Selected Atherosclerosis-Related Diseases May Differentially Affect the Relationship between Plasma Advanced Glycation End Products, Receptor sRAGE, and Uric Acid
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.1.1. AAA and AIOD Patients
2.1.2. CKD Patients
2.2. Sample Collection
2.3. Laboratory Analysis
2.3.1. AGEs Assay Kit (Cell Biolabs, Inc., San Diego, CA, USA)
2.3.2. Receptor sRAGE (RayBiotech, Norcross, Peachtree Corners, GA, USA)
2.3.3. hsCRP (DRG International Inc., Springfield Township, NJ, USA)
2.4. Statistical Analysis
3. Results
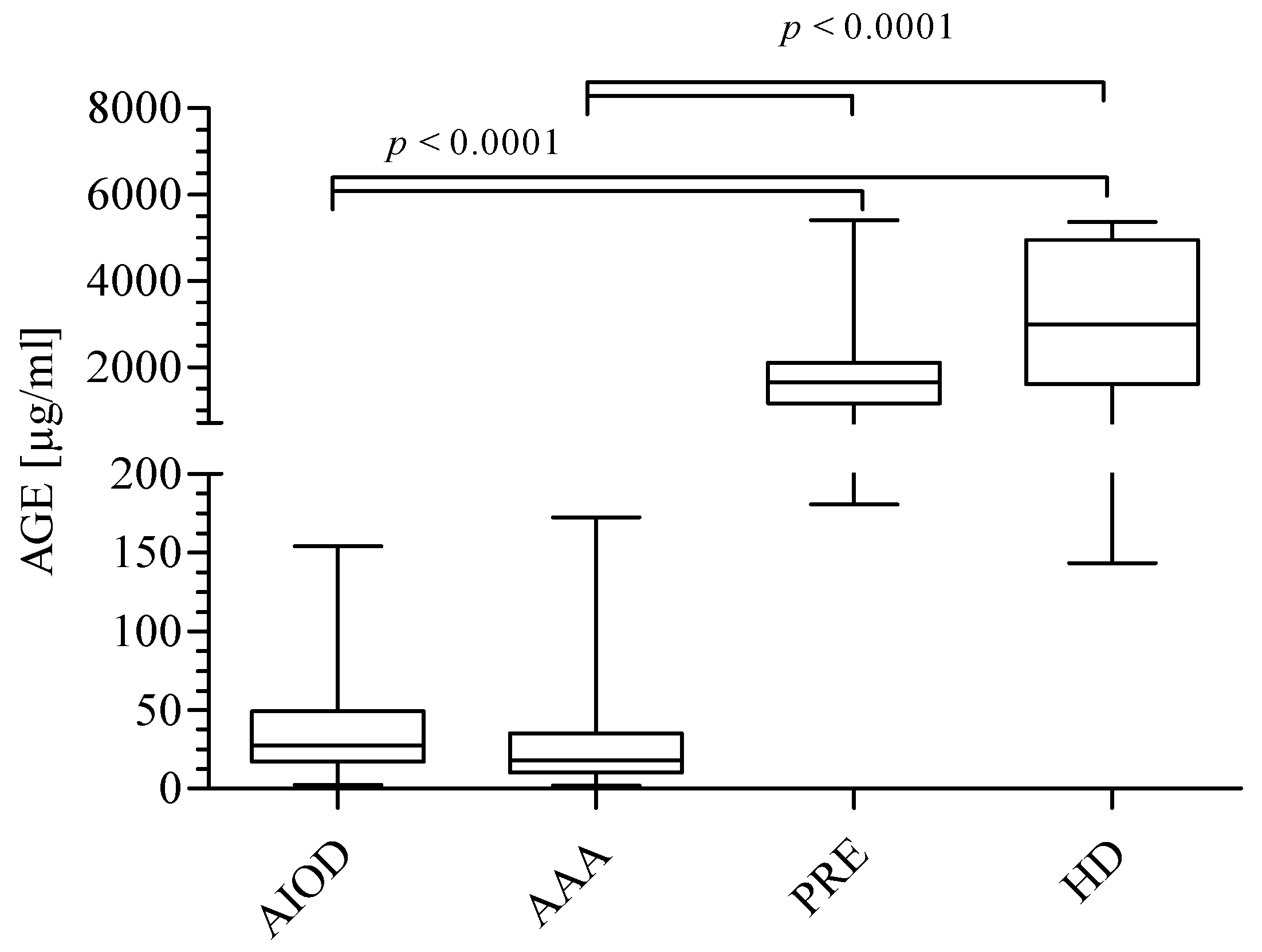
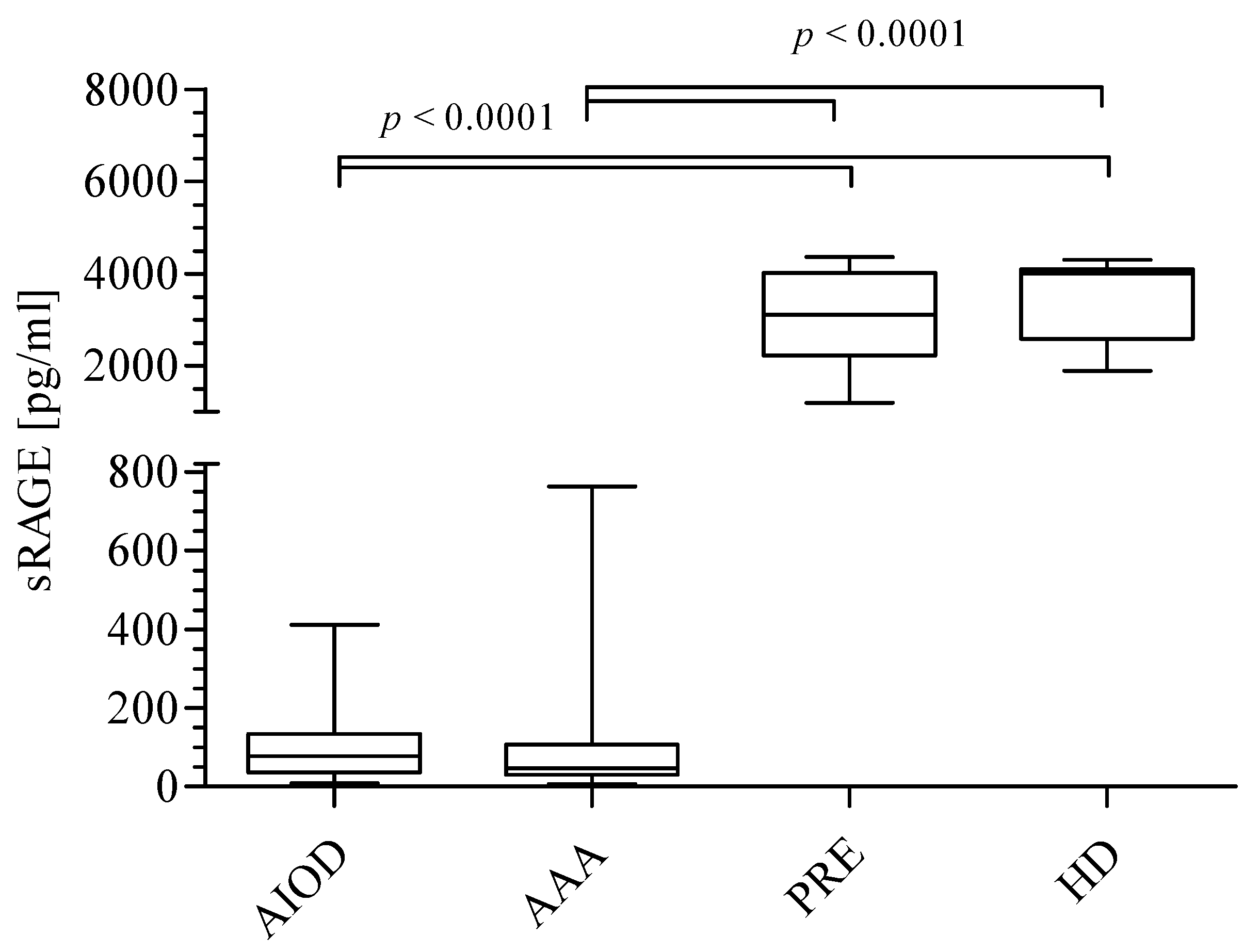
3.1. AGEs, sRAGE, AGEs/sRAGE Ratio, and UA Level in the Studied Groups
3.2. The association of AGEs, sRAGE, AGEs/sRAGE Ratio, and UA with Age, Gender, hsCRP, as well as estimated glomerular filtration rate (eGFR) in Studied Groups
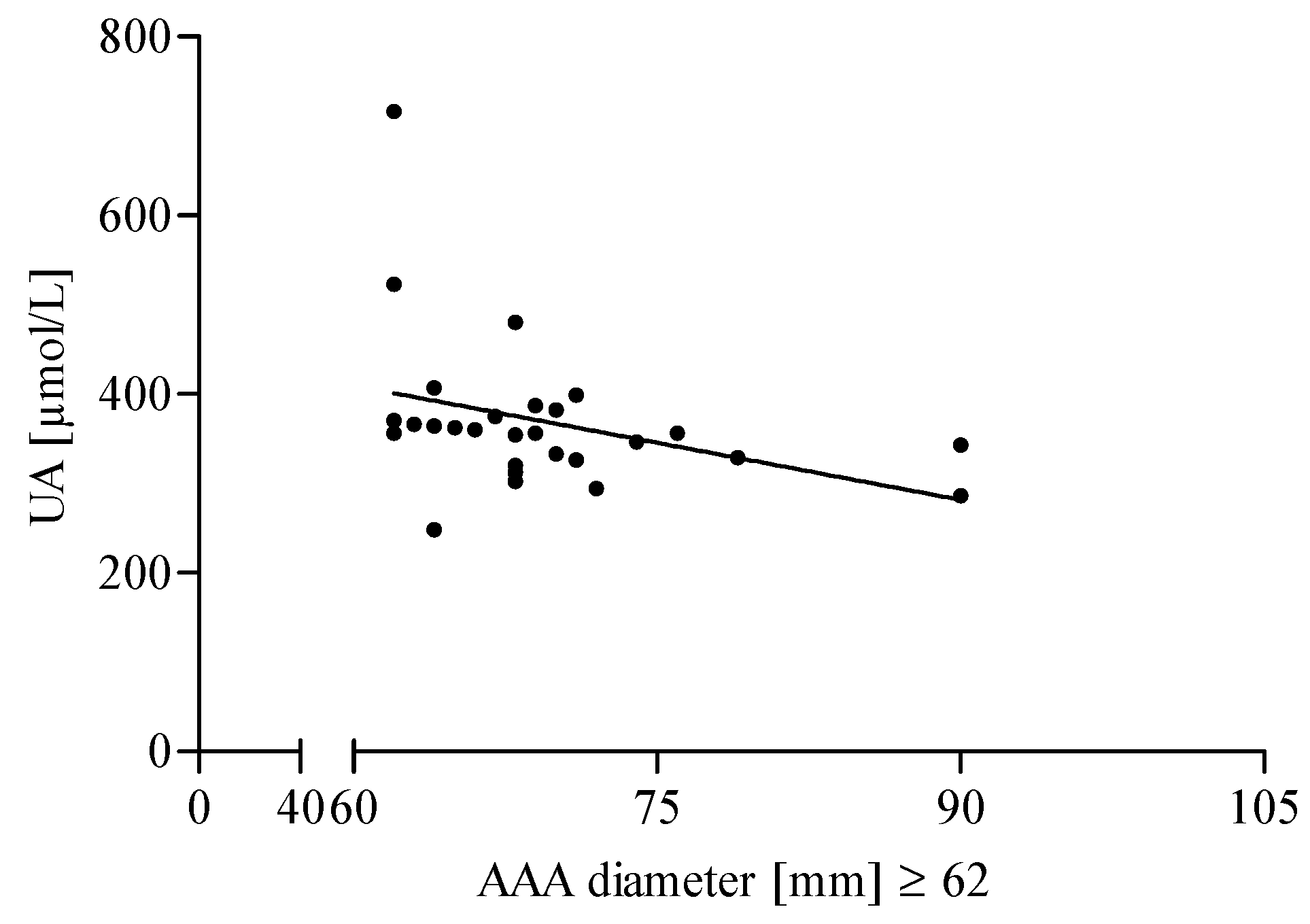
3.3. The Association of AGEs and UA Level with the Diameter of the Aneurysm in AAA Patients
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysms |
| AIOD | aortoiliac occlusive disease |
| PRE | pre-dialyzed patients (CKD stage 3–4) |
| HD | hemodialyzed patients (CKD stage 5) |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| AGEs | advanced glycation end products |
| sRAGE | soluble receptor for advanced glycation end products |
| UA | uric acid |
| RAGE | receptor for advanced glycation end products |
| eGFR | estimated glomerular filtration rate |
| hsCRP | high-sensitivity C-reactive protein |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalousová, M.; Zima, T.; Tesař, V.; Dusilová-Sulková, S.; Škrha, J. Advanced glycoxidation end products in chronic diseases—Clinical chemistry and genetic background. Mutat. Res. 2005, 579, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryszczyńska, B.; Budzyń, M.; Begier-Krasińska, B.; Osińska, A.; Boruczkowski, M.; Kaczmarek, M.; Bukowska, A.; Iskra, M.; Kasprzak, M.P. Association between advanced glycation end products, soluble RAGE receptor, and endothelium dysfunction, evaluated by circulating endothelial cells and endothelial progenitor cells in patients with mild and resistant hypertension. Int. J. Mol. Sci. 2019, 20, 3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef]
- Simm, A.; Bartling, B.; Silber, R.E. RAGE: A new pleiotropic antagonistic gene? Ann. N. Y. Acad. Sci. 2004, 1019, 228–231. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, J.H. Receptor for advanced glycation end products (RAGE), its ligands, and soluble RAGE: Potential biomarkers for diagnosis and therapeutic targets for human renal diseases. Genom. Inform. 2013, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.J.; Flyvbjerg, A.; Bjerre, M. Soluble receptor for advanced glycation end product: A biomarker for acute coronary syndrome. BioMed. Res. Int. 2015, 2015, 815942. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Park, E.Y.; Mun, H.; Chang, E.; Ko, J.Y.; Kim, D.Y.; Park, J.H. Soluble receptor for advanced glycation end products inhibits disease progression in autosomal dominant polycystic kidney disease by down-regulating cell proliferation. FASEB J. 2015, 29, 3506–3514. [Google Scholar] [CrossRef]
- Tahara, N.; Yamagishi, S.I.; Tahara, A.; Ishibashi, M.; Hayabuchi, N.; Takeuchi, M.; Imaizumi, T. Adiponectin is inversely associated with ratio of serum levels of AGEs to sRAGE and vascular inflammation. Int. J. Cardiol. 2012, 158, 461–462. [Google Scholar] [CrossRef]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushiyama, A.; Nakatsu, Y.; Matsunaga, Y.; Yamamotoya, T.; Mori, K.; Ueda, K.; Inoue, Y.; Sakoda, H.; Fujishiro, M.; Ono, H.; et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediat. Inflamm. 2016, 2016, 8603164. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.; Grossman, E.; Goldbourt, U. Uric acid variability at midlife as an independent predictor of coronary heart disease and all-cause mortality. PLoS ONE 2019, 14, e0220532. [Google Scholar] [CrossRef] [Green Version]
- Cortese, F.; Giordano, P.; Scicchitano, P.; Faienza, M.F.; De Pergola, G.; Calculli, G.; Meliota, G.; Ciccone, M.M. Uric acid: From a biological advantage to a potential danger. A focus on cardiovascular effects. Vascul. Pharmacol. 2019, 120, 106565. [Google Scholar] [CrossRef]
- Cai, W.; Duan, X.M.; Liu, Y.; Yu, J.; Tang, Y.L.; Liu, Z.L.; Jiang, S.; Zhang, C.P.; Liu, J.Y.; Xu, J.X. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed. Res. Int. 2017, 2017, 4391920. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Iskra, M. Advanced oxidation protein products and carbonylated proteins as biomarkers of oxidative stress in selected atherosclerosis-mediated diseases. Biomed. Res. Int. 2017, 2017, 4975264. [Google Scholar] [CrossRef] [Green Version]
- Kraśniak, A.; Drożdż, M.; Pasowicz, M.; Chmiel, G.; Michałek, M.; Szumilak, D.; Podolec, P.; Klimeczek, P.; Konieczyńska, M.; Wicher-Muniak, E.; et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2007, 22, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Luczak, M.; Formanowicz, D.; Pawliczak, E.; Wanic-Kossowska, M.; Wykretowicz, A.; Figlerowicz, M. Chronic kidney disease-related atherosclerosis-proteomic studies of blood plasma. Proteome Sci. 2011, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Luczak, M.; Suszynska-Zajczyk, J.; Marczak, L.; Formanowicz, D.; Pawliczak, E.; Wanic-Kossowska, M.; Stobiecki, M. Label-free quantitative proteomics reveals differences in molecular mechanism of atherosclerosis related and non-related to chronic kidney disease. Int. J. Mol. Sci. 2016, 17, 631. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic toxicity of advanced glycation end products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlassara, H. Advanced glycation end-products and atherosclerosis. Ann. Med. 1996, 28, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Hofmann, M.A.; Ziegler, R.; Nawroth, P.P. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc. Res. 1998, 37, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Fuh, H.; Donnelly, T.; Cybulsky, M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol. Med. 1995, 1, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleniuc, M.; Secara, I.; Onofriescu, M.; Hogas, S.; Voroneanu, L.; Siriopol, D.; Covic, A. Consequences of advanced glycation end products accumulation in chronic kidney disease and clinical usefulness of their assessment using a non-invasive technique–skin autofluorescence. Maedica 2011, 6, 298. [Google Scholar] [PubMed]
- Sakata, N.; Imanaga, Y.; Meng, J.; Tachikawa, Y.; Takebayashi, S.; Nagai, R.; Horiuchi, S. Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 1999, 142, 67–77. [Google Scholar] [CrossRef]
- Isoyama, N.; Leurs, P.; Qureshi, A.R.; Bruchfeld, A.; Anderstam, B.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Lindholm, B. Plasma S100A12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease Stage 5 patients. Nephrol. Dial. Transplant. 2015, 30, 84–91. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 001–011. [Google Scholar]
- Prasad, K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: Myth or reality. Int. J. Angiol. 2014, 23, 011–016. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.L.; Carraro, C.C.; Belló-Klein, A.; Kalil, A.N.; Aerts, N. Oxidative stress in human aorta of patients with advanced aortoiliac occlusive disease. Braz. J. Cardiovasc. Surg. 2016, 31, 428–433. [Google Scholar]
- Koole, D.; van Herwaarden, J.A.; Schalkwijk, C.G.; Lafeber, F.P.; Vink, A.; Smeets, M.B.; Pasterkamp, G.; Moll, F. L A potential role for glycated cross-links in abdominal aortic aneurysm disease. J. Vasc. Surg. 2017, 65, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikołajczyk-Stecyna, A.; Korcz, M.; Gabriel, K.; Pawlaczyk, G.; Oszkinis, G.; Słomski, R. Risk factors in abdominal aortic aneurysm and aortoiliac occlusive disease and differences between them in the Polish population. Sci. Rep. 2013, 3, 3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, K. Dyslipoproteinemia and peripheral arterial occlusive disease. Angiology 2004, 55, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Mochizuki, T.; Takei, T.; Tsuchiya, K.; Nitta, K. Serum uric acid levels and long-term outcomes in chronic kidney disease. Heart Vessel. 2014, 29, 504–512. [Google Scholar] [CrossRef]
- Luo, Q.; Xia, X.; Li, B.; Lin, Z.; Yu, X.; Huang, F. Serum uric acid and cardiovascular mortality in chronic kidney disease: A meta-analysis. BMC Nephrol. 2019, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Muiesan, M.L.; Agabiti-Rosei, C.; Paini, A.; Salvetti, M. Uric acid and cardiovascular disease: An update. Eur. Cardiol. 2016, 11, 54. [Google Scholar] [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Rahmani, J.; Bellissimo, N.; Hekmatdoost, A. Serum uric acid and risk of cardiovascular mortality: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc. Disord. 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Murea, M.; Tucker, B.M. The physiology of uric acid and the impact of end-stage kidney disease and dialysis. Semin. Dial. 2019, 32, 47–57. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Gibson, S. Febuxostat and increased dialysis as a treatment for severe tophaceous gout in a hemodialysis patient. Case Rep. Nephrol. 2016, 2016, 9106935. [Google Scholar] [CrossRef] [Green Version]
- El-Saeed, G.S.; Fadel, F.; Elshamaa, M.F.; Galal, R.E.; Elghoroury, E.A.; Thabet, E.H.; Abdelrahman, S.M. Advanced glycation end products and soluble receptor as markers of oxidative stress in children on hemodialysis. Ren. Fail. 2015, 37, 1452–1456. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Kato, I.; Doi, T.; Yonekura, H.; Ohashi, S.; Takeuchi, M.; Watanabe, T.; Yamagishi, S.; Sakurai, S.; Takasawa, S.; et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J. Clin. Investig. 2001, 108, 261–268. [Google Scholar] [CrossRef]
- Duk-Hee, K.; Sung-Kyu, H. Uric Acid Puzzle: Dual Role as Anti-oxidantand Pro-oxidant. Electrolyte Blood Press. 2014, 12, 1–6. [Google Scholar]
- Kamogawa, E.; Sueishi, Y. A multiple free-radical scavenging (MULTIS) study on the antioxidant capacity of a neuroprotective drug, edaravone as compared with uric acid, glutathione, and trolox. Bioorg. Med. Chem. Lett. 2014, 24, 1376–1379. [Google Scholar] [CrossRef]
- Gersch, C.; Palii, S.P.; Kim, K.M.; Angerhofer, A.; Johnson, R.J.; Henderson, G.N. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 2009, 28, 118–149. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: A review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef] [Green Version]
- Esen, A.M.; Akcakoyun, M.; Esen, O.; Acar, G.; Emiroglu, Y.; Pala, S.; Kargin, R.; Karapinar, H.; Ozcan, O.; Barutcu, I. Uric acid as a marker of oxidative stress in dilatation of the ascending aorta. Am. J. Hypertens. 2011, 24, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Patetsios, P.; Rodino, W.; Wisselink, W.; Bryan, D.; Kirwin, J.D.; Panetta, T.F. Identification of uric acid in aortic aneurysms and atherosclerotic artery. Ann. N. Y. Acad. Sci. 1996, 800, 243–245. [Google Scholar] [CrossRef]
- De Vos, L.C.; Lefrandt, J.D.; Dullaart, R.P.; Zeebregts, C.J.; Smit, A.J. Advanced glycation end products: An emerging biomarker for adverse outcome in patients with peripheral artery disease. Atherosclerosis 2016, 254, 291–299. [Google Scholar] [CrossRef] [Green Version]



| Parameters | AAA (70 Patients) No. (%) | AIOD (20 Patients) No. (%) | PRE (50 Patients) No. (%) | HD (35 Patients) No. (%) |
|---|---|---|---|---|
| Age (mean ± SD) | 70.25 ± 8.65 | 63.78 ± 6.80 | 71.6 ± 13.12 | 54.03 ± 16.18 |
| Gender (male/female) | 55/ 15 | 14 / 6 | 27/23 | 24/11 |
| Hypertension | 48 (68) | 12 (60) | 50 (100) | 34 (100) |
| Hypercholesterolemia | 18 (25) | 3 (15) | 50 (100) | 34 (100) |
| Coronary artery disease | 30 (44) | 8 (40) | 17 (34) | 34 (100) |
| Previous myocardial infarction | 12 (17) | 3 (15) | 8 (16) | 9 (26.5) |
| Cerebrovascular accident | 7 (10) | 1 (5) | 1 (2) | 4 (11.8) |
| Kidney disease | 8 (11.5) | 2 (10) | 50 (100) | 34 (100) |
| Pulmonary disease | 7 (10) | 4 (20) | 0 | 0 |
| Medications | ||||
| β-blocker | 32 (46) | 10 (50) | 29 (58) | 20 (58.8) |
| ACEIs | 35 (50) | 8 (40) | 25 (50) | 11 (32) |
| Statins | 37 (52.8) | 15 (70) | 39 (78) | 27 (79.4) |
| NSAIDs | 70 (100) | 20 (100) | 40 (80) | 11 (32.4) |
| Parameters | AAA (70 Patients) | AIOD (20 Patients) | PRE (50 Patients) | HD (35 Patients) |
|---|---|---|---|---|
| Total cholesterol (TC) (mmol/L) | 4.80 ± 2.64 | 4.44 ± 1.67 | 5.12 ± 1.21 | 4.22 ± 1.30 a |
| LDL-cholesterol (LDL-C) (mmol/L) | 2.40 (1.75–3.03) | 2.50 (1.80–4.00) | 3.24 (2.28–3.80) b,c | 2.45 (1.36–3.00) |
| HDL-cholesterol (HDL-C) (mmol/L) | 1.23 (1.01–1.50) | 1.04 (0.95–1.32) | 0.88 (0.80–1.81) b | 1.06 (0.77–1.21) d |
| Triacylglycerols (TAG) (mmol/L) | 1.63 ± 0.89 | 1.40 ± 0.53 | 1.63 ± 0.42 | 1.44 ± 0.50 |
| Red blood cells (RBC) (1012/L) | 4.60 ± 0.54 | 4.70 ± 0.75 | 3.65 ± 0.60 b,c | 3.42 ± 0.50 d,e |
| White blood cells (WBC) (109/L) | 8.23 ± 3.44 | 9.20 ± 2.65 | 6.72 ± 2.20 b,c | 6.54 ± 1.60 |
| eGFR (mL/min/1.73 m2) | 70.00 ± 18.00 | 76.90 ± 13.90 | 25.00 ± 10.70 b,c | 7.60 ± 3.14 a,d,e |
| hsCRP (mg/L) | 9.93 (3.41–13.70) | 7.54 (3.67–13.56) | 8.65 (3.53–11.80) | 10.70 (8.60–12.10) |
| Parameter | AAA | AIOD | PRE | HD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male n = 55 | Female n = 15 | p | Male n = 14 | Female n = 6 | p | Male n = 27 | Female n = 23 | p | Male n = 24 | Female n = 11 | p | |
| AGEs | 17.25 (8.18–38.40) | 18.50 (13.45–46.67) | 0.2928 | 29.86 (22.93–56.82) | 17.01 (13.65–33.97) | 0.0798 | 1630 (1096–2300) | 1665 (1090–1907) | 0.8661 | 2929 (1488–4820) | 3027 (1709–5083) | 0.5715 |
| sRAGE | 54.22 (31.32–127.60) | 42.90 (21.43–77.18) | 0.2240 | 96.51 ± 102.60 | 115.40 ± 76.10 | 0.6920 | 3025 ± 1268 | 2845 ± 800 | 0.5835 | 4042 (2887–4139) | 3994 (2589–4088) | 0.5048 |
| AGEs/sRAGE | 0.300 (0.099–0.474) | 0.491 (0.229–2.397) | 0.0417 | 0.354 (0.276–0.972) | 0.264 (0.115–1.581) | 0.7441 | 0.459 (0.322–1.029) | 0.606 (0.314–0.794) | 0.7359 | 0.797 ± 0.399 | 0.969 ± 0.612 | 0.3549 |
| UA | 353.1 ± 121.2 | 319.50 ± 48.97 | 0.3146 | 309.50 ± 98.45 | 343.60 ± 71.00 | 0.4925 | 384.40 ± 107.20 | 390.40 ± 79.53 | 0.8351 | 390.30 ± 92.84 | 364.10 ± 69.22 | 0.4489 |
| AGEs | ||||
| r | Group of Patients | p Value | 95% Confidence Intervals | |
| sRAGE | 0.2801 | AAA | 0.0217 | 0.03549 to 0.4931 |
| sRAGE | 0.4267 | HD | 0.0149 | 0.08090 to 0.6808 |
| UA | −0.4442 | HD | 0.0394 | −0.6339 to −0.01975 |
| sRAGE | 0.3271 | CKD | 0.0031 | 0.1092 to 0.5150 |
| sRAGE | 0.2162 | CVD | 0.0431 | 0.000745 to 0.4125 |
| sRAGE | ||||
| r | Group of Patients | p Value | 95% Confidence Intervals | |
| AGEs | 0.2801 | AAA | 0.0217 | 0.03549 to 0.4931 |
| AGEs | 0.4267 | HD | 0.0149 | 0.08090 to 0.6808 |
| eGFR | 0.3247 | PRE | 0.0244 | 0.03594 to 0.5634 |
| AGEs | 0.3271 | CKD | 0.0031 | 0.1092 to 0.5150 |
| AGEs | 0.2162 | CVD | 0.0431 | 0.000745 to 0.4125 |
| eGFR | −0.2302 | CVD | 0.0386 | −0.4325 to −0.00597 |
| AGEs/sRAGE | ||||
| r | Group of Patients | p Value | 95% Confidence Intervals | |
| UA | −0.3829 | HD | 0.0305 | −0.6455 to −0.03939 |
| eGFR | ||||
| r | Group of Patients | p Value | 95% Confidence Intervals | |
| UA | −0.3210 | AAA | 0.0056 | −0.5183 to −0.09132 |
| UA | −0.3156 | HD | 0.0392 | −0.5627 to −0.01681 |
| sRAGE | 0.3247 | PRE | 0.0244 | 0.03594 to 0.5634 |
| UA | ||||
| r | Group of Patients | p Value | 95% Confidence Intervals | |
| eGFR | −0.3210 | AAA | 0.0056 | −0.5183 to −0.09132 |
| eGFR | −0.3156 | HD | 0.0392 | −0.5627 to −0.01681 |
| AGEs/sRAGE | −0.3829 | HD | 0.0305 | −0.6455 to −0.03939 |
| eGFR | −0.2616 | CVD | 0.0133 | −0.4506 to −0.05016 |
| CKD | ||||||||
| AGEs | sRAGE | UA | ||||||
| Parameter | Coefficient | p | Parameter | Coefficient | p | Parameter | Coefficient | p |
| age gender hsCRP eGFR | −6.386 8.495 16.680 −32.410 | 0.4523 0.9711 0.1663 <0.0001 | age gender hsCRP eGFR AGEs | –10.810 136.700 2.674 11.030 0.221 | 0.2505 0.5796 0.8401 0.3734 0.0071 | age gender hsCRP eGFR AGEs | −0.311 20.150 −0.749 −1.246 −0.012 | 0.6348 0.2649 0.4214 0.0004 0.0580 |
| CVD | ||||||||
| AGEs | sRAGE | UA | ||||||
| Parameter | Coefficient | p | Parameter | Coefficient | p | Parameter | Coefficient | p |
| age gender hsCRP eGFR | 0.184 −16.210 0.172 −0.009 | 0.7744 0.2904 0.8211 0.9777 | age gender hsCRP eGFR AGEs | −0.355 80.780 −2.156 −3.062 1.443 | 0.8592 0.0955 0.3654 0.0029 0.0006 | age gender hsCRP eGFR AGEs | −0.720 43.080 −0.569 −1.926 −0.066 | 0.6010 0.1942 0.7272 0.0061 0.8089 |
| ALL | ||||||||
| AGEs | sRAGE | UA | ||||||
| Parameter | Coefficient | p | Parameter | Coefficient | p | Parameter | Coefficient | p |
| age gender hsCRP eGFR | –6.386 8.495 16.680 −32.410 | 0.4523 0.9711 0.1663 <0.0001 | age gender hsCRP eGFR AGEs | −10.730 −7.769 −12.400 −29.310 0.399 | 0.1097 0.9663 0.1934 <0.0001 <0.0001 | age gender hsCRP eGFR AGEs | −0.311 20.150 −0.749 −1.246 −0.012 | 0.6348 0.2649 0.4214 0.0004 0.0580 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryszczyńska, B.; Budzyń, M.; Formanowicz, D.; Wanic-Kossowska, M.; Formanowicz, P.; Majewski, W.; Iskra, M.; Kasprzak, M.P. Selected Atherosclerosis-Related Diseases May Differentially Affect the Relationship between Plasma Advanced Glycation End Products, Receptor sRAGE, and Uric Acid. J. Clin. Med. 2020, 9, 1416. https://doi.org/10.3390/jcm9051416
Gryszczyńska B, Budzyń M, Formanowicz D, Wanic-Kossowska M, Formanowicz P, Majewski W, Iskra M, Kasprzak MP. Selected Atherosclerosis-Related Diseases May Differentially Affect the Relationship between Plasma Advanced Glycation End Products, Receptor sRAGE, and Uric Acid. Journal of Clinical Medicine. 2020; 9(5):1416. https://doi.org/10.3390/jcm9051416
Chicago/Turabian StyleGryszczyńska, Bogna, Magdalena Budzyń, Dorota Formanowicz, Maria Wanic-Kossowska, Piotr Formanowicz, Wacław Majewski, Maria Iskra, and Magdalena P. Kasprzak. 2020. "Selected Atherosclerosis-Related Diseases May Differentially Affect the Relationship between Plasma Advanced Glycation End Products, Receptor sRAGE, and Uric Acid" Journal of Clinical Medicine 9, no. 5: 1416. https://doi.org/10.3390/jcm9051416







