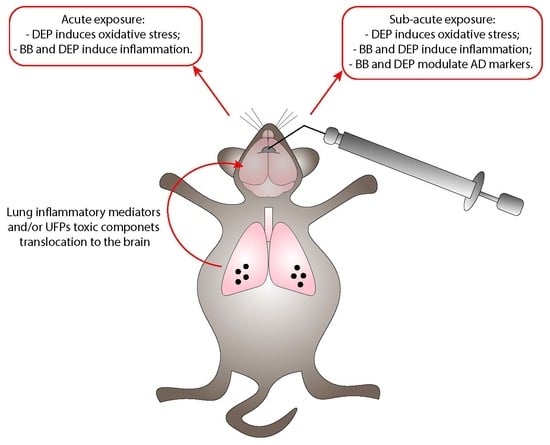
Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset
Abstract
:1. Introduction
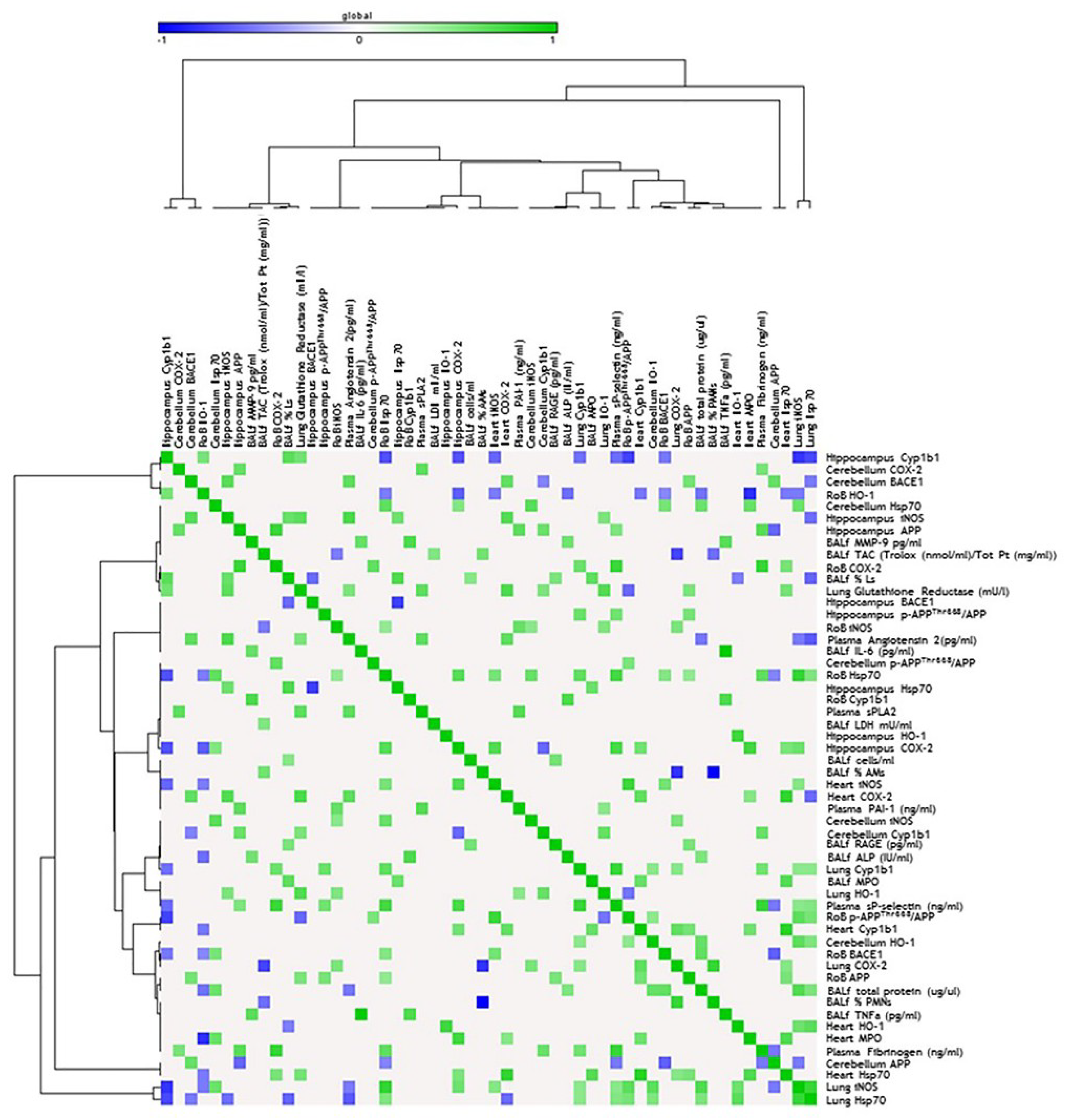
2. Results
2.1. BB and DEP Exposure Does Not Affect Brain Morphology
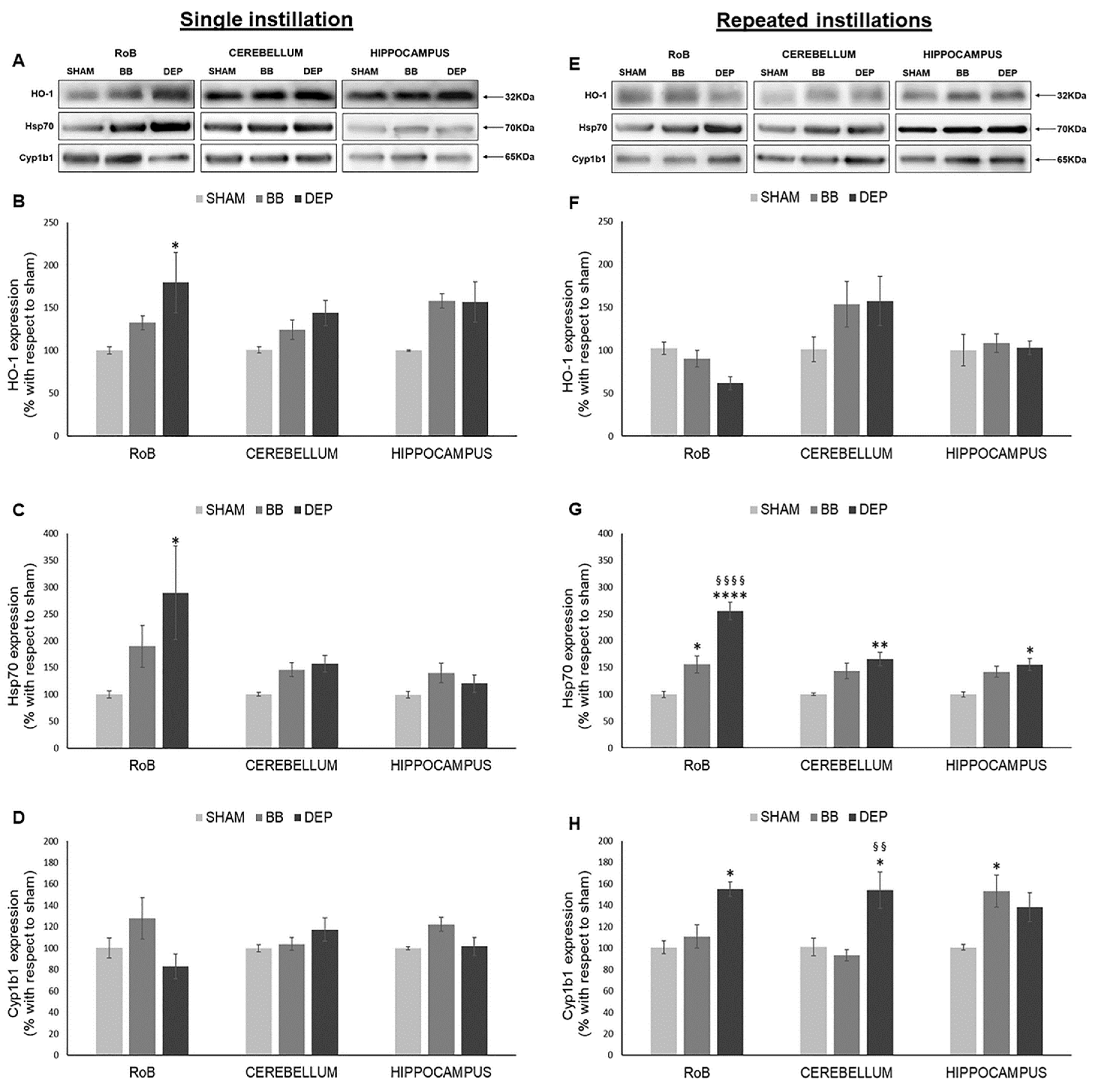
2.2. Induction of Oxidative Stress Related Proteins under BB and DEP Exposure
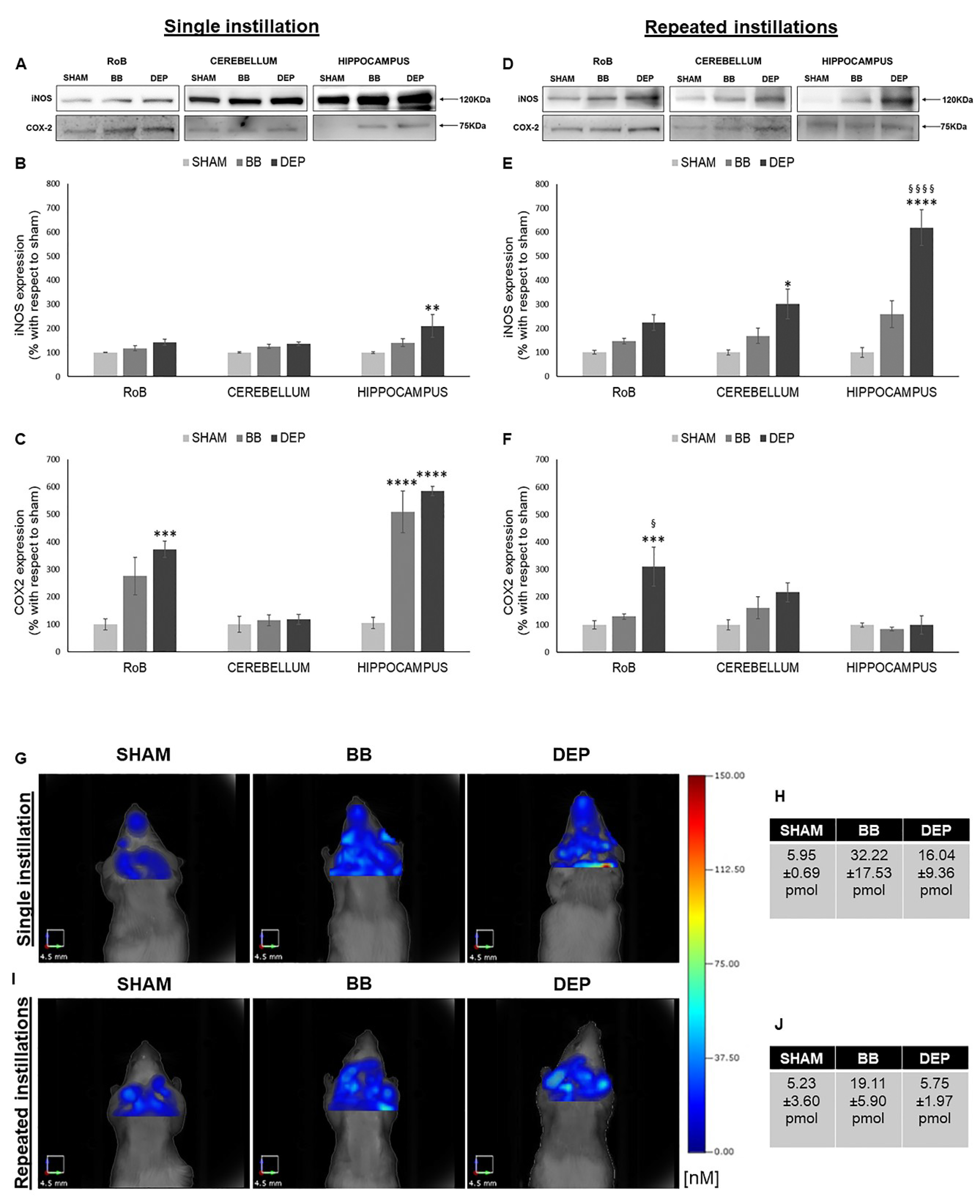
2.3. Induction of Inflammation-Related Proteins under BB and DEP Treatment
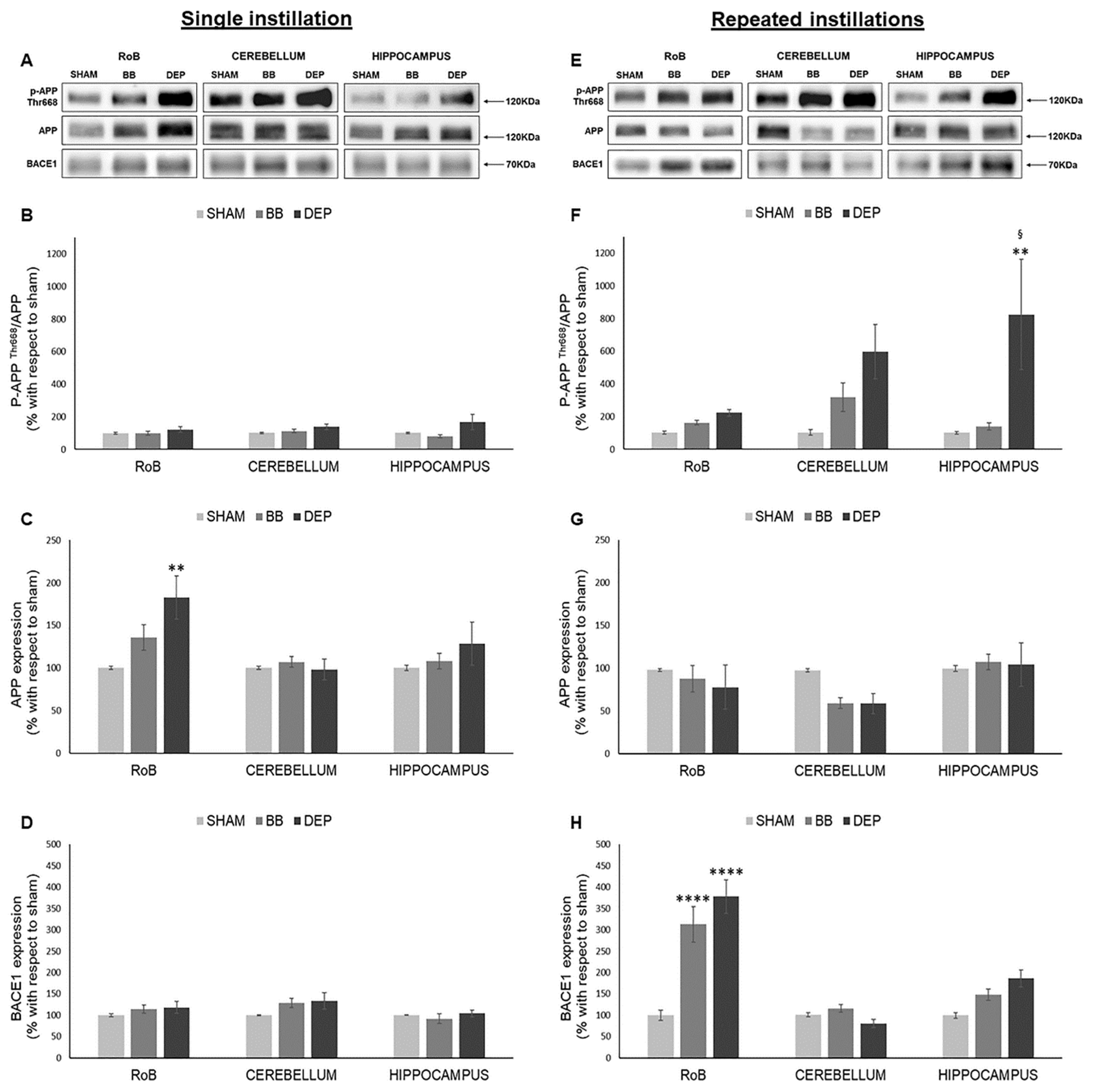
2.4. Evaluation of Amyloidogenic Precursor Protein Processing
3. Discussion
3.1. DEP Treatment Induces Higher Oxidative Stress Response Than BB in Mouse Brain
3.2. Both BB and DEP Treatment Induces Inflammation in Mouse Brain
3.3. UFP Treatment Induces Changes of AD-Related Proteins in Mouse Brain
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. UFPs Characterization
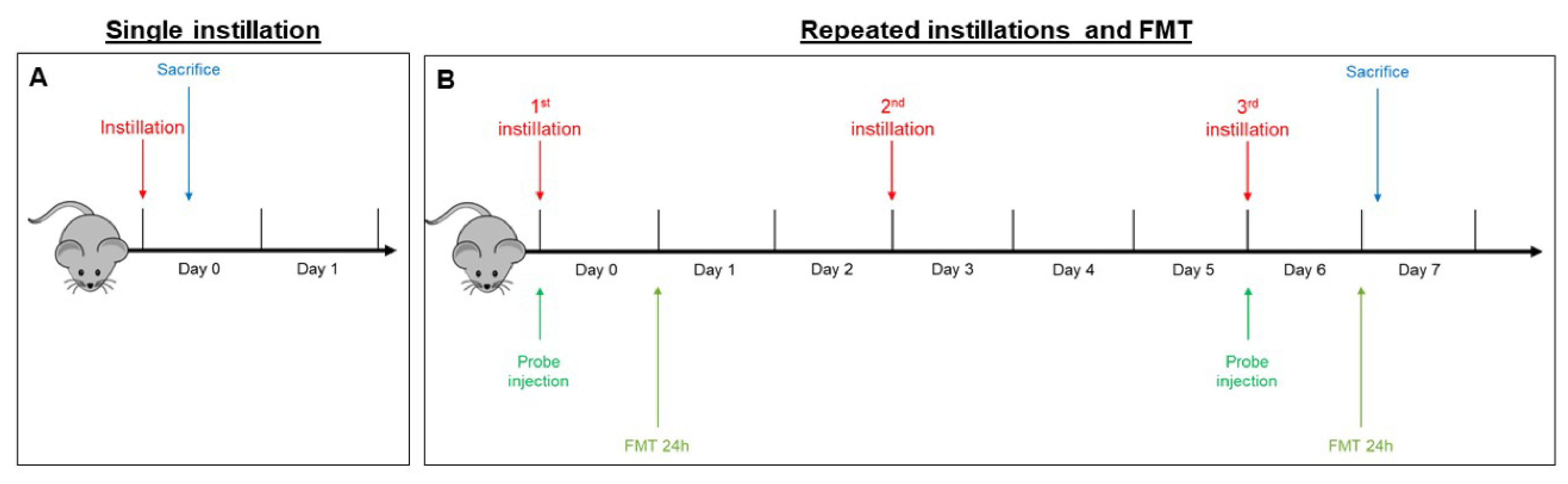
4.4. Intratracheal Instillation
- In the sub-acute exposure setting, the intratracheal instillation was performed on days 0, 3, and 6, for a total of three instillations. Mice were euthanized 24 h following the last instillation [33,84,85], to understand if the effect of the single individual instillation was amplified when repeated or if a normal condition was restored (Figure 6B).
4.5. Fluorescent Molecular Tomography
4.6. Brain Dissection
4.7. Tissues Homogenization
4.8. Histopathological Analyses
4.9. SDS-PAGE Electrophoresis and Immunoblotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, K.K.; Miller, M.R.; Shah, A.S.V. Air Pollution and Stroke. J. Stroke 2018, 20, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Cassee, F.R.; Heroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Schmid, O.; Moller, W.; Semmler-Behnke, M.; Ferron, G.A.; Karg, E.; Lipka, J.; Schulz, H.; Kreyling, W.G.; Stoeger, T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 2009, 14 (Suppl. 1), 67–73. [Google Scholar] [CrossRef] [PubMed]
- ARPA Lombardia. INEMAR: INventario EMissioni ARia. Available online: http://inemar.arpalombardia.it/inemar/webdata/elab_standard_reg.seam (accessed on 31 March 2020).
- Longhin, E.M.; Mantecca, P.; Gualtieri, M. Fifteen Years of Airborne Particulates in Vitro Toxicology in Milano: Lessons and Perspectives Learned. Int. J. Mol. Sci. 2020, 21, 2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovelli, S.; Cattaneo, A.; Borghi, F.; Spinazzè, A.; Campagnolo, D.; Limbeck, A.; Cavallo, D.M. Mass Concentration and Size-Distribution of Atmospheric Particulate Matter in an Urban Environment. Aerosol Air Qual. Res. 2017, 17, 1142–1155. [Google Scholar] [CrossRef]
- Zheng, M.; Cass, G.R.; Ke, L.; Wang, F.; Schauer, J.J.; Edgerton, E.S.; Russell, A.G. Source apportionment of daily fine particulate matter at Jefferson Street, Atlanta, GA, during summer and winter. J. Air Waste Manag. Assoc. 2007, 57, 228–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhin, E.; Gualtieri, M.; Capasso, L.; Bengalli, R.; Mollerup, S.; Holme, J.A.; Ovrevik, J.; Casadei, S.; Di Benedetto, C.; Parenti, P.; et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ. Pollut. 2016, 215, 366–375. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Forsberg, B.; Annesi-Maesano, I.; Blomberg, A.; Bolling, A.; Boman, C.; Bonlokke, J.; Brauer, M.; Bruce, N.; Heroux, M.E.; et al. Health impacts of anthropogenic biomass burning in the developed world. Eur. Respir. J. 2015, 46, 1577–1588. [Google Scholar] [CrossRef] [Green Version]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.V.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.F.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Respir. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Sydbom, A.; Blomberg, A.; Parnia, S.; Stenfors, N.; Sandstrom, T.; Dahlen, S.E. Health effects of diesel exhaust emissions. Eur. Respir. J. 2001, 17, 733–746. [Google Scholar] [CrossRef]
- IARC. Air Pollution and Cancer; IARC Library Cataloguing in Publication Data: Lyon, France, 2013; ISBN 978-92-832-2166-1.
- Oberdorster, G.; Utell, M.J. Ultrafine particles in the urban air: To the respiratory tract and beyond? Environ. Health Perspect. 2002, 110, A440–A441. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Wallenborn, J.G.; McGee, J.K.; Schladweiler, M.C.; Ledbetter, A.D.; Kodavanti, U.P. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol. Sci. 2007, 98, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [Green Version]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. 4), 547–551. [Google Scholar]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdorster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health A 2002, 65, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Zia, S.; Subramaniyan, D.; Al-Amri, I.; Al Kindi, M.A.; Ali, B.H. Interaction of diesel exhaust particles with human, rat and mouse erythrocytes in vitro. Cell Physiol. Biochem. 2012, 29, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schurch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Feng, W.Y.; Wang, M.; Shi, J.W.; Zhang, F.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F.; Huang, Y.Y.; Xie, Y.N.; et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: Micro-distribution, chemical states, and histopathological observation. Biol. Trace Elem. Res. 2007, 118, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Elder, A.; Rinderknecht, A. Nanoparticles and the brain: Cause for concern? J. Nanosci. Nanotechnol. 2009, 9, 4996–5007. [Google Scholar] [CrossRef] [Green Version]
- Calderon-Garciduenas, L.; Calderon-Garciduenas, A.; Torres-Jardon, R.; Avila-Ramirez, J.; Kulesza, R.J.; Angiulli, A.D. Air pollution and your brain: What do you need to know right now. Prim. Health Care Res. Dev. 2015, 16, 329–345. [Google Scholar] [CrossRef] [Green Version]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef]
- Seagrave, J.; McDonald, J.D.; Reed, M.D.; Seilkop, S.K.; Mauderly, J.L. Responses to subchronic inhalation of low concentrations of diesel exhaust and hardwood smoke measured in rat bronchoalveolar lavage fluid. Inhal. Toxicol. 2005, 17, 657–670. [Google Scholar] [CrossRef]
- Totlandsdal, A.I.; Ovrevik, J.; Cochran, R.E.; Herseth, J.I.; Bolling, A.K.; Lag, M.; Schwarze, P.; Lilleaas, E.; Holme, J.A.; Kubatova, A. The occurrence of polycyclic aromatic hydrocarbons and their derivatives and the proinflammatory potential of fractionated extracts of diesel exhaust and wood smoke particles. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2014, 49, 383–396. [Google Scholar] [CrossRef]
- Farina, F.; Lonati, E.; Milani, C.; Massimino, L.; Ballarini, E.; Donzelli, E.; Crippa, L.; Marmiroli, P.; Botto, L.; Corsetto, P.A.; et al. In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. Int. J. Mol. Sci. 2019, 20, 2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Mora-Tiscareno, A.; Styner, M.; Gomez-Garza, G.; Zhu, H.; Torres-Jardon, R.; Carlos, E.; Solorio-Lopez, E.; Medina-Cortina, H.; Kavanaugh, M.; et al. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J. Alzheimers Dis. 2012, 31, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Solt, A.C.; Henriquez-Roldan, C.; Torres-Jardon, R.; Nuse, B.; Herritt, L.; Villarreal-Calderon, R.; Osnaya, N.; Stone, I.; Garcia, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [PubMed]
- Lee, M.S.; Kao, S.C.; Lemere, C.A.; Xia, W.; Tseng, H.C.; Zhou, Y.; Neve, R.; Ahlijanian, M.K.; Tsai, L.H. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 2003, 163, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.A.; Kim, H.S.; Ha, T.Y.; Ha, J.W.; Shin, K.Y.; Jeong, Y.H.; Lee, J.P.; Park, C.H.; Kim, S.; Baik, T.K.; et al. Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol. Cell. Biol. 2006, 26, 4327–4338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlofs-Nijland, M.E.; van Berlo, D.; Cassee, F.R.; Schins, R.P.; Wang, K.; Campbell, A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part. Fibre Toxicol. 2010, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Van Berlo, D.; Albrecht, C.; Knaapen, A.M.; Cassee, F.R.; Gerlofs-Nijland, M.E.; Kooter, I.M.; Palomero-Gallagher, N.; Bidmon, H.J.; van Schooten, F.J.; Krutmann, J.; et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch. Toxicol. 2010, 84, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Levesque, S.; Taetzsch, T.; Lull, M.E.; Kodavanti, U.; Stadler, K.; Wagner, A.; Johnson, J.A.; Duke, L.; Kodavanti, P.; Surace, M.J.; et al. Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 2011, 119, 1149–1155. [Google Scholar] [CrossRef] [Green Version]
- Farina, F.; Milani, C.; Botto, L.; Lonati, E.; Bulbarelli, A.; Palestini, P. Involvement of MEK-ERK1-2 pathway in the anti-oxidant response in C6 glioma cells after diesel exhaust particles exposure. Toxicol. Lett. 2016, 250–251, 57–65. [Google Scholar] [CrossRef]
- Milani, C.; Corsetto, P.A.; Farina, F.; Botto, L.; Lonati, E.; Massimino, L.; Rizzo, A.M.; Bulbarelli, A.; Palestini, P. Early evidence of stress in immortalized neurons exposed to diesel particles: The role of lipid reshaping behind oxidative stress and inflammation. Toxicology 2018, 409, 63–72. [Google Scholar] [CrossRef]
- Farina, F.; Lonati, E.; Brambilla, A.; Dal Magro, R.; Milani, C.; Botto, L.; Sancini, G.; Palestini, P.; Bulbarelli, A. Diesel exhaust particles (DEP) pre-exposure contributes to the anti-oxidant response impairment in hCMEC/D3 during post-oxygen and glucose deprivation damage. Toxicol. Lett. 2017, 274, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kocbach Bolling, A.; Pagels, J.; Yttri, K.E.; Barregard, L.; Sallsten, G.; Schwarze, P.E.; Boman, C. Health effects of residential wood smoke particles: The importance of combustion conditions and physicochemical particle properties. Part. Fibre Toxicol. 2009, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaitis, C.P.; Leong, B.K.; Rop, D.A.; Aaron, C.S. Validation of intratracheal instillation as an alternative for aerosol inhalation toxicity testing. J. Appl. Toxicol. 1999, 19, 133–140. [Google Scholar] [CrossRef]
- Block, M.L.; Calderon-Garciduenas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Chen, J. Heme oxygenase in neuroprotection: From mechanisms to therapeutic implications. Rev. Neurosci. 2014, 25, 269–280. [Google Scholar] [CrossRef]
- Rojas, M.; Godschalk, R.; Alexandrov, K.; Cascorbi, I.; Kriek, E.; Ostertag, J.; Van Schooten, F.J.; Bartsch, H. Myeloperoxidase—463A variant reduces benzo[a]pyrene diol epoxide DNA adducts in skin of coal tar treated patients. Carcinogenesis 2001, 22, 1015–1018. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, J.K.; Park, S.H.; Kim, B.G.; Jang, A.S.; Oh, S.H.; Lee, J.H.; Suh, M.W.; Park, M.K. Effects of inhaled particulate matter on the central nervous system in mice. Neurotoxicology 2018, 67, 169–177. [Google Scholar] [CrossRef]
- Kido, T.; Bai, N.; Yatera, K.; Suzuki, H.; Meredith, A.; Mukae, H.; Rosenfeld, M.E.; van Eeden, S.F. Diesel exhaust inhalation induces heat shock protein 70 expression in vivo. Inhal. Toxicol. 2011, 23, 593–601. [Google Scholar] [CrossRef]
- Graff, D.W.; Cascio, W.E.; Brackhan, J.A.; Devlin, R.B. Metal particulate matter components affect gene expression and beat frequency of neonatal rat ventricular myocytes. Environ. Health Perspect. 2004, 112, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Rossner, P., Jr.; Binkova, B.; Sram, R.J. Heat shock proteins hsp32 and hsp70 as biomarkers of an early response? In vitro induction of heat shock proteins after exposure of cell culture to carcinogenic compounds and their real mixtures. Mutagenes. Res. 2003, 542, 105–116. [Google Scholar]
- Mossman, B.T.; Borm, P.J.; Castranova, V.; Costa, D.L.; Donaldson, K.; Kleeberger, S.R. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part. Fibre Toxicol. 2007, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kure, E.H.; Andreassen, A.; Ovrebo, S.; Grzybowska, E.; Fiala, Z.; Strozyk, M.; Chorazy, M.; Haugen, A. Benzo(a)pyrene-albumin adducts in humans exposed to polycyclic aromatic hydrocarbons in an industrial area of Poland. Occup. Environ. Med. 1997, 54, 662–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henriquez-Roldan, C.; Schoonhoven, R.; Acuna-Ayala, H.; Villarreal-Calderon, A.; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol. Pathol. 2003, 31, 524–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Reed, W.; Maronpot, R.R.; Henriquez-Roldan, C.; Delgado-Chavez, R.; Calderon-Garciduenas, A.; Dragustinovis, I.; Franco-Lira, M.; Aragon-Flores, M.; Solt, A.C.; et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 2004, 32, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Sancini, G.; Battaglia, C.; Tinaglia, V.; Mantecca, P.; Camatini, M.; Palestini, P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS ONE 2013, 8, e56636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Zhao, J.; Ge, J.; Yang, J.; Song, X.; Wang, C.; Mao, J.; Zhang, Y.; Zou, Y.; Liu, Y.; et al. Particulate Matter Facilitates C6 Glioma Cells Activation and the Release of Inflammatory Factors Through MAPK and JAK2/STAT3 Pathways. Neurochem. Res. 2016, 41, 1969–1981. [Google Scholar] [CrossRef]
- Malyshev, I.; Manukhina, E.B.; Mikoyan, V.D.; Kubrina, L.N.; Vanin, A.F. Nitric oxide is involved in heat-induced HSP70 accumulation. FEBS Lett. 1995, 370, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, L.J.; Fan, S.J.; Jiang, H.; Pan, F. Swimming exercise effects on the expression of HSP70 and iNOS in hippocampus and prefrontal cortex in combined stress. Neurosci. Lett. 2010, 476, 99–103. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharm. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) inhibitors: Future therapeutic strategies for epilepsy management. J. Neuroinflamm. 2019, 16, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, D.P.; Puig, K.L.; Gorr, M.W.; Wold, L.E.; Combs, C.K. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS ONE 2015, 10, e0127102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissinen, L.; Kahari, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, H.A.; Lucero, J.; Guyot, A.C.; Herbert, L.M.; McDonald, J.D.; Mabondzo, A.; Lund, A.K. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part. Fibre Toxicol. 2013, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Lucero, J.; Suwannasual, U.; Herbert, L.M.; McDonald, J.D.; Lund, A.K. The role of the lectin-like oxLDL receptor (LOX-1) in traffic-generated air pollution exposure-mediated alteration of the brain microvasculature in Apolipoprotein (Apo) E knockout mice. Inhal. Toxicol. 2017, 29, 266–281. [Google Scholar] [CrossRef]
- Latronico, T.; Brana, M.T.; Merra, E.; Fasano, A.; Di Bari, G.; Casalino, E.; Liuzzi, G.M. Impact of manganese neurotoxicity on MMP-9 production and superoxide dismutase activity in rat primary astrocytes. Effect of resveratrol and therapeutical implications for the treatment of CNS diseases. Toxicol. Sci. 2013, 135, 218–228. [Google Scholar] [CrossRef]
- Becaria, A.; Lahiri, D.K.; Bondy, S.C.; Chen, D.; Hamadeh, A.; Li, H.; Taylor, R.; Campbell, A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol. 2006, 176, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Maloney, B.; Sambamurti, K.; Zawia, N.; Lahiri, D.K. Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr. Alzheimer Res. 2012, 9, 589–599. [Google Scholar] [CrossRef]
- Bondy, S.C. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology 2016, 341–343, 41–46. [Google Scholar] [CrossRef]
- Claeysen, S.; Cochet, M.; Donneger, R.; Dumuis, A.; Bockaert, J.; Giannoni, P. Alzheimer culprits: Cellular crossroads and interplay. Cell. Signal. 2012, 24, 1831–1840. [Google Scholar] [CrossRef]
- Pajak, B.; Kania, E.; Orzechowski, A. Killing Me Softly: Connotations to Unfolded Protein Response and Oxidative Stress in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1805304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, E.; Small, D.H. Insights into the physiological function of the beta-amyloid precursor protein: Beyond Alzheimer’s disease. J. Neurochem. 2014, 129, 756–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirouac, L.; Rajic, A.J.; Cribbs, D.H.; Padmanabhan, J. Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer’s Disease. eNeuro 2017, 4, 0149-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchsinger, J.A.; Reitz, C.; Honig, L.S.; Tang, M.X.; Shea, S.; Mayeux, R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005, 65, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Sancini, G.; Farina, F.; Battaglia, C.; Cifola, I.; Mangano, E.; Mantecca, P.; Camatini, M.; Palestini, P. Health risk assessment for air pollutants: Alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS ONE 2014, 9, e109685. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Zwier, R.; Junginger, H.E.; Borchard, G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur. J. Pharm. Biopharm. 2005, 61, 214–218. [Google Scholar] [CrossRef]
- Mantecca, P.; Sancini, G.; Moschini, E.; Farina, F.; Gualtieri, M.; Rohr, A.; Miserocchi, G.; Palestini, P.; Camatini, M. Lung toxicity induced by intratracheal instillation of size-fractionated tire particles. Toxicol. Lett. 2009, 189, 206–214. [Google Scholar] [CrossRef]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Mantecca, P.; Gallinotti, D.; Camatini, M.; Palestini, P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol. Lett. 2011, 202, 209–217. [Google Scholar] [CrossRef]
- Kaewamatawong, T.; Shimada, A.; Morita, T.; Banlunara, W.; Bintvihok, A. Acute and Subacute Pulmonary Effects of Diesel Exhaust Particles in Mice: Pathological Changes and Translocation Pathways to the Circulation. Thai J. Vet. Med. 2009, 39, 311–318. [Google Scholar]
- Win-Shwe, T.T.; Fujitani, Y.; Sone, H.; Furuyama, A.; Nitta, H.; Hirano, S. Effects of acute single intranasal instillation of secondary organic aerosol on neurological and immunological biomarkers in the brain and lung of BALB/c mice. J. Toxicol. Sci. 2013, 38, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Happo, M.S.; Salonen, R.O.; Halinen, A.I.; Jalava, P.I.; Pennanen, A.S.; Dormans, J.A.; Gerlofs-Nijland, M.E.; Cassee, F.R.; Kosma, V.M.; Sillanpaa, M.; et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal. Toxicol. 2010, 22, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Saunders, V.; Breysse, P.; Clark, J.; Sproles, A.; Davila, M.; Wills-Karp, M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ. Health Perspect. 2010, 118, 640–646. [Google Scholar] [CrossRef]
- Tsurumi, C.; Esser, N.; Firat, E.; Gaedicke, S.; Follo, M.; Behe, M.; Elsasser-Beile, U.; Grosu, A.L.; Graeser, R.; Niedermann, G. Non-invasive in vivo imaging of tumor-associated CD133/prominin. PLoS ONE 2010, 5, e15605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spijker, S. Dissection of Rodent Brain Regions. In Neuroproteomics; Li, K.W., Ed.; Humana Press: New York, NY, USA; Heidelberg, Germany, 2011; Volume 57, pp. 23–26. [Google Scholar]
- Hagihara, H.; Toyama, K.; Yamasaki, N.; Miyakawa, T. Dissection of hippocampal dentate gyrus from adult mouse. J. Vis. Exp. 2009, 33, e1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Shen, Y. An old method facing a new challenge: Re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013, 92, 747–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritz, C.P. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics 2017, 17, 1600189. [Google Scholar] [CrossRef] [Green Version]
- Daffara, R.; Botto, L.; Beretta, E.; Conforti, E.; Faini, A.; Palestini, P.; Miserocchi, G. Endothelial cells as early sensors of pulmonary interstitial edema. J. Appl. Physiol. (1985) 2004, 97, 1575–1583. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Kocbach, A.; Namork, E.; Schwarze, P.E. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology 2008, 24, 123–132. [Google Scholar] [CrossRef]

| Element | Unit | DEP | BB |
|---|---|---|---|
| Al | ng/µg | 135 ± 4 | ND |
| K | ng/µg | 50 ± 0.02 | 195 ± 12.5 |
| Ca | ng/µg | 198 ± 8 | 70 ± 4 |
| Fe | ng/µg | 4 ± 0.001 | ND |
| Zn | ng/µg | 70 ± 2 | 4 ± 0.001 |
| Cr | ng/µg | 0.04 ± 0.001 | ND |
| Mn | ng/µg | 0.03 ± 0.001 | 0.42 ± 0.03 |
| V | ng/µg | 0.05 ± 0.007 | ND |
| Ni | ng/µg | 0.02 ± 0.001 | ND |
| Pb | ng/µg | 0.02 ± 0.001 | ND |
| Total PAHs | ng/mg | 600 ± 150 | 50 ± 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, C.; Farina, F.; Botto, L.; Massimino, L.; Lonati, E.; Donzelli, E.; Ballarini, E.; Crippa, L.; Marmiroli, P.; Bulbarelli, A.; et al. Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. Int. J. Mol. Sci. 2020, 21, 3699. https://doi.org/10.3390/ijms21103699
Milani C, Farina F, Botto L, Massimino L, Lonati E, Donzelli E, Ballarini E, Crippa L, Marmiroli P, Bulbarelli A, et al. Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. International Journal of Molecular Sciences. 2020; 21(10):3699. https://doi.org/10.3390/ijms21103699
Chicago/Turabian StyleMilani, Chiara, Francesca Farina, Laura Botto, Luca Massimino, Elena Lonati, Elisabetta Donzelli, Elisa Ballarini, Luca Crippa, Paola Marmiroli, Alessandra Bulbarelli, and et al. 2020. "Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset" International Journal of Molecular Sciences 21, no. 10: 3699. https://doi.org/10.3390/ijms21103699