[68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy
Abstract
:1. Introduction
- establish the PET Tracer [68Ga]-NODAGA-RGD for imaging angiogenesis in a murine model of myocardial infarction;
- assess the effect of CiC transplantation on LV-remodeling post infarction measured by cardiac MRI (CMR); and,
- evaluate whether αvβ3-integrin expression in the infarct, as measured by [68Ga]-NODAGA-RGD-PET, predicts cardiac function following cell therapy.
2. Materials and Methods
2.1. Stem Cell Culture and Cardiovascular Differentiation
2.2. Animal Model
- 1)
- SHAM (n = 6)
- 2)
- MI (n = 6)
- 3)
- MI + blocked (n = 3)
- 4)
- MIC (n = 5)
- 5)
- MI only (n = 6)
- 6)
- MIC (n = 6)
2.3. PET Imaging
2.4. PET Image Analysis
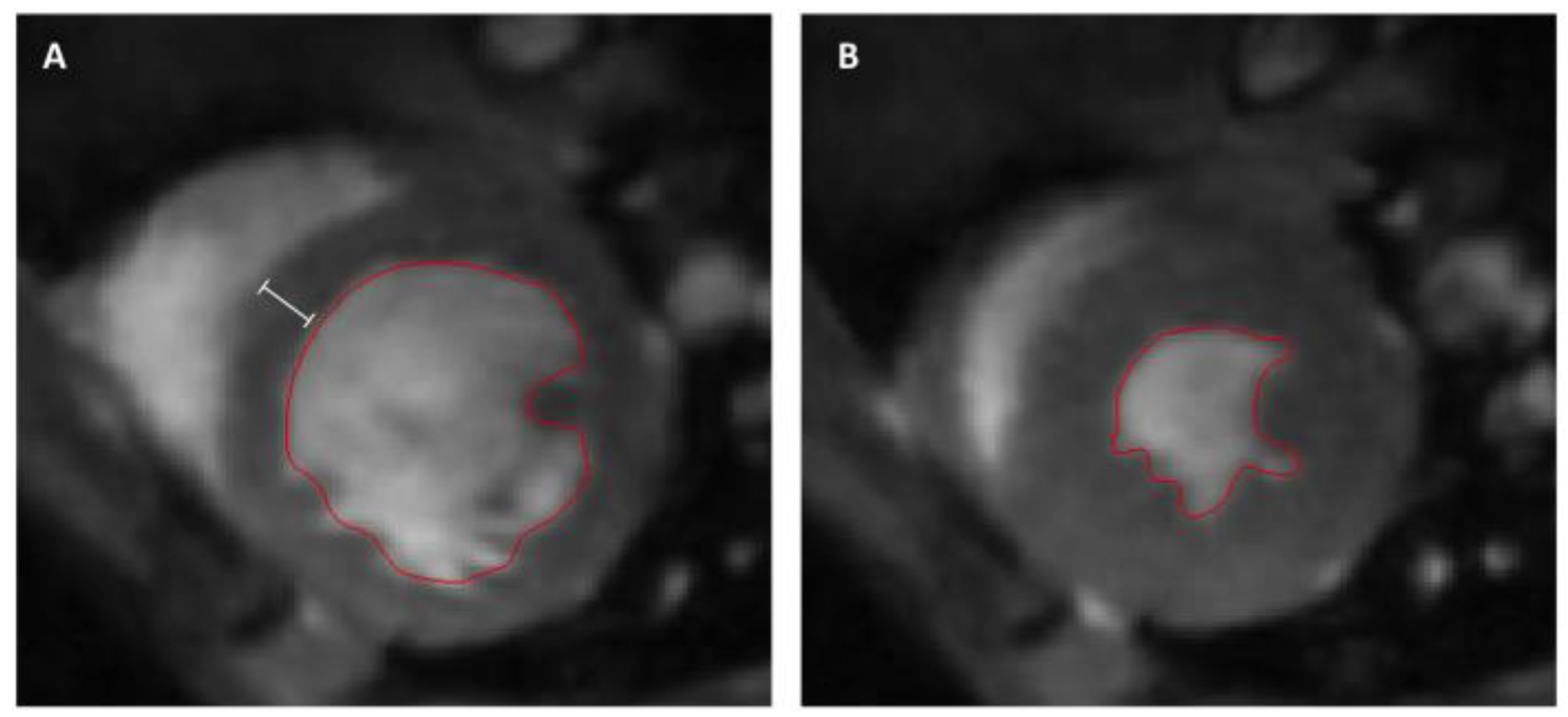
2.5. Cardiac Magnetic Resonance Imaging and Analysis
2.6. Histology
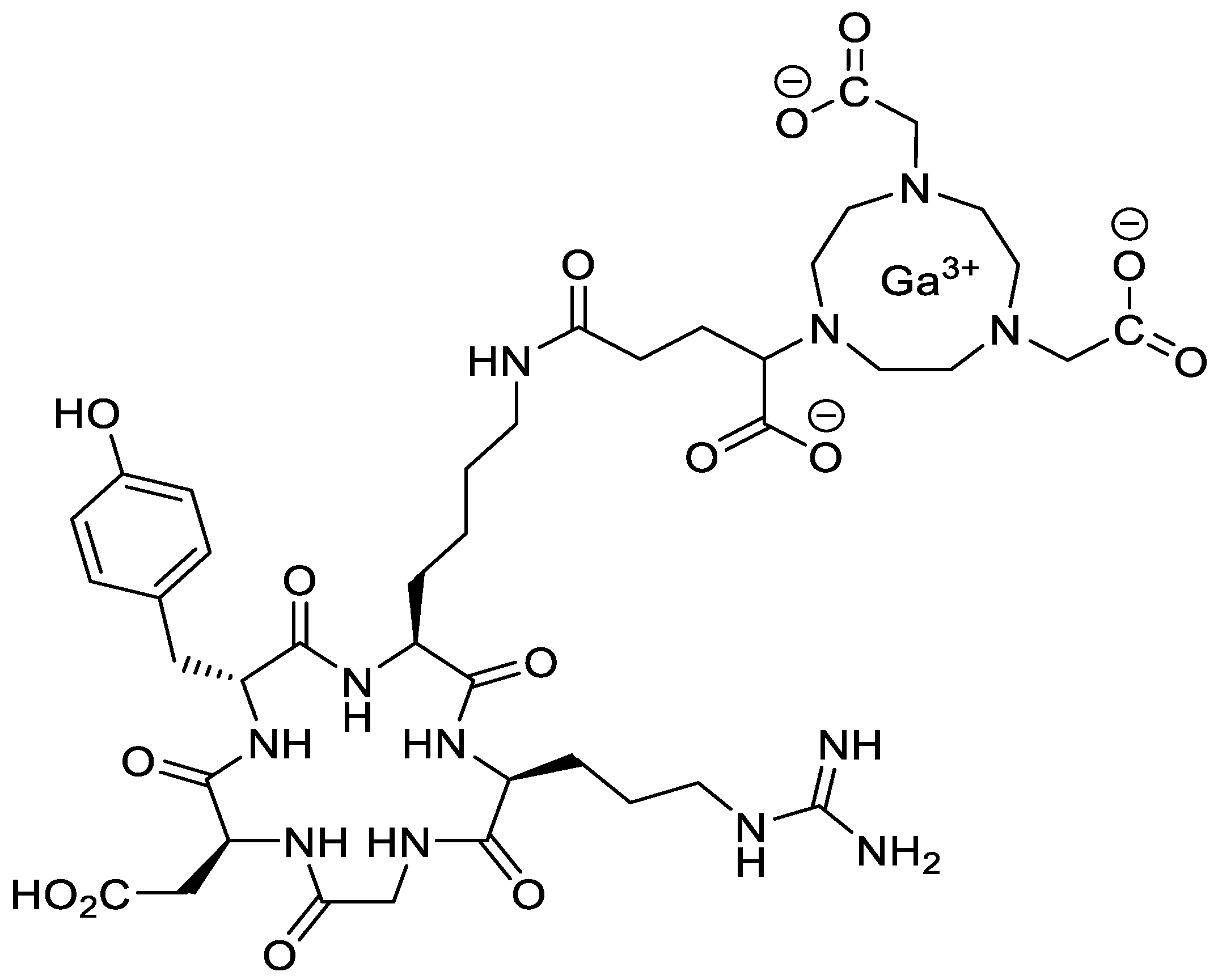
2.7. Tracer Preparation
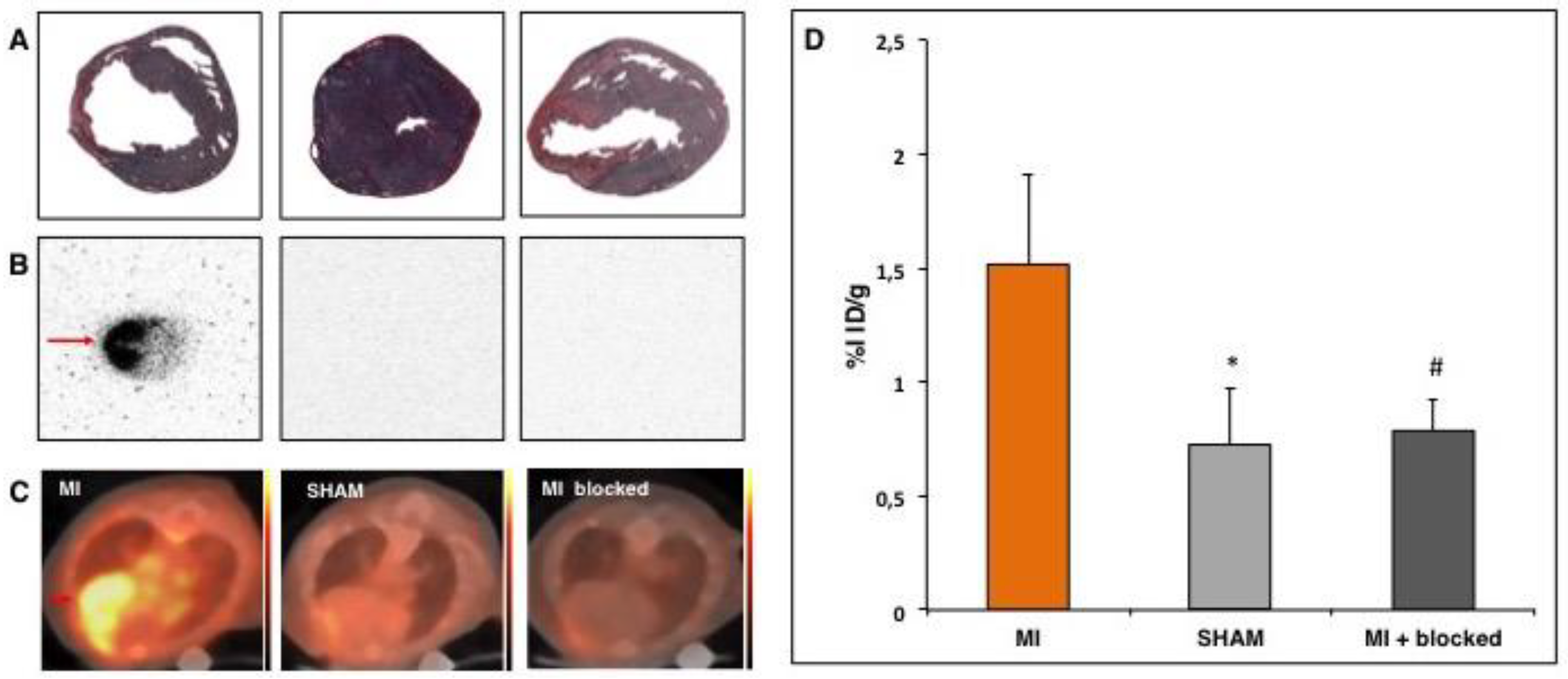
2.8. Autoradiography
2.9. Statistics
3. Results
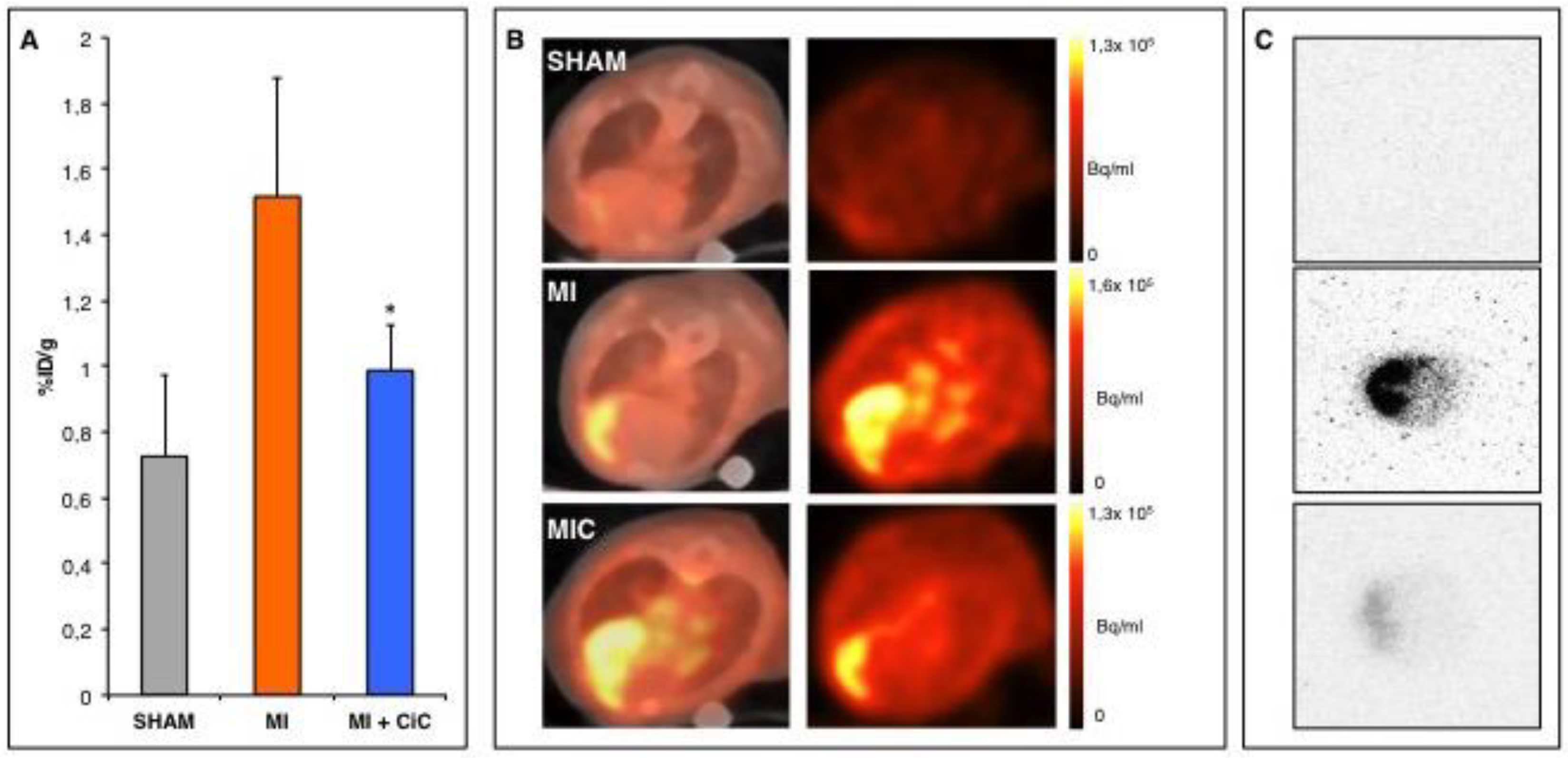
3.1. PET- Imaging of αvβ3-Integrin after Myocardial Infarction
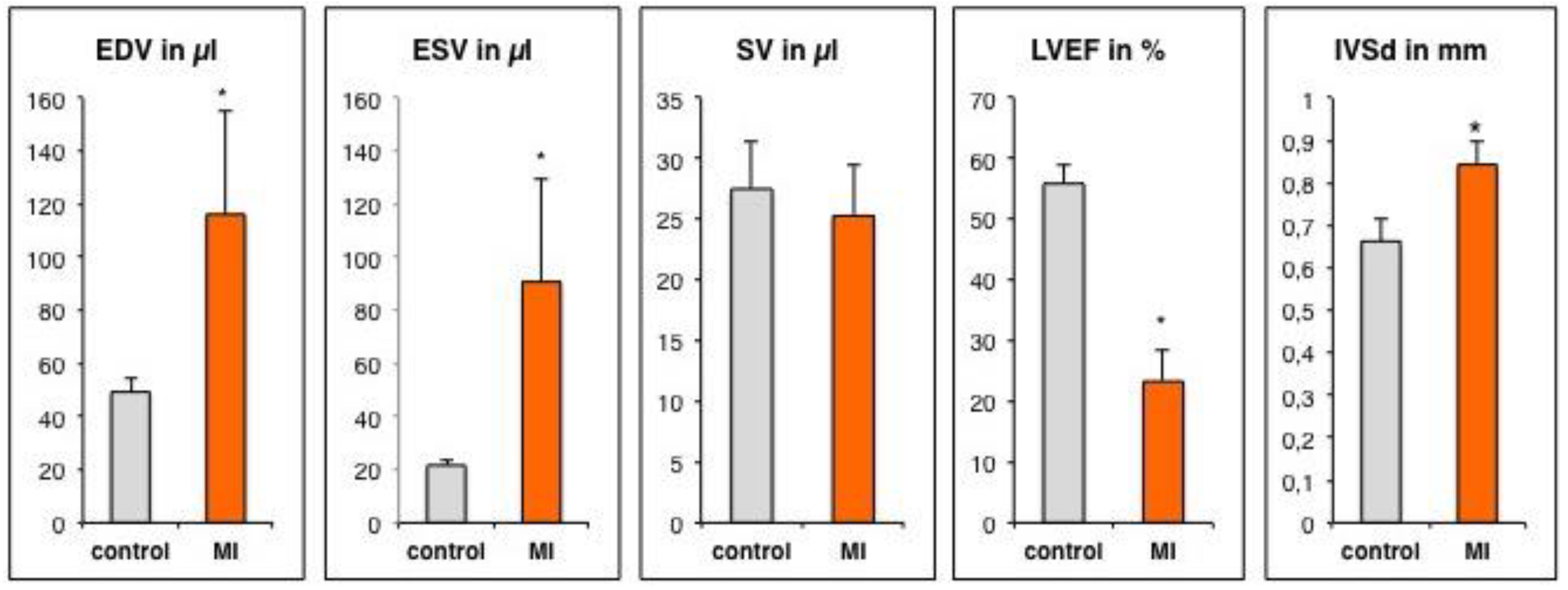
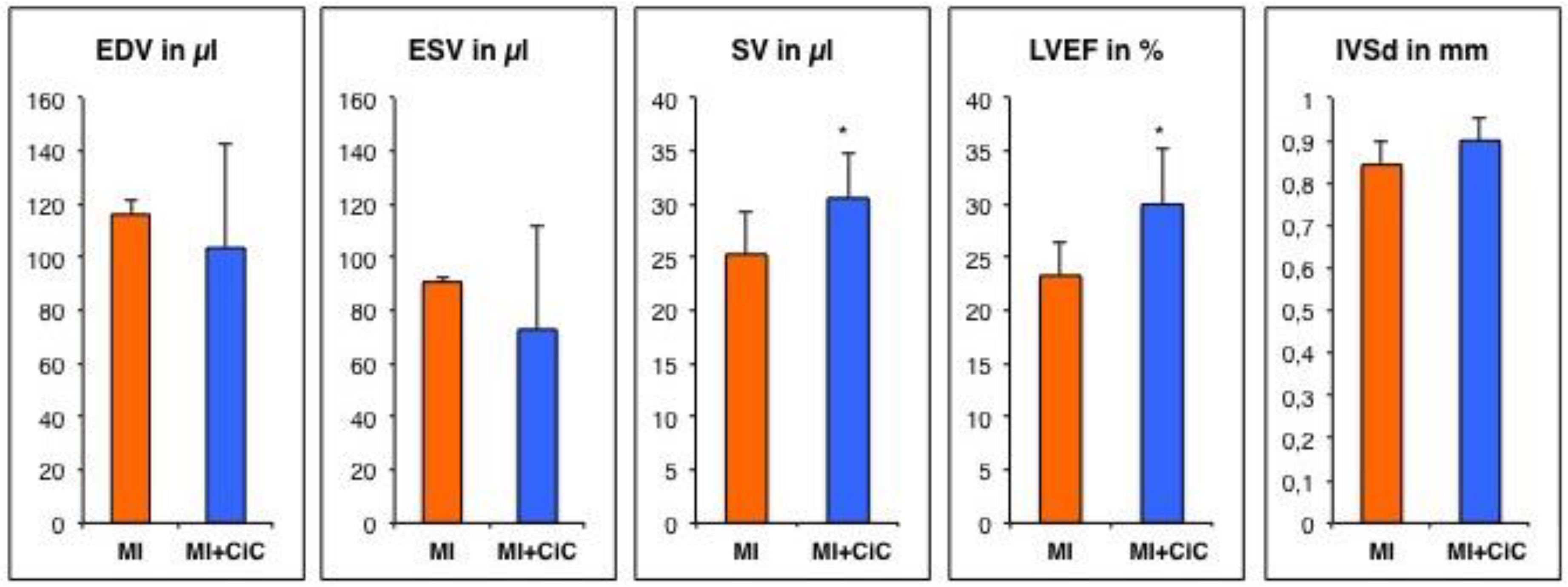
3.2. Assessment of Left Ventricular Remodeling Following AMI by the Use of MRI
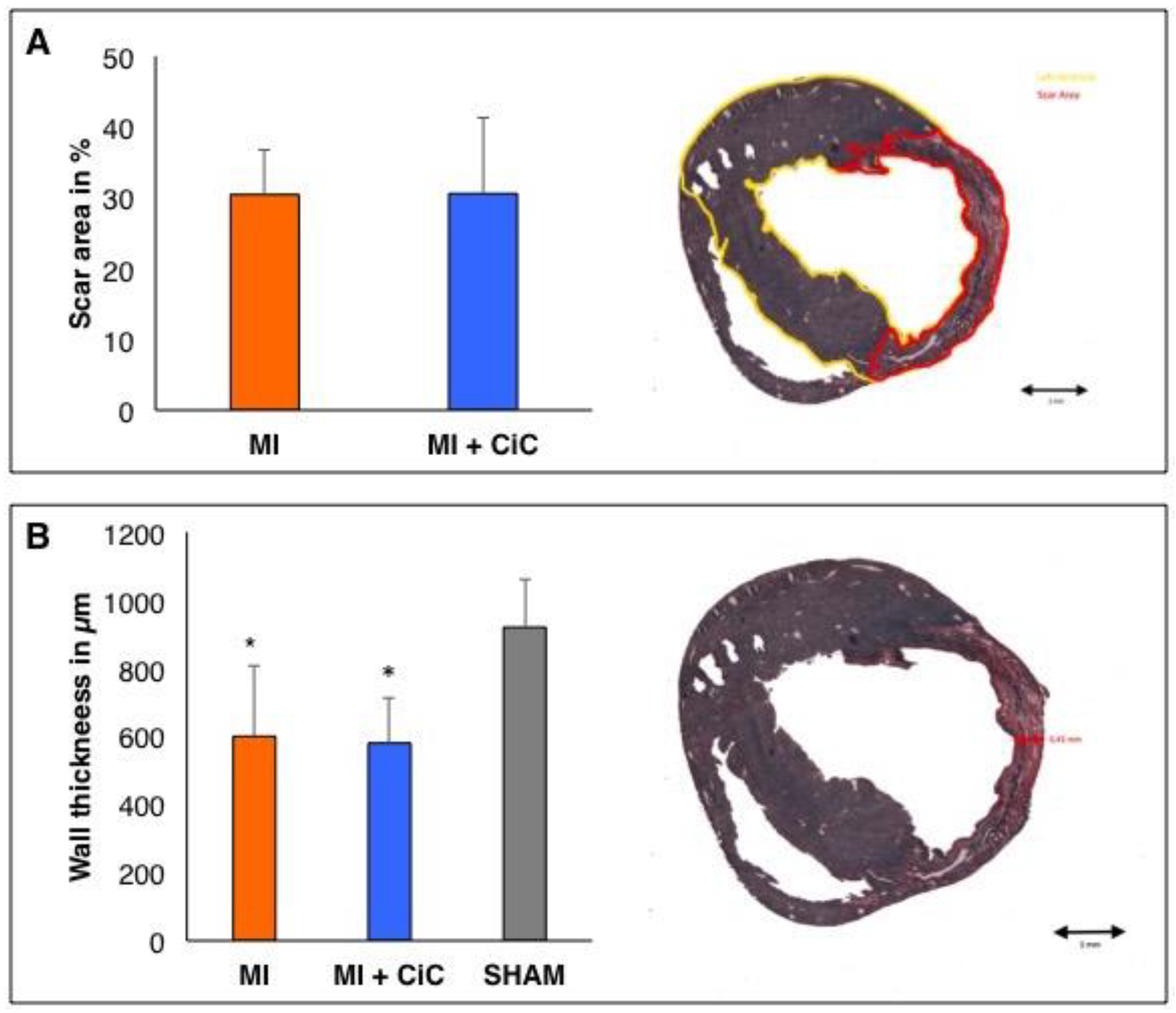
3.3. Effect of Cell Therapy on Cardiac Remodeling Following AMI
3.4. Effect of CiC Transplantation on Angiogenesis and Correlation with LV-Remodelling
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LAD | left anterior descending coronary artery |
| MI | myocardial infarction |
| CiC | cardiac induced cells |
| mESCs | murine embryonic stem cells |
| EB | embryoid bodies |
| LVEF | left ventricular ejection fraction |
| LV | left ventricle |
| PET | positron emission tomography |
| SPECT | single photon emission tomography |
| CMR | Cardiac magnetic resonance |
| CT | computer tomography |
| VOI | volumes of interest |
| IVSd | diastolic interventricular septal thickness |
| SD | standard deviation |
| EDV | end-diastolic volume |
| ESV | end-systolic volume |
| SV | stroke volume |
References
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The mechanistic basis of infarct healing. Antioxid. Redox Signal. 2006, 8, 1907–1939. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochain, C.; Channon, K.M.; Silvestre, J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef] [Green Version]
- Van der Laan, A.M.; Piek, J.J.; van Royen, N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat. Rev. Cardiol. 2009, 6, 515–523. [Google Scholar] [CrossRef]
- Mandic, L.; Traxler, D.; Gugerell, A.; Zlabinger, K.; Lukovic, D.; Pavo, N.; Goliasch, G.; Spannbauer, A.; Winkler, J.; Gyongyosi, M. Molecular Imaging of Angiogenesis in Cardiac Regeneration. Curr. Cardiovasc. Imaging Rep. 2016, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Hodivala-Dilke, K. αvβ3 integrin and angiogenesis: A moody integrin in a changing environment. Curr. Opin. Cell Biol. 2008, 20, 514–519. [Google Scholar] [CrossRef]
- Hendrikx, G.; De Saint-Hubert, M.; Dijkgraaf, I.; Bauwens, M.; Douma, K.; Wierts, R.; Pooters, I.; Van den Akker, N.M.; Hackeng, T.M.; Post, M.J.; et al. Molecular imaging of angiogenesis after myocardial infarction by (111)In-DTPA-cNGR and (99m)Tc-sestamibi dual-isotope myocardial SPECT. EJNMMI Res. 2015, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Gronman, M.; Tarkia, M.; Kiviniemi, T.; Halonen, P.; Kuivanen, A.; Savunen, T.; Tolvanen, T.; Teuho, J.; Kakela, M.; Metsala, O.; et al. Imaging of αvβ3 integrin expression in experimental myocardial ischemia with [(68)Ga]NODAGA-RGD positron emission tomography. J. Transl. Med. 2017, 15, 144. [Google Scholar] [CrossRef]
- Laitinen, I.; Notni, J.; Pohle, K.; Rudelius, M.; Farrell, E.; Nekolla, S.G.; Henriksen, G.; Neubauer, S.; Kessler, H.; Wester, H.J.; et al. Comparison of cyclic RGD peptides for αvβ3 integrin detection in a rat model of myocardial infarction. EJNMMI Res. 2013, 3, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherif, H.M.; Saraste, A.; Nekolla, S.G.; Weidl, E.; Reder, S.; Tapfer, A.; Rudelius, M.; Higuchi, T.; Botnar, R.M.; Wester, H.J.; et al. Molecular imaging of early αvβ3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J. Nucl. Med. 2012, 53, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Wei, H.J.; Lin, K.J.; Lin, W.W.; Wang, C.W.; Pan, W.Y.; Hwang, S.M.; Chang, Y.; Sung, H.W. Multimodality noninvasive imaging for assessing therapeutic effects of exogenously transplanted cell aggregates capable of angiogenesis on acute myocardial infarction. Biomaterials 2015, 73, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Gaebel, R.; Doering, P.; Mueller, P.; Lemcke, H.; Stenzel, J.; Lindner, T.; Kurth, J.; Steinhoff, G.; Vollmar, B.; et al. 18F-FDG PET-Based Imaging of Myocardial Inflammation Predicts a Functional Outcome Following Transplantation of mESC-Derived Cardiac Induced Cells in a Mouse Model of Myocardial Infarction. Cells 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, W.; Dunmore, J.H.; Fairchild-Huntress, V.; Fang, Q.; Auerbach, A.B.; Huszar, D.; Joyner, A.L. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques 2000, 29, 1024–1028, 1030, 1032. [Google Scholar] [CrossRef]
- Thiele, F.; Voelkner, C.; Krebs, V.; Muller, P.; Jung, J.J.; Rimmbach, C.; Steinhoff, G.; Noack, T.; David, R.; Lemcke, H. Nkx2.5 Based Ventricular Programming of Murine ESC-Derived Cardiomyocytes. Cell. Physiol. Biochem. 2019, 53, 337–354. [Google Scholar] [CrossRef]
- Rimmbach, C.; Jung, J.J.; David, R. Generation of murine cardiac pacemaker cell aggregates based on ES-cell-programming in combination with Myh6-promoter-selection. J. Vis. Exp. 2015, e52465. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.; Gaebel, R.; Lemcke, H.; Wiekhorst, F.; Hausburg, F.; Lang, C.; Zarniko, N.; Westphal, B.; Steinhoff, G.; David, R. Intramyocardial fate and effect of iron nanoparticles co-injected with MACS((R)) purified stem cell products. Biomaterials 2017, 135, 74–84. [Google Scholar] [CrossRef]
- Lang, C.; Lehner, S.; Todica, A.; Boening, G.; Franz, W.M.; Bartenstein, P.; Hacker, M.; David, R. Positron emission tomography based in-vivo imaging of early phase stem cell retention after intramyocardial delivery in the mouse model. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1730–1738. [Google Scholar] [CrossRef]
- Heiberg, E.; Sjogren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med. Imaging 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Thoms, M. The quantum efficiency of radiographic imaging with image plates. Nucl. Instrum. Methods Phys. Res. 1996, 378, 598–611. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maeda, K.; Takefuji, M.; Kikuchi, R.; Morishita, Y.; Hirashima, M.; Murohara, T. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci. Rep. 2017, 7, 7156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrikx, G.; Voo, S.; Bauwens, M.; Post, M.J.; Mottaghy, F.M. SPECT and PET imaging of angiogenesis and arteriogenesis in pre-clinical models of myocardial ischemia and peripheral vascular disease. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2433–2447. [Google Scholar] [CrossRef] [Green Version]
- Knetsch, P.A.; Petrik, M.; Griessinger, C.M.; Rangger, C.; Fani, M.; Kesenheimer, C.; von Guggenberg, E.; Pichler, B.J.; Virgolini, I.; Decristoforo, C.; et al. [68Ga]NODAGA-RGD for imaging αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1303–1312. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar]
- Haubner, R.; Weber, W.A.; Beer, A.J.; Vabuliene, E.; Reim, D.; Sarbia, M.; Becker, K.F.; Goebel, M.; Hein, R.; Wester, H.J.; et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005, 2, e70. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of αvβ3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Lang, L.; Guo, N.; Cao, F.; Quan, Q.; Hu, S.; Kiesewetter, D.O.; Niu, G.; Chen, X. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18F-AlF-NOTA-PRGD2. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Hedhli, J.; Czerwinski, A.; Schuelke, M.; Ploska, A.; Sowinski, P.; Hood, L.; Mamer, S.B.; Cole, J.A.; Czaplewska, P.; Banach, M.; et al. Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of αvβ3 Integrin Activity. Sci. Rep. 2017, 7, 3185. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Dobrucki, L.W.; Sadeghi, M.M.; Zhang, J.; Bourke, B.N.; Cavaliere, P.; Song, J.; Chow, C.; Jahanshad, N.; van Royen, N.; et al. Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation 2005, 111, 3255–3260. [Google Scholar] [CrossRef] [Green Version]
- Hedhli, J.; Slania, S.L.L.; Ploska, A.; Czerwinski, A.; Konopka, C.J.; Wozniak, M.; Banach, M.; Dobrucki, I.T.; Kalinowski, L.; Dobrucki, L.W. Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice. Sci. Rep. 2018, 8, 5401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohle, K.; Notni, J.; Bussemer, J.; Kessler, H.; Schwaiger, M.; Beer, A.J. 68Ga-NODAGA-RGD is a suitable substitute for (18)F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl. Med. Biol. 2012, 39, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Virag, J.I.; Murry, C.E. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am. J. Pathol. 2003, 163, 2433–2440. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, M.L.; Escobar, G.P.; Dobrucki, L.W.; Goshorn, D.K.; Bouges, S.; Mingoia, J.T.; McClister, D.M., Jr.; Su, H.; Gannon, J.; MacGillivray, C.; et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H232–H239. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Frangogiannis, N.G. Macrophages in the Remodeling Failing Heart. Circ. Res. 2016, 119, 776–778. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.I.; Wolfien, M.; Langenbach, A.; Muller, P.; Wolkenhauer, O.; Yavari, A.; Ince, H.; Steinhoff, G.; Krause, B.J.; David, R.; et al. Cardiac Cell Therapies for the Treatment of Acute Myocardial Infarction: A Meta-Analysis from Mouse Studies. Cell. Physiol. Biochem. 2017, 42, 254–268. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Jenkins, W.S.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T.; et al. Cardiac αvβ3 integrin expression following acute myocardial infarction in humans. Heart 2017, 103, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Vandervelde, S.; van Amerongen, M.J.; Tio, R.A.; Petersen, A.H.; van Luyn, M.J.; Harmsen, M.C. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc. Pathol. 2006, 15, 83–90. [Google Scholar] [CrossRef]
- Minatoguchi, S.; Takemura, G.; Chen, X.H.; Wang, N.; Uno, Y.; Koda, M.; Arai, M.; Misao, Y.; Lu, C.; Suzuki, K.; et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation 2004, 109, 2572–2580. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, C.I.; Döring, P.; Gäbel, R.; Vasudevan, P.; Lemcke, H.; Müller, P.; Stenzel, J.; Lindner, T.; Joksch, M.; Kurth, J.; et al. [68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy. Cells 2020, 9, 1358. https://doi.org/10.3390/cells9061358
Lang CI, Döring P, Gäbel R, Vasudevan P, Lemcke H, Müller P, Stenzel J, Lindner T, Joksch M, Kurth J, et al. [68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy. Cells. 2020; 9(6):1358. https://doi.org/10.3390/cells9061358
Chicago/Turabian StyleLang, Cajetan Immanuel, Piet Döring, Ralf Gäbel, Praveen Vasudevan, Heiko Lemcke, Paula Müller, Jan Stenzel, Tobias Lindner, Markus Joksch, Jens Kurth, and et al. 2020. "[68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy" Cells 9, no. 6: 1358. https://doi.org/10.3390/cells9061358





