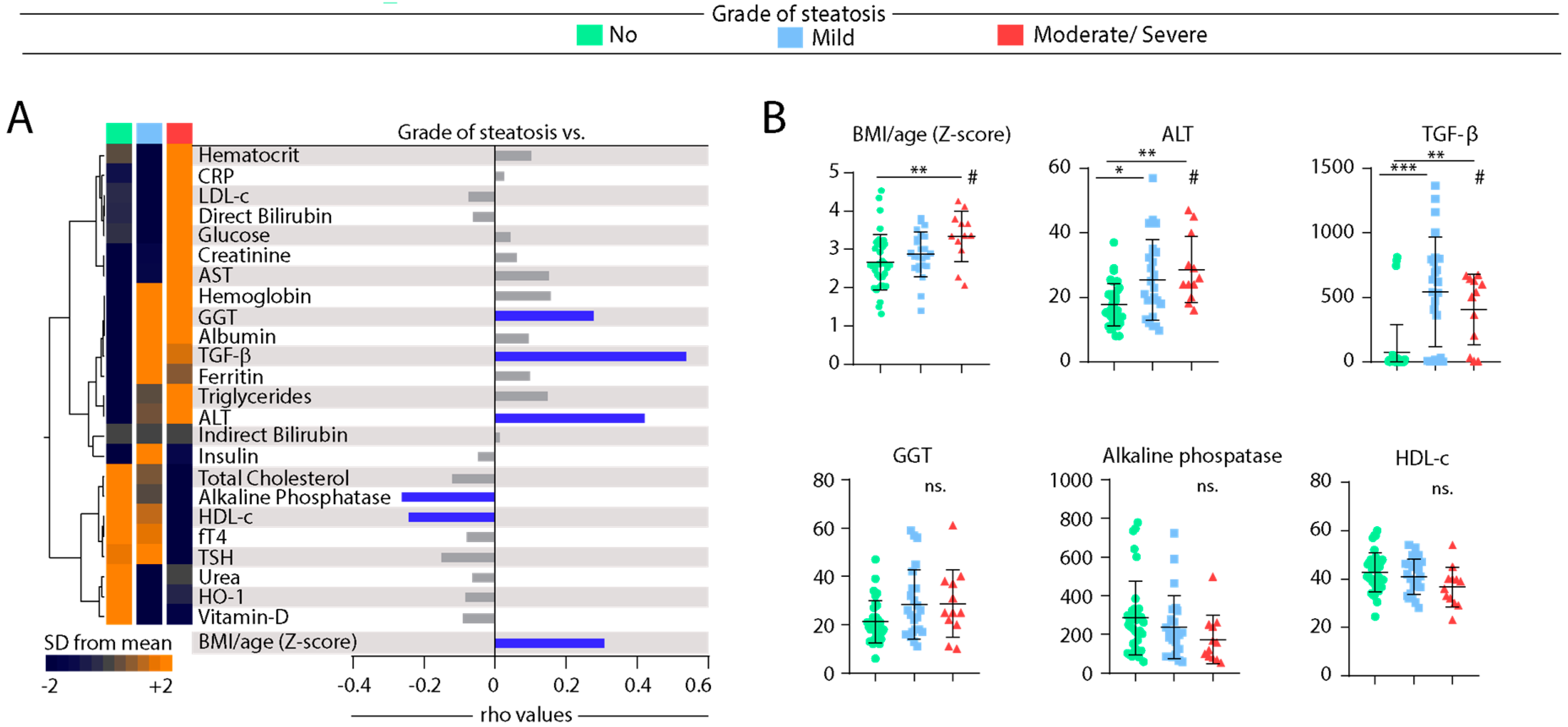
3.4. Associations between Grade of Hepatic Steatosis and Values of Biochemical Parameters in Peripheral Blood
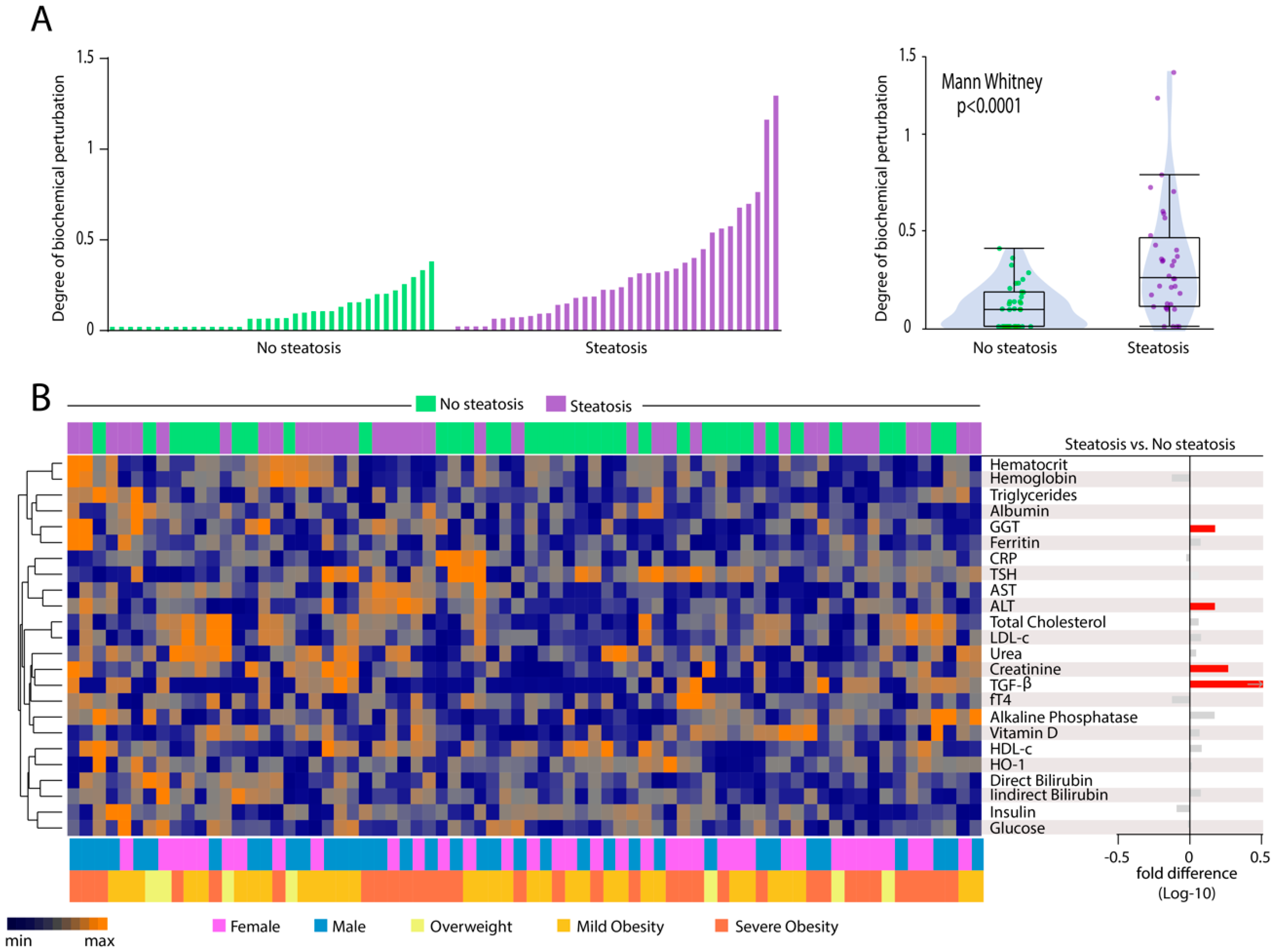
3.5. Patients with Hepatic Steatosis Display Higher Degree of Biochemical Perturbation in Peripheral Blood
3.6. Heightened Plasma Levels of TGF-β Hallmarks Hepatic Steatosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alkhouri, N.; Alisi, A.; Della Corte, C.; Fitzpatrick, E.; Raponi, M.; Dhawan, A. Nonalcoholic fatty liver disease: A challenge for pediatricians. JAMA Pediatr. 2015, 169, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [Green Version]
- Draijer, L.; Benninga, M.; Koot, B. Pediatric NAFLD: An overview and recent developments in diagnostics and treatment. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 447–461. [Google Scholar] [CrossRef]
- Mann, J.P.; Valenti, L.; Scorletti, E.; Byrne, C.D.; Nobili, V. Nonalcoholic Fatty Liver Disease in Children. Semin. Liver Dis. 2018, 38, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Assuncao, S.N.F.; Sorte, N.C.B.; Alves, C.D.; Mendes, P.S.A.; Alves, C.R.B.; Silva, L.R. Nonalcoholic fatty liver disease (NAFLD) pathophysiology in obese children and adolescents: Update. Nutr. Hosp. 2017, 34, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Clemente, M.G.; Mandato, C.; Poeta, M.; Vajro, P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J. Gastroenterol. 2016, 22, 8078–8093. [Google Scholar] [CrossRef]
- Fang, Y.L.; Chen, H.; Wang, C.L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Holterman, A.X.; Guzman, G.; Fantuzzi, G.; Wang, H.; Aigner, K.; Browne, A.; Holterman, M. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity (Silver Spring) 2013, 21, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holterman, A.; Gurria, J.; Tanpure, S.; DiSomma, N. Nonalcoholic fatty liver disease and bariatric surgery in adolescents. Semin. Pediatr. Surg. 2014, 23, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: Position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Uppal, V.; Mansoor, S.; Furuya, K.N. Pediatric Non-alcoholic Fatty Liver Disease. Curr. Gastroenterol. Rep. 2016, 18, 24. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Hepatic steatosis: Innocent bystander or guilty party? Hepatology 1998, 27, 1463–1466. [Google Scholar] [CrossRef]
- Esler, W.P.; Bence, K.K. Metabolic Targets in Nonalcoholic Fatty Liver Disease. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 247–267. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- Thamer, C.; Tschritter, O.; Haap, M.; Shirkavand, F.; Machann, J.; Fritsche, A.; Schick, F.; Haring, H.; Stumvoll, M. Elevated serum GGT concentrations predict reduced insulin sensitivity and increased intrahepatic lipids. Horm. Metab. Res. 2005, 37, 246–251. [Google Scholar] [CrossRef]
- Assunção, S.N.F.; Sorte, N.C.A.B.; Alves, C.A.D.; Mendes, P.S.A.; Alves, C.R.B.; Silva, L.R. Inflammatory cytokines and non-alcoholic fatty liver disease (NAFLD) in obese children and adolescents. Nutr. Hosp. 2018, 35, 78–83. [Google Scholar] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef]
- Yuan, X.W.; Li, D.D.; Liu, L.D.; Zhang, Y.; Zhao, W.; Cui, L.Y.; Yang, Y.; Nan, Y.M. Application of heme oxygenase 1 in the diagnosis of non-alcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Junge, J.M.; Drummond, G.S. Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharm. Sci. 2016, 37, 17–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gressner, A.M.; Weiskirchen, R.; Breitkopf, K.; Dooley, S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002, 7, d793–d807. [Google Scholar] [CrossRef]
- Flisiak, R.; Pytel-Krolczuk, B.; Prokopowicz, D. Circulating transforming growth factor beta(1) as an indicator of hepatic function impairment in liver cirrhosis. Cytokine 2000, 12, 677–681. [Google Scholar] [CrossRef]
- de Onis, M.; Garza, C.; Onyango, A.W.; Rolland-Cachera, M.F.; de pédiatrie, l.C.d.d.f. WHO growth standards for infants and young children. Arch. Pediatr. 2009, 16, 47–53. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Freedman, D.S.; Serdula, M.K.; Srinivasan, S.R.; Berenson, G.S. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: The Bogalusa Heart Study. Am. J. Clin. Nutr. 1999, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinhaes, C.L.; Arriaga, M.B.; de Almeida, B.L.; Oliveira, J.V.; Santos, C.S.; Calcagno, J.I.; Carvalho, T.X.; Giovanetti, M.; Alcantara, L.C.J.; de Siqueira, I.C.; et al. Newborns with Zika virus-associated microcephaly exhibit marked systemic inflammatory imbalance. J. Infect. Dis. 2020, 221, 1567. [Google Scholar] [CrossRef] [PubMed]
- Vinhaes, C.L.; Oliveira-de-Souza, D.; Silveira-Mattos, P.S.; Nogueira, B.; Shi, R.; Wei, W.; Yuan, X.; Zhang, G.; Cai, Y.; Barry, C.E., 3rd; et al. Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: A prospective cohort study. Cytokine 2019, 123, 154759. [Google Scholar] [CrossRef]
- Oliveira-de-Souza, D.; Vinhaes, C.L.; Arriaga, M.B.; Kumar, N.P.; Cubillos-Angulo, J.M.; Shi, R.; Wei, W.; Yuan, X.; Zhang, G.; Cai, Y.; et al. Molecular degree of perturbation of plasma inflammatory markers associated with tuberculosis reveals distinct disease profiles between Indian and Chinese populations. Sci. Rep. 2019, 9, 8002. [Google Scholar] [CrossRef] [Green Version]
- Cruz, L.A.B.; Moraes, M.O.A.; Queiroga-Barros, M.R.; Fukutani, K.F.; Barral-Netto, M.; Andrade, B.B. Chronic hepatitis B virus infection drives changes in systemic immune activation profile in patients coinfected with Plasmodium vivax malaria. PLoS Negl. Trop. Dis. 2019, 13, e0007535. [Google Scholar] [CrossRef]
- Andrade, B.B.; Singh, A.; Narendran, G.; Schechter, M.E.; Nayak, K.; Subramanian, S.; Anbalagan, S.; Jensen, S.M.; Porter, B.O.; Antonelli, L.R.; et al. Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog. 2014, 10, e1004433. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Diehl, A.M. Nonalcoholic Steatohepatitis. Annu. Rev. Med. 2017, 68, 85–98. [Google Scholar] [CrossRef]
- Ore, A.; Akinloye, O.A. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina (Kaunas) 2019, 55, 26. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles from Hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappini, F.; Barrier, A.; Saffroy, R.; Domart, M.C.; Dagues, N.; Azoulay, D.; Sebagh, M.; Franc, B.; Chevalier, S.; Debuire, B.; et al. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab. Investig. 2006, 86, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, B.; Nath, L.R. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J. Recept. Signal Transduct. 2020, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Hadhoud, E.; Ayman, A.-R.; Fawzy, E.; Fawzy, M. Transforming Growth Factor Beta One and Non Alcoholic Fatty Liver Disease. Afro-Egypt J. Infect. Endem. Dis. 2014, 4, 136–142. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Manion, M.; Andrade, B.B.; DerSimonian, R.; Gu, W.; Rupert, A.; Musselwhite, L.W.; Sierra-Madero, J.G.; Belaunzaran-Zamudio, P.F.; Sanne, I.; Lederman, M.M.; et al. Country of residence is associated with distinct inflammatory biomarker signatures in HIV-infected patients. J. Virus Erad. 2017, 3, 24–33. [Google Scholar]
- Mendonca, V.R.; Queiroz, A.T.; Lopes, F.M.; Andrade, B.B.; Barral-Netto, M. Networking the host immune response in Plasmodium vivax malaria. Malar J. 2013, 12, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.J.; Tran, T.; Kaufman, H.; Niles, J.; Gish, R. Increasing metabolic co-morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PLoS ONE 2019, 14, e0220612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S.; Watanabe, N.; Numata, M.; Ogawa, T.; Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. J. Gastroenterol. 2003, 38, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1160–1171. [Google Scholar] [CrossRef]
- Alisi, A.; Nobili, V.; Ceccarelli, S.; Panera, N.; De Stefanis, C.; De Vito, R.; Vitali, R.; Bedogni, G.; Balsano, C.; Cucchiara, S.; et al. Plasma high mobility group box 1 protein reflects fibrosis in pediatric nonalcoholic fatty liver disease. Expert Rev. Mol. Diagn. 2014, 14, 763–771. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yoo, Y.J.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Kim, I.H.; Lee, S.Y.; Kim, B.H.; Kim, J.W.; et al. Multiparametric MR is a Valuable Modality for Evaluating Disease Severity of Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2020, 11, e00157. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barretto, J.R.; Boa-Sorte, N.; Vinhaes, C.L.; Malta-Santos, H.; Rebouças-Silva, J.; Ramos, C.F.; Torres-Nascimento, M.A.S.; Borges, V.M.; Andrade, B.B. Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study. Nutrients 2020, 12, 1650. https://doi.org/10.3390/nu12061650
Barretto JR, Boa-Sorte N, Vinhaes CL, Malta-Santos H, Rebouças-Silva J, Ramos CF, Torres-Nascimento MAS, Borges VM, Andrade BB. Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study. Nutrients. 2020; 12(6):1650. https://doi.org/10.3390/nu12061650
Chicago/Turabian StyleBarretto, Junaura R., Ney Boa-Sorte, Caian L. Vinhaes, Hayna Malta-Santos, Jessica Rebouças-Silva, Camila F. Ramos, Monica A. S. Torres-Nascimento, Valeria M. Borges, and Bruno B. Andrade. 2020. "Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study" Nutrients 12, no. 6: 1650. https://doi.org/10.3390/nu12061650
APA StyleBarretto, J. R., Boa-Sorte, N., Vinhaes, C. L., Malta-Santos, H., Rebouças-Silva, J., Ramos, C. F., Torres-Nascimento, M. A. S., Borges, V. M., & Andrade, B. B. (2020). Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study. Nutrients, 12(6), 1650. https://doi.org/10.3390/nu12061650




