Validation of a Simple HPLC–UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Artificial Saliva
2.4. Instrumentation and Chromatographic Conditions
2.5. Preparation of Standards
2.6. Application to Real Samples
2.7. Sample Size Calculation
3. Results
3.1. Chromatography
3.2. Method Validation
3.2.1. Linearity and Sensitivity
3.2.2. Accuracy and Precision
3.3. Application to Real Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, H.; Koh, H.; Alcaraz, M.G.R.; Schmidlin, P.R.; Davis, D. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. In Cochrane Database of Systematic Reviews; Alcaraz, M.G.R., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Alexander, G.; Hopcraft, M.S.; Tyas, M.J.; Wong, R.H.K. Dentists’ restorative decision-making and implications for an ‘amalgamless’ profession. Part 4: Clinical factor. Aust. Dent. J. 2017, 62, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J. Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J. Occup. Med. Toxicol. 2011, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Liaw, Y.; Metussin, A.P.; Lynch, C.D.; Wilson, N.; Blum, I.R.; Bunton, P.A. The teaching of posterior composites: A survey of dental schools in Oceania. J. Dent. 2019, 84, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Livadiotou, D.; Palaghias, G.; Papadoyannis, I. A simple and rapid HPLC method for the direct determination of residual monomers released from dental polymeric materials in blood serum and urine. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 201–207. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Söderholm, K.-J.; Mariotti, A. Bis-Gma–based Resins in Dentistry: Are They Safe? J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamada, T.; Lynch, C.D.; Wilson, N.H.F. Teaching of posterior composites in dental schools in Japan—30 years and beyond. J. Dent. 2018, 76, 19–23. [Google Scholar] [CrossRef]
- Bettencourt, A.F.; Neves, C.B.; de Almeida, M.S.; Pinheiro, L.M.; e Oliveira, S.A.; Lopes, L.P.; Castro, M.F. Biodegradation of acrylic based resins: A review. Dent. Mater. 2010, 26, e171–e180. [Google Scholar] [CrossRef]
- Chavali, R.; Nejat, A.H.; Lawson, N.C. Machinability of CAD-CAM materials. J. Prosthet. Dent. 2017, 118, 194–199. [Google Scholar] [CrossRef]
- Fasbinder, D.J. Materials for chairside CAD/CAM restorations. Compend. Contin. Educ. Dent. 2010, 31, 702–704, 706, 708–709. [Google Scholar] [PubMed]
- Hussain, B.; Khai, M.; Thieu, L.; Johnsen, G.F.; Reseland, J.E.; Haugen, H.J. Can CAD / CAM resin blocks be considered as substitute for conventional resins ? Dent. Mater. 2017, 33, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Bansal, R.; Burgess, J.O. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent. Mater. 2016, 32, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Alamoush, R.A.; Satterthwaite, J.D.; Silikas, N.; Watts, D.C. Viscoelastic stability of pre-cured resin-composite CAD/CAM structures. Dent. Mater. 2019, 35, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- de Mendonca, A.F.; Shahmoradi, M. Microstructural and Mechanical Characterization of CAD/CAM Materials for Monolithic Dental Restorations. J Prosthodont. 2019, 28, e587–e594. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Dionysopoulos, D.; Tolidis, K.; Kouros, P.; Koliniotou-Koumpia, E.; Tsitrou, E. Structural Integrity Evaluation of Large MOD Restorations Fabricated With a Bulk-Fill and a CAD/CAM Resin Composite Material. Oper. Dent. 2019, 44, 312–321. [Google Scholar] [CrossRef]
- Phan, A.C.; Tang, M.L.; Nguyen, J.F.; Ruse, N.D.; Sadoun, M. High-temperature high-pressure polymerized urethane dimethacrylate - Mechanical properties and monomer release. Dent. Mater. 2014, 30, 350–356. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. (Paris) 2013, 74, 211–220. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Löfroth, M.; Ghasemimehr, M.; Falk, A.; von Steyern, P.V. Bisphenol A in dental materials—Existence, leakage and biological effects. Heliyon 2019, 5, e01711. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Hans Scheers, Lode Godderis, P Hoet, Van Meerbeek, B. Erratum: How much do resin-based dental materials release? A meta-analytical approach (Dental Materials 27 (8) (2011) 723-747)). Dent. Mater. 2013, 29, 919. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, Y.; Lukanov, L.; Filipov, I.; Vladimirov, S. High-performance liquid chromatographic determination of unreacted monomers and other residues contained in dental composites. J. Biochem. Biophys. Methods 2008, 70, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O.; Kçnig, A.; Hellwig, E.; Kümmerer, K. Long-term release of monomers from modern dental-composite materials. Eur. J. Oral Sci. 2009, 117, 68–75. [Google Scholar] [CrossRef]
- Lempel, E.; Czibulya, Z.; Kunsági-Máté, S.; Szalma, J.; Sümegi, B.; Böddi, K. Quantification of conversion degree and monomer elution from dental composite using HPLC and micro-Raman spectroscopy. Chromatographia 2014, 77, 1137–1144. [Google Scholar] [CrossRef]
- Vervliet, P.; De Nys, S.; Boonen, I.; Duca, R.C.; Elskens, M.; Van Landuyt, K.L.; Covaci, A. Qualitative analysis of dental material ingredients, composite resins and sealants using liquid chromatography coupled to quadrupole time of flight mass spectrometry. J. Chromatogr. A 2018, 1576, 90–100. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Kerezoudi, C.; Tolika, E.; Palaghias, G. A simple isocratic HPLC method for the simultaneous determination of the five most common residual monomers released from resin-based dental restorative materials. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 740–749. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent. Mater. 2015, 31, 1587–1598. [Google Scholar] [CrossRef]
- Putzeys, E.; De Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Meerbeek, B.V.; Van Landuyt, K.L. Long-term elution of monomers from resin-based dental composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Hadjicharalampous, M.; Palaghias, G.; Papadoyannis, I. Development and validation of an isocratic HPLC method for the simultaneous determination of residual monomers released from dental polymeric materials in artificial saliva. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 511–523. [Google Scholar] [CrossRef]
- Kuroda, N.; Kinoshita, Y.; Sun, Y.; Wada, M.; Kishikawa, N.; Nakashima, K.; Nakazawa, H.; Makino, T. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003, 30, 1743–1749. [Google Scholar] [CrossRef]
- Mourouzis, P.; Andreasidou, E.; Samanidou, V.; Tolidis, K. Short-term and long-term release of monomers from newly developed resin-modified ceramics and composite resin CAD-CAM blocks. J. Prosthet. Dent. 2019, 123, 339–348. [Google Scholar] [CrossRef]
- Sulaiman, T.A. Materials in digital dentistry—A review. J. Esthet. Restor. Dent. 2020, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.L.; Powers, J.M. Restorative Dental Materials Restorative Dental Materials; Elsevier Health Sciences: Amsterdam, The Netherlands, 2002; Volune 135. [Google Scholar]
- Sakaguchi, R.L.; Powers, J.M. (Eds.) Craig’s Restorative Dental Materials, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Söderholm, K.J.M.; Yang, M.C.K.; Garcea, I. Filler particle leachability of experimental dental composites. Eur. J. Oral Sci. 2000, 108, 555–560. [Google Scholar] [CrossRef]
- Kerezoudi, C.; Gogos, C.; Samanidou, V.; Tziafas, D.; Palaghias, G. Evaluation of monomer leaching from a resin cement through dentin by a novel model. Dent. Mater. 2016, 32, e297–e305. [Google Scholar] [CrossRef]
- De Nys, S.; Putzeys, E.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Meerbeek, B.V.; et al. A novel high sensitivity UPLC-MS/MS method for the evaluation of bisphenol A leaching from dental materials. Sci. Rep. 2018, 8, 6981. [Google Scholar] [CrossRef]
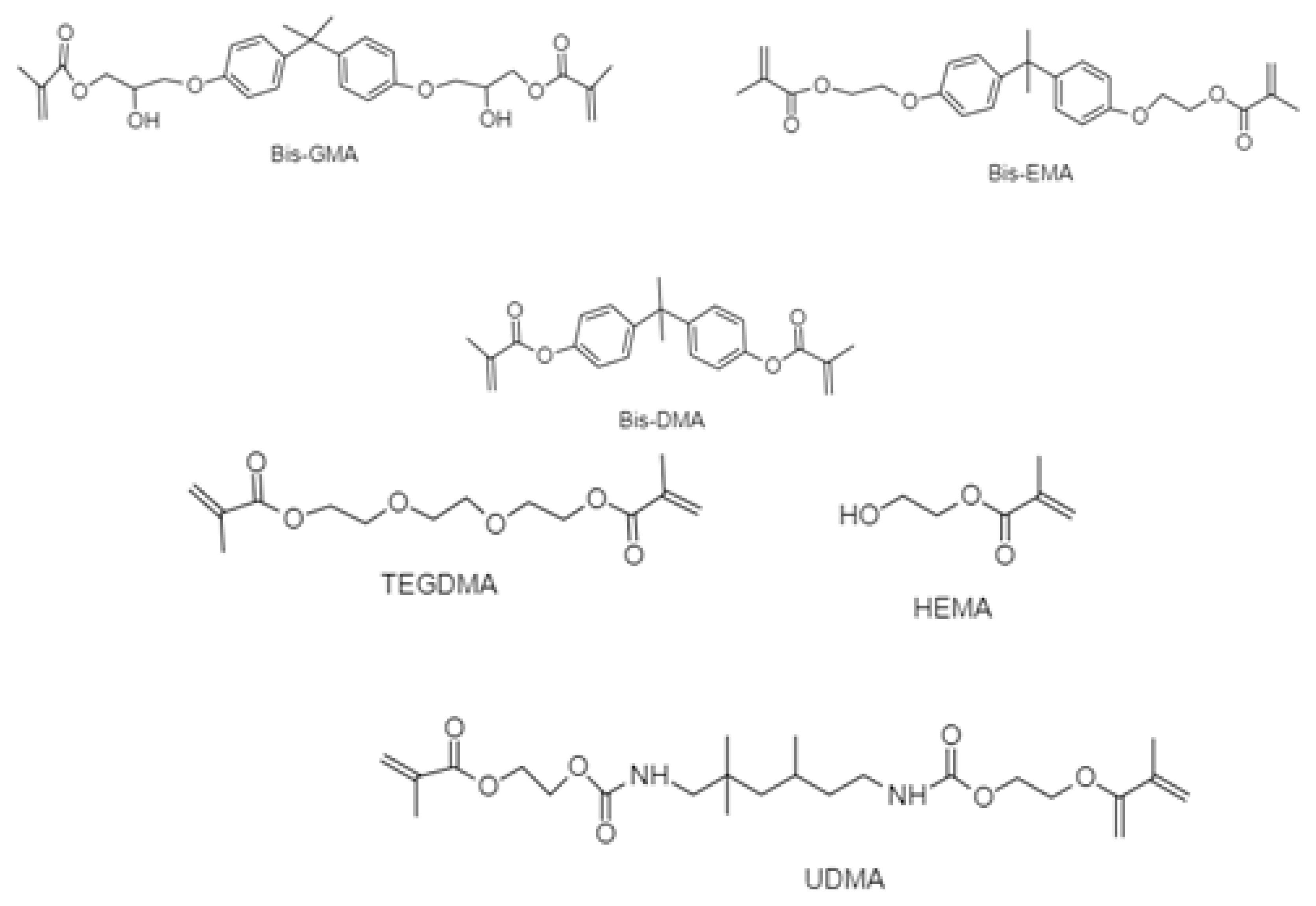
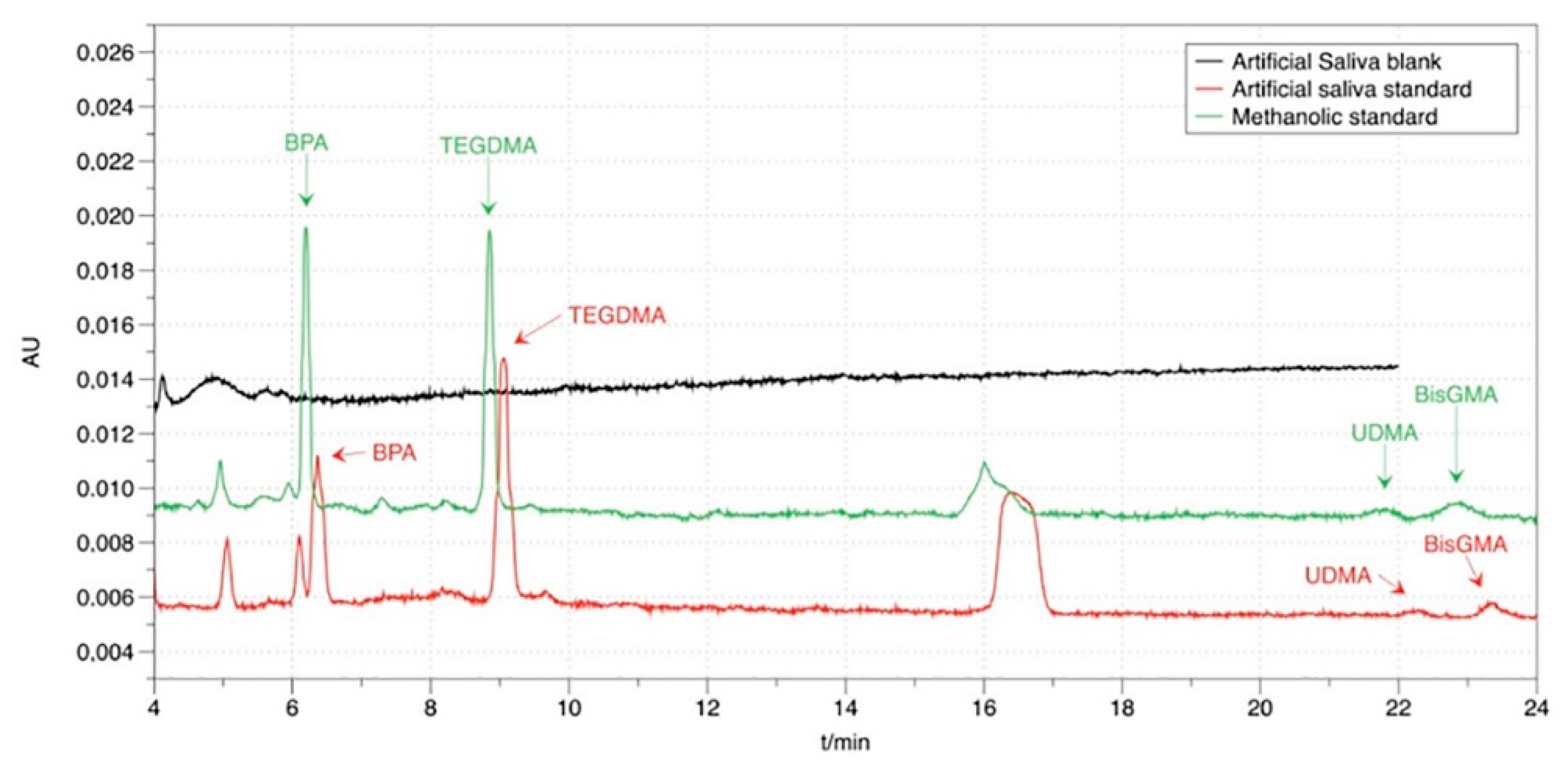
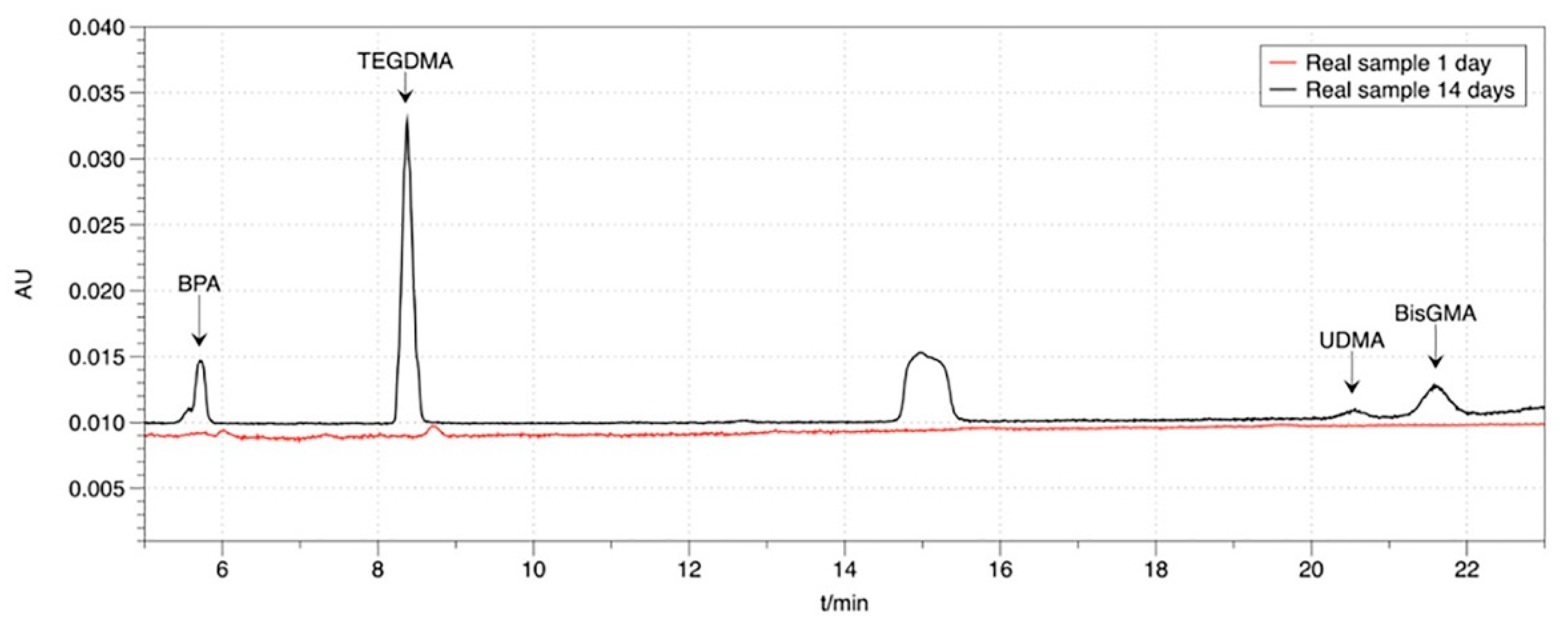
| Material | Type | Composition | Manufacture Lot Number | |
|---|---|---|---|---|
| Matrix | Fillers | |||
| ENAMIC | Polymer Infiltrated Ceramic Material | UDMA, TEGDMA (14 wt %–25 v/v) | Feldspar ceramic enriched with aluminium oxide (75% v/v), (86 wt %) | Vita Zahnfabrik, H. Rauter GmbH & Co KG, Germany LOT 56802 |
| AVENCIA | Nano- ceramic block | UDMA, TEGDMA | SiO2 (40 nm), Al2O3 (20 nm) (62 wt %) | Kuraray, Noritake Dental Inc., Hattersheim, Germany LOT 000318 |
| Tetric CAD | Nano-hybrid block | (28.4 wt %) BisGMA, UDMA, TEGDMA | (64 wt %) barium aluminium silicate glass mean size <1 μm, (7.1 wt %) silicon dioxide with an average particle size of <20 nm. | Ivoclar Vivadent Schaan, Liechtenstein. LOTX29398 |
| Tetric | Nano-hybrid composite | (18.8 wt %) BisGMA, TEGDMA, UDMA | Barium glass filler, Ytterbium trifluoride, mixed oxide (63.5 wt %), polymer (17 wt %), additive, catalysts, pigments, stabilizers (0.7 wt %) Particle size: 0.04–3 μm | Ivoclar Vivadent Schaan, Liechtenstein. LOTV23649 |
| Analytes | Equation | Correlation Coefficient | LOD (ng/μL) | LOQ (ng/μL) |
|---|---|---|---|---|
| BPA | Y = 0.096 × + 0.017 | 0.997 | 0.06 | 0.2 |
| TEGDMA | Y = 0.112 × − 0.041 | 0.998 | 0.06 | 0.2 |
| UDMA | Y = 0.021 × − 0.017 | 0.994 | 0.06 | 0.2 |
| Bis-GMA | Y = 0.065 × − 0.010 | 0.991 | 0.06 | 0.2 |
| Monomers | Intra-Day Precision RSD (%) N = 4 | Recovery (%) ± SD | ||
| 0.5 ng/μL | 5 ng/μL | 10 ng/μL | ||
| BPA | 14.2 | 4.4 | 0.4 | 101 ± 5.1 |
| TEGDMA | 6.7 | 3.4 | 0.8 | 102 ± 22.3 |
| UDMA | 7.5 | 6.7 | 0.1 | 106 ± 13.1 |
| Bis-GMA | 15.0 | 8.1 | 0.3 | 98 ± 10.4 |
| Monomers | Inter-Day Precision RSD (%) N = 3 × 3 | Recovery (%) ± SD | ||
| 0.5 ng/μL | 5 ng/μL | 10 ng/μL | ||
| BPA | 11.9 | 14.6 | 3.1 | 101 ± 5.1 |
| TEGDMA | 14.4 | 15.0 | 0.9 | 102 ± 22.3 |
| UDMA | 6.0 | 14.6 | 12.3 | 106 ± 13.1 |
| Bis-GMA | 3.5 | 10.4 | 13.9 | 98 ± 10.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantopoulou, E.-I.; Plastiras, O.-E.; Mourouzis, P.; Samanidou, V. Validation of a Simple HPLC–UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva. Methods Protoc. 2020, 3, 35. https://doi.org/10.3390/mps3020035
Diamantopoulou E-I, Plastiras O-E, Mourouzis P, Samanidou V. Validation of a Simple HPLC–UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva. Methods and Protocols. 2020; 3(2):35. https://doi.org/10.3390/mps3020035
Chicago/Turabian StyleDiamantopoulou, Elisavet-Ioanna, Orfeas-Evanggelos Plastiras, Petros Mourouzis, and Victoria Samanidou. 2020. "Validation of a Simple HPLC–UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva" Methods and Protocols 3, no. 2: 35. https://doi.org/10.3390/mps3020035
APA StyleDiamantopoulou, E.-I., Plastiras, O.-E., Mourouzis, P., & Samanidou, V. (2020). Validation of a Simple HPLC–UV Method for the Determination of Monomers Released from Dental Resin Composites in Artificial Saliva. Methods and Protocols, 3(2), 35. https://doi.org/10.3390/mps3020035








