TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model
Abstract
:1. Introduction
2. Results
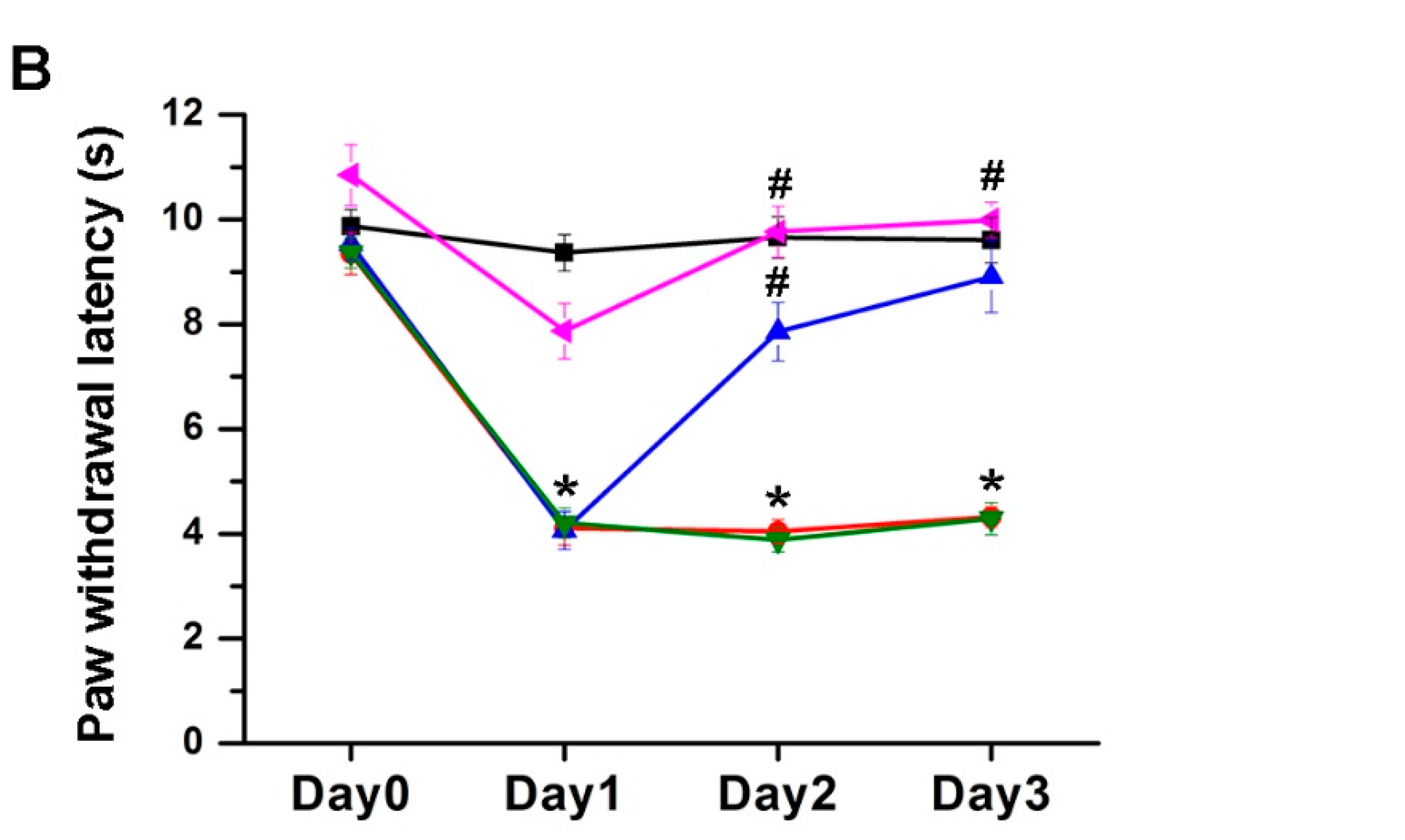
2.1. The Effect of EA Treatment on Mechanical and Thermal Behavior
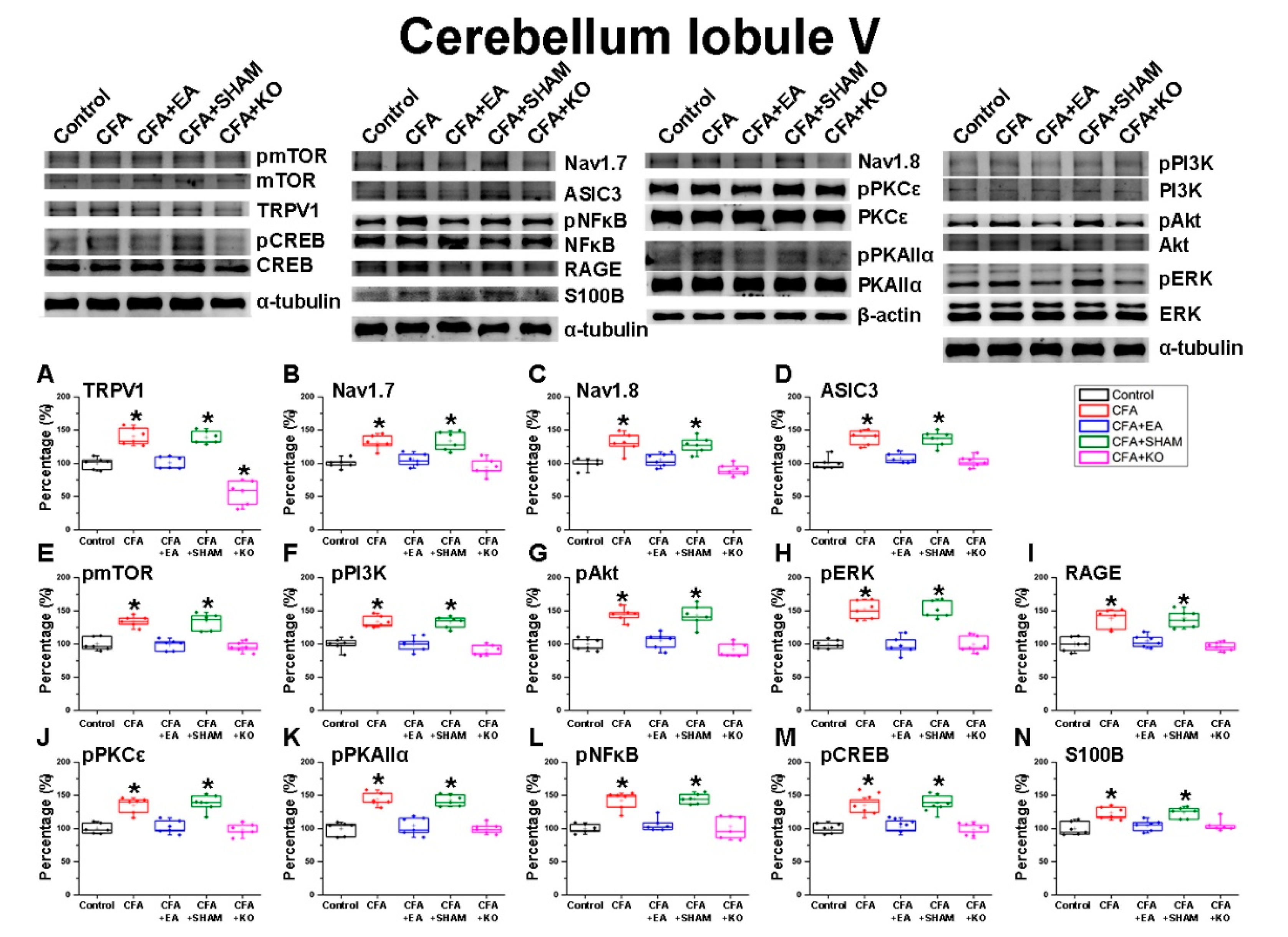
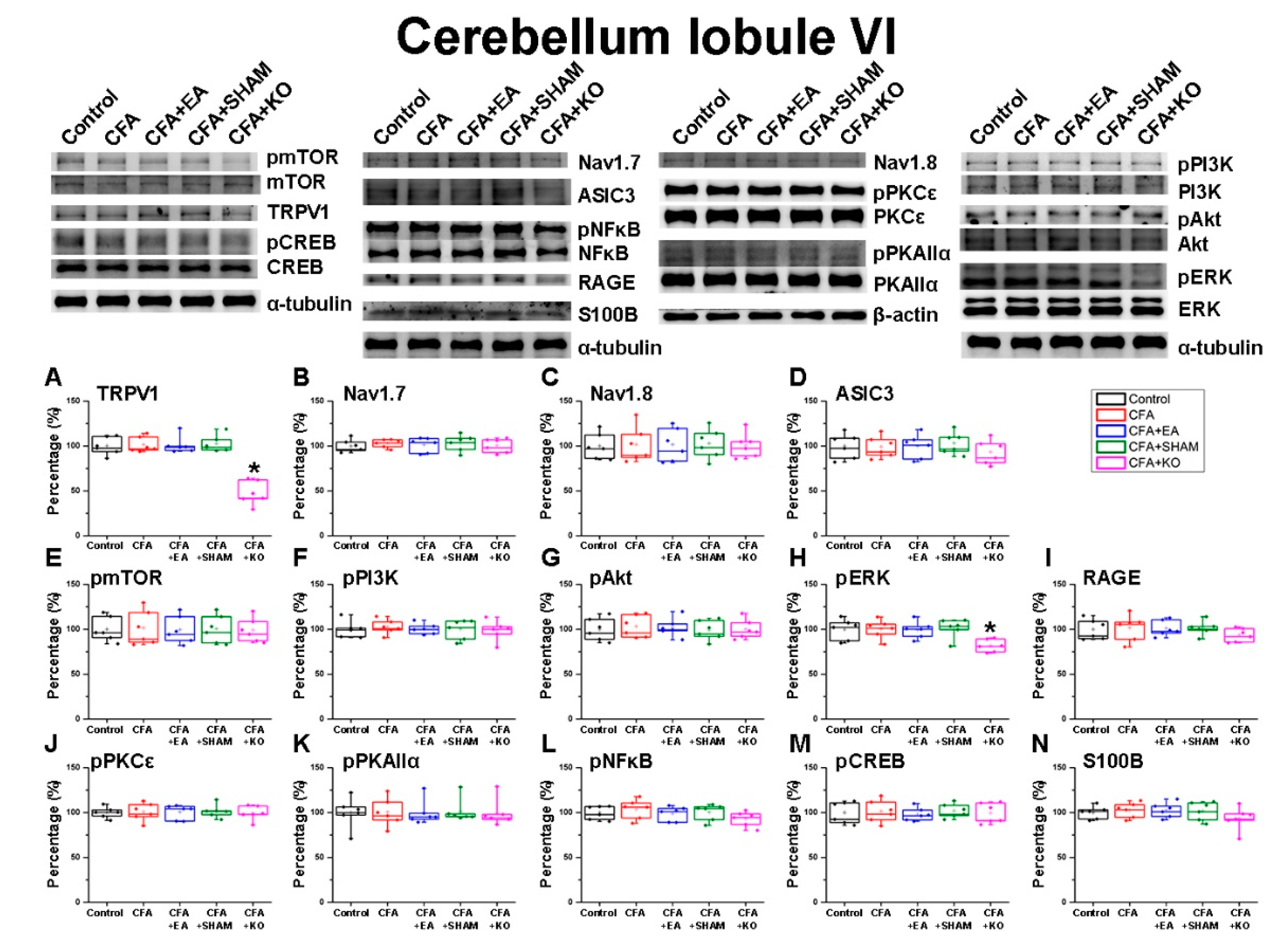
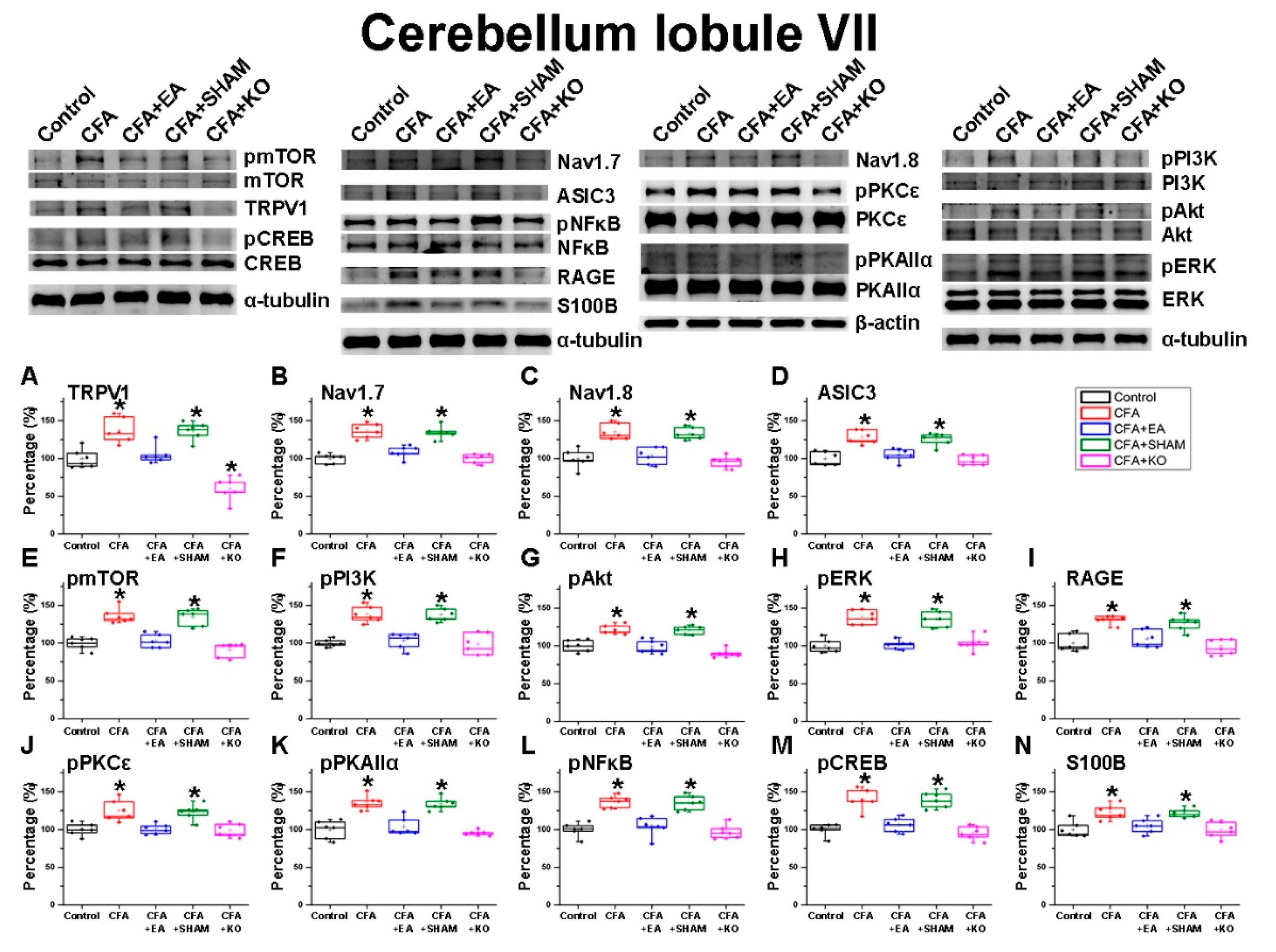
2.2. The Effect of EA Treatment on Nociceptor and Its Downstream in the Cerebellum Lobules V, VI and VII
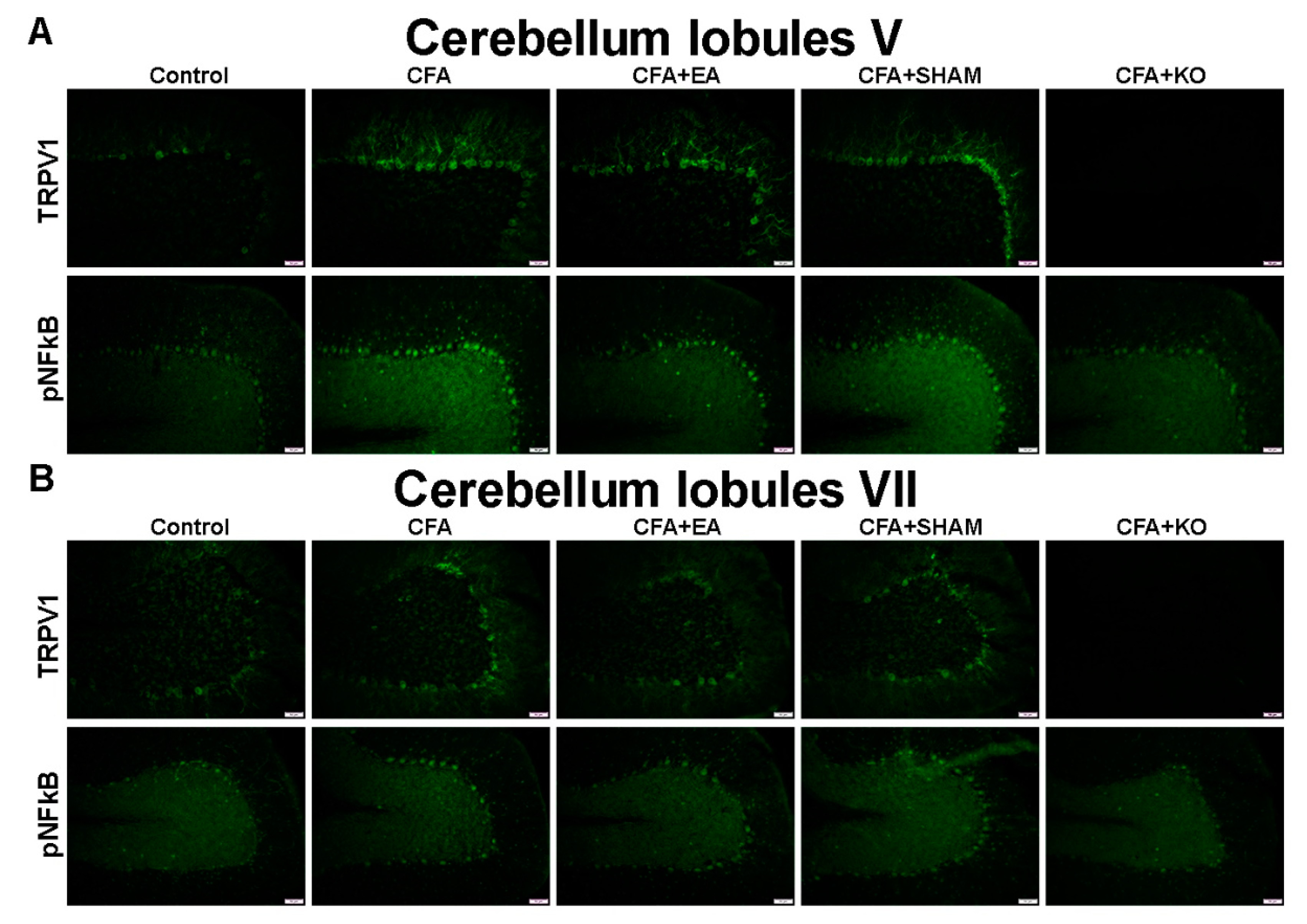
2.3. The Effect of EA Treatment on Protein Expression Alteration in the Cerebellum Lobules V, VIa, VIb and VII via Immunofluorescence Technique
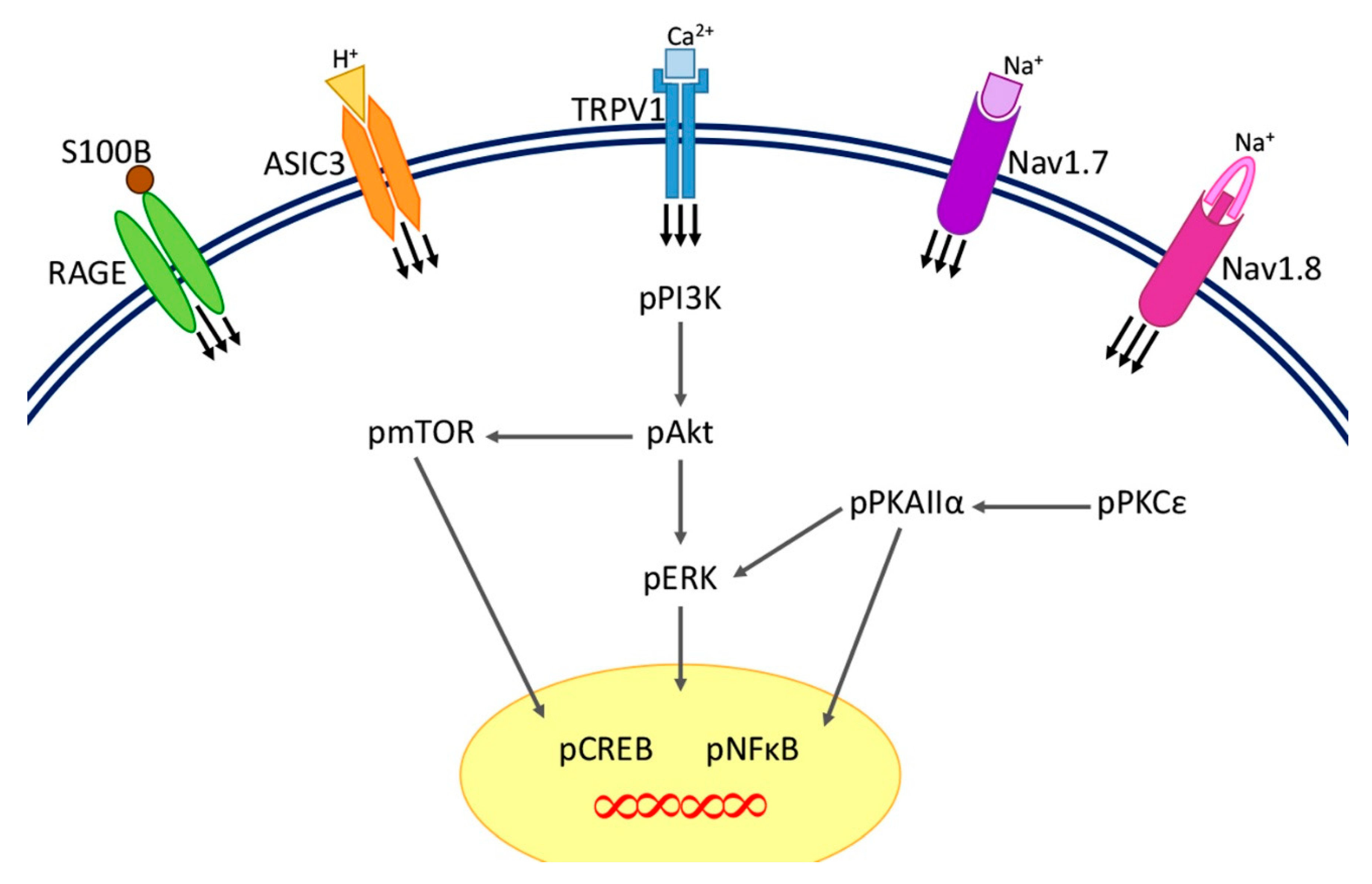
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Behavioral Examination
4.3. EA Treatment
4.4. Immunoblotting
4.5. Immunofluorescence
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woolf, C.J. What is this thing called pain? J. Clin. Invest. 2010, 120120, 3742–3744. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laing, R.J.; Dhaka, A. ThermoTRPs and Pain. Neuroscientist 2016, 22, 171–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharm. 2017, 96, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Feehan, A.K.; Morgenweck, J.; Zhang, X.; Amgott-Kwan, A.T.; Zadina, J.E. Novel Endomorphin Analogs Are More Potent and Longer-Lasting Analgesics in Neuropathic, Inflammatory, Postoperative, and Visceral Pain Relative to Morphine. J. Pain 2017, 18, 1526–1541. [Google Scholar] [CrossRef]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Yang, E.S.; Li, P.W.; Nilius, B.; Li, G. Ancient Chinese medicine and mechanistic evidence of acupuncture physiology. Pflugers Arch 2011, 462, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Benharash, P. Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Meridian. Stud. 2014, 7, 190–193. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Hahm, T.S. The effect of 2 Hz and 100 Hz electrical stimulation of acupoint on ankle sprain in rats. J. Korean Med. Sci. 2007, 22, 347–351. [Google Scholar] [CrossRef]
- Oh, B.; Eade, T.; Kneebone, A.; Hruby, G.; Lamoury, G.; Pavlakis, N.; Clarke, S.; Zaslawski, C.; Marr, I.; Costa, D.; et al. Acupuncture in oncology: The effectiveness of acupuncture may not depend on needle retention duration. Integrative Cancer Ther. 2018, 17, 458–466. [Google Scholar] [CrossRef]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apps, R.; Garwicz, M. Anatomical and physiological foundations of cerebellar information processing. Nat. Rev. Neurosci. 2005, 6, 297–311. [Google Scholar] [CrossRef]
- Itō, M. The Cerebellum: Brain for an Implicit Self; FT Press: Upper Saddle River, NJ, USA, 2012. [Google Scholar] [CrossRef] [Green Version]
- Moulton, E.A.; Schmahmann, J.D.; Becerra, L.; Borsook, D. The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev. 2010, 65, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saab, C.Y.; Willis, W.D. The cerebellum: Organization, functions and its role in nociception. Brain Res Brain Res. Rev. 2003, 42, 85–95. [Google Scholar] [CrossRef]
- Kameda, T.; Zvick, J.; Vuk, M.; Sadowska, A.; Tam, W.K.; Leung, V.Y.; Bölcskei, K.; Helyes, Z.; Applegate, L.A.; Hausmann, O.N.; et al. Expression and Activity of TRPA1 and TRPV1 in the Intervertebral Disc: Association with Inflammation and Matrix Remodeling. Int. J. Mol. Sci. 2019, 20, 1767. [Google Scholar] [CrossRef] [Green Version]
- Oehler, B.; Kistner, K.; Martin, C.; Schiller, J.; Mayer, R.; Mohammadi, M.; Sauer, R.S.; Filipovic, M.R.; Nieto, F.R.; Jan, K.; et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci. Rep. 2017, 7, 5447. [Google Scholar] [CrossRef] [Green Version]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Yekkirala, A.; Yaksh, T.L. Basic/Translational Development of Forthcoming Opioid- and Nonopioi-Targeted Pain Therapeutics. Anesth. Analg. 2017, 125, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Lee, K.B.; Shin, J.S.; Lee, J.; Kim, M.R.; Ha, I.H.; Ko, Y.; Lee, Y.J. Effectiveness of Acupuncture and Electroacupuncture for Chronic Neck Pain: A Systematic Review and Meta-Analysis. Am. J. Chin. Med. 2017, 45, 1573–1595. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Quiroz-Gonzalez, S.; Torres-Rosas, R. Nerve Stimulation: Immunomodulation and Control of Inflammation. Trends Mol. Med. 2017, 23, 1103–1120. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Kühnel, M.; Filippopulos, F.; Blum, B.; Eggert, T.; Straube, A. Altered experimental pain perception after cerebellar infarction. Pain 2014, 155, 1303–1312. [Google Scholar] [CrossRef]
- Moberget, T.; Ivry, R.B. Cerebellar contributions to motor control and language comprehension: Searching for common computational principles. Ann. NY Acad. Sci. 2016, 1369, 154–171. [Google Scholar] [CrossRef]
- Diano, M.; D’Agata, F.; Cauda, F.; Costa, T.; Geda, E.; Sacco, K.; Duca, S.; Torta, D.M.; Geminiani, G.C. Cerebellar Clustering and Functional Connectivity During Pain Processing. Cerebellum 2016, 15, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Coombes, S.A.; Misra, G. Pain and motor processing in the human cerebellum. Pain 2016, 157, 117–127. [Google Scholar] [CrossRef]
- Zhang, R.X.; Ren, K. Animal Models of inflammatory Pain. In Animal Models of Pain; Springer: Totowa, NJ, USA, 2011; pp. 23–40. [Google Scholar]
- Dai, Y. TRPs and pain. Semin. Immunopathol. 2016, 38, 277–291. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharm. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.L.; Peng, Y.Y.; Peng, B.W. Modulation of neuroinflammation: Role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav. Immun. 2017, 64, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E. Noradrenergic inhibition of presynaptic TRPV1 channels: A new pathway of pain control. J. Physiol. 2017, 595, 2413–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Lazaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezey, E.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Tóth, A.; Boczán, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Edes, I.; Csiba, L.; Blumberg, P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef]
- Han, P.; Korepanova, A.V.; Vos, M.H.; Moreland, R.B.; Chiu, M.L.; Faltynek, C.R. Quantification of TRPV1 protein levels in rat tissues to understand its physiological roles. J. Mol. Neurosci. 2013, 50, 23–32. [Google Scholar] [CrossRef]
- Roberts, J.C.; Davis, J.B.; Benham, C.D. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004, 995, 176–183. [Google Scholar] [CrossRef]
- Sluka, K.A.; Gregory, N.S. The dichotomized role for acid sensing ion channels in musculoskeletal pain and inflammation. Neuropharmacology 2015, 94, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Srebro, D.; Vuckovic, S.; Prostran, M. Participation of peripheral TRPV1, TRPV4, TRPA1 and ASIC in a magnesium sulfate-induced local pain model in rat. Neuroscience 2016, 339, 1–11. [Google Scholar] [CrossRef]
- Neto, J.O.B.; Garcia, J.B.S.; de Souza Cartágenes, M.D.S.; Amaral, A.G.; Onuchic, L.F.; Ashmawi, H.A. Influence of androgenic blockade with flutamide on pain behaviour and expression of the genes that encode the NaV1.7 and NaV1.8 voltage-dependent sodium channels in a rat model of postoperative pain. J. Transl. Med. 2019, 17, 287. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S. Nav1.7 and Nav1.8: Role in the pathophysiology of pain. Mol. Pain 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, E.; Fritz, G.; Vetter, S.W.; Heizmann, C.W. Binding of S100 proteins to RAGE: An update. Biochim. Biophys. Acta 2009, 1793, 993–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, J.; Svensson, C.I. Role of extracellular damage-associated molecular pattern molecules (DAMPs) as mediators of persistent pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 251–279. [Google Scholar]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Tsubota, M.; Ishikura, H.; Sekiguchi, F.; Terada, Y.; Tsujiuchi, T.; Lui, K.; Nishibori, M.; Kawabata, A. Macrophage-derived HMGB1 as a Pain Mediator in the Early Stage of Acute Pancreatitis in Mice: Targeting RAGE and CXCL12/CXCR4 Axis. J. Neuroimmune. Pharmacol. 2017, 12, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Falk, S.; Santana-Varela, S.; Bogdanov, Y.D.; Nassar, M.A.; Heegaard, A.M.; Wood, J.N. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014, 6, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Tang, N.Y.; Lin, Y.W.; Li, T.C.; Liu, H.J.; Hsieh, C.L. Effect of electroacupuncture on rats with chronic constriction injury-induced neuropathic pain. Sci. World J. 2014, 2014, 129875. [Google Scholar] [CrossRef]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P.; Lin, Y.W. Electroacupuncture Attenuates CFA-induced Inflammatory Pain by suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice. Sci. Rep. 2017, 7, 42531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.G.; Hsieh, C.L.; Lin, Y.W. Analgesic Effect of Electroacupuncture in a Mouse Fibromyalgia Model: Roles of TRPV1, TRPV4, and pERK. PLoS ONE 2015, 10, e0128037. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Gao, J.; He, H.; Liu, C.; Liu, Y.; Li, J.; Wang, J. Identification of differentially expressed non-coding RNAs and mRNAs involved in Qi stagnation and blood stasis syndrome. Exp. Ther. Med. 2019, 17, 1206–1223. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.K.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010, 13, 883–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.Y.; Chen, W.H.; Hsieh, C.L.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complement Altern Med. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.; Sen, S.; Bral, M.; Reddy, S.; Bradley, K.K.; Cornett, E.M.; Fox, C.J.; Kaye, A.D. The Role of Acupuncture in Pain Management. Curr. Pain Headache Rep. 2016, 20, 22. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inprasit, C.; Lin, Y.-W. TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model. Int. J. Mol. Sci. 2020, 21, 3312. https://doi.org/10.3390/ijms21093312
Inprasit C, Lin Y-W. TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model. International Journal of Molecular Sciences. 2020; 21(9):3312. https://doi.org/10.3390/ijms21093312
Chicago/Turabian StyleInprasit, Chanya, and Yi-Wen Lin. 2020. "TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model" International Journal of Molecular Sciences 21, no. 9: 3312. https://doi.org/10.3390/ijms21093312
APA StyleInprasit, C., & Lin, Y.-W. (2020). TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model. International Journal of Molecular Sciences, 21(9), 3312. https://doi.org/10.3390/ijms21093312





