RETRACTED: Experimental Study on the Influence of Apigenin K and Melatonin in Socket Preservation as Bone Stimulators: An Experimental Study in Beagle Dogs
Abstract
Featured Application
Abstract
1. Introduction
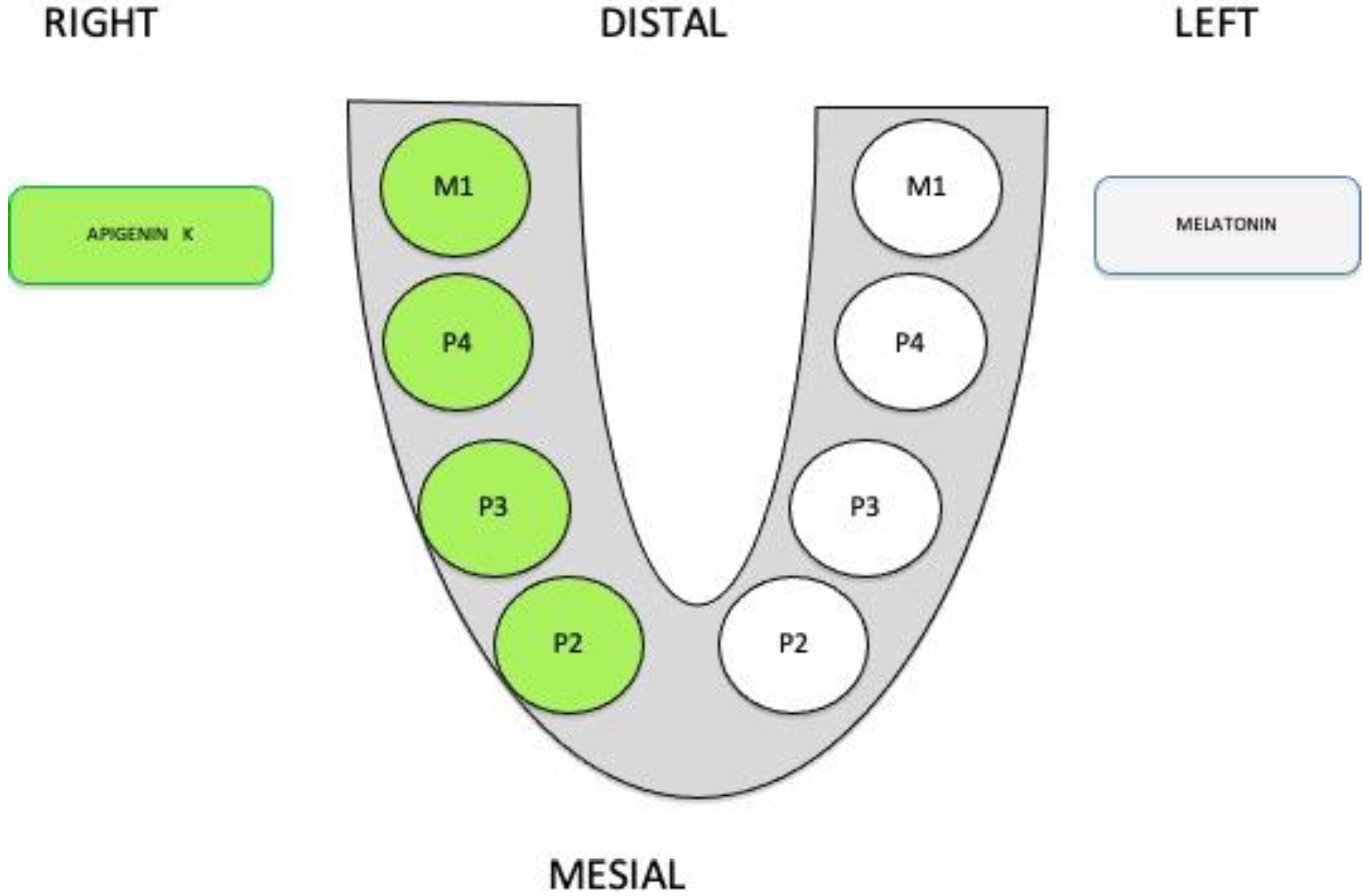
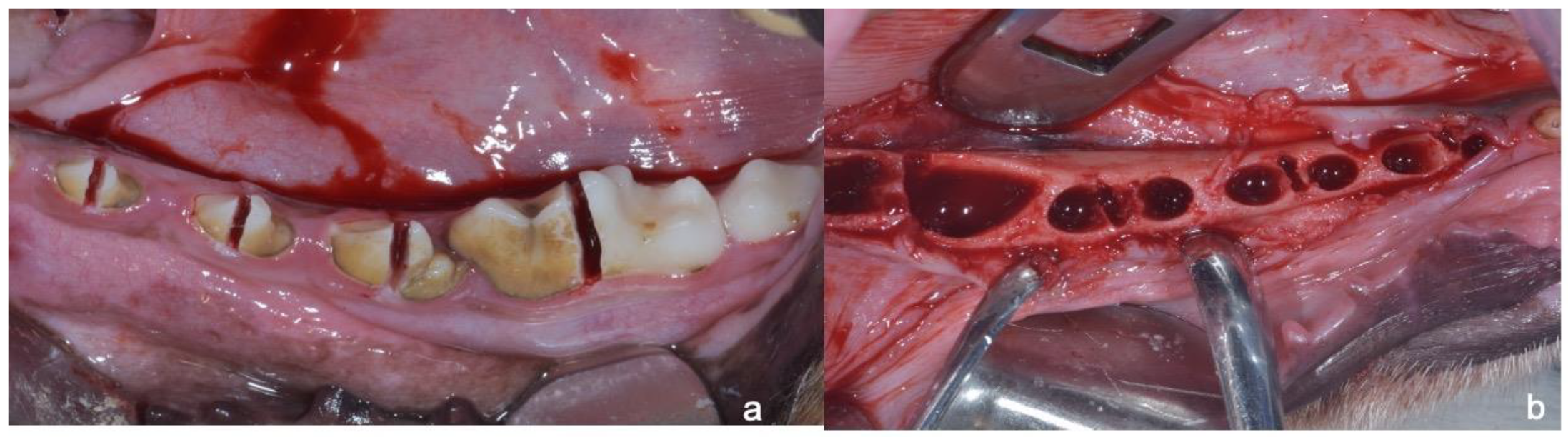
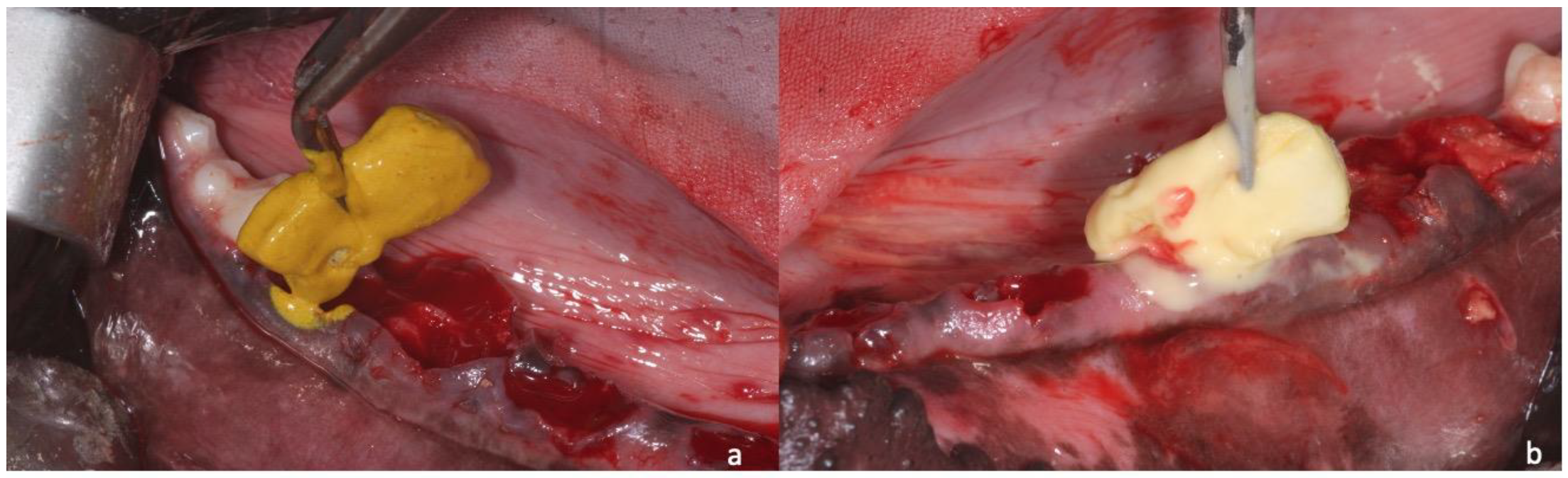
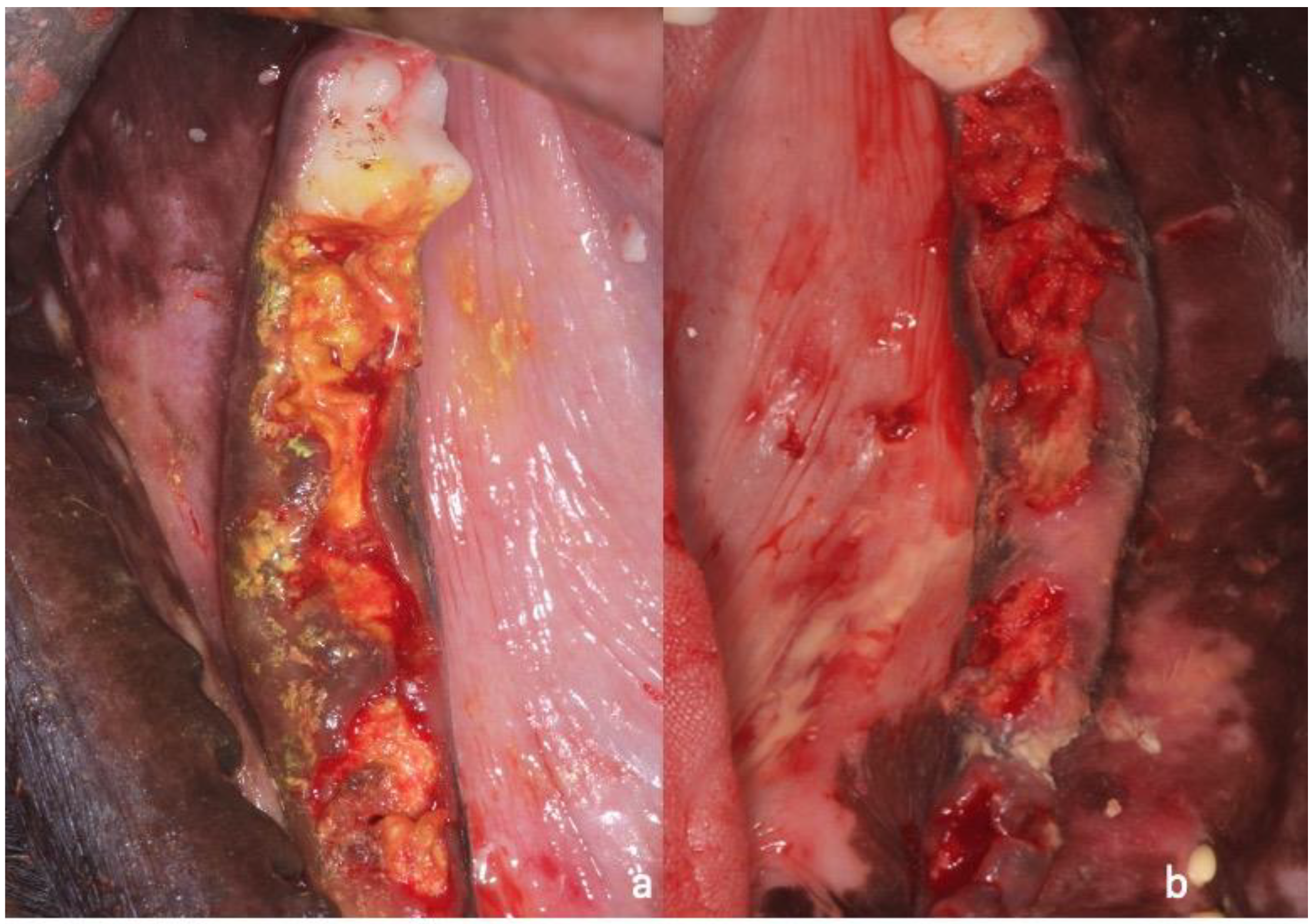
2. Materials and Methods
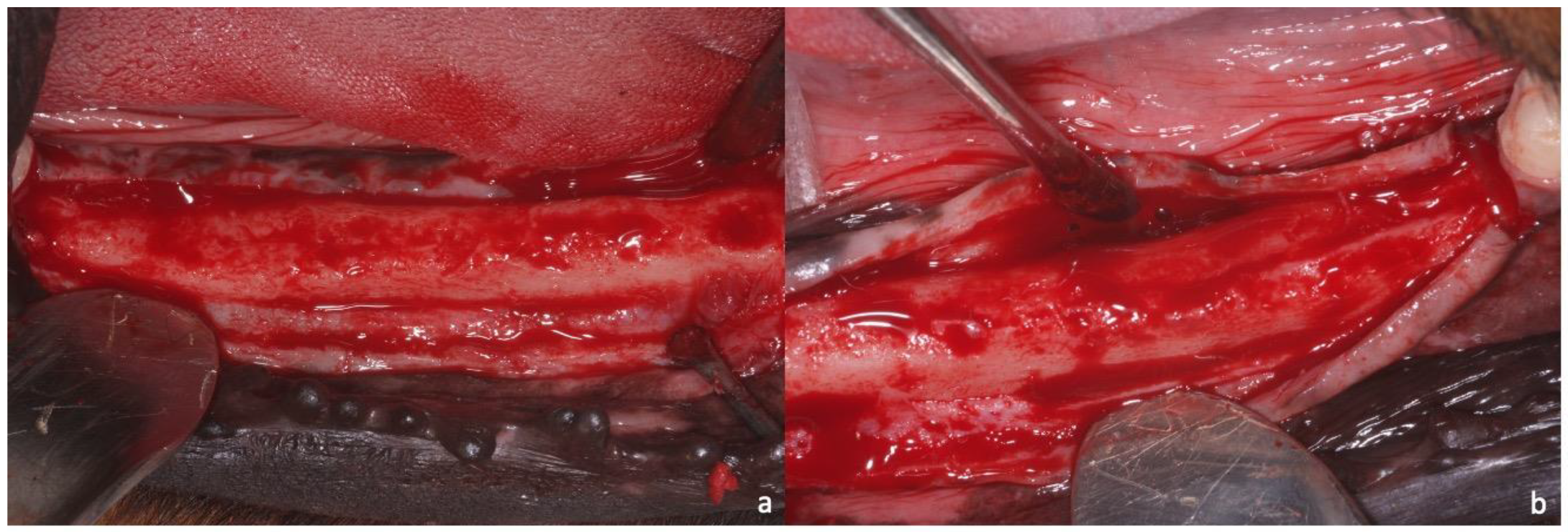
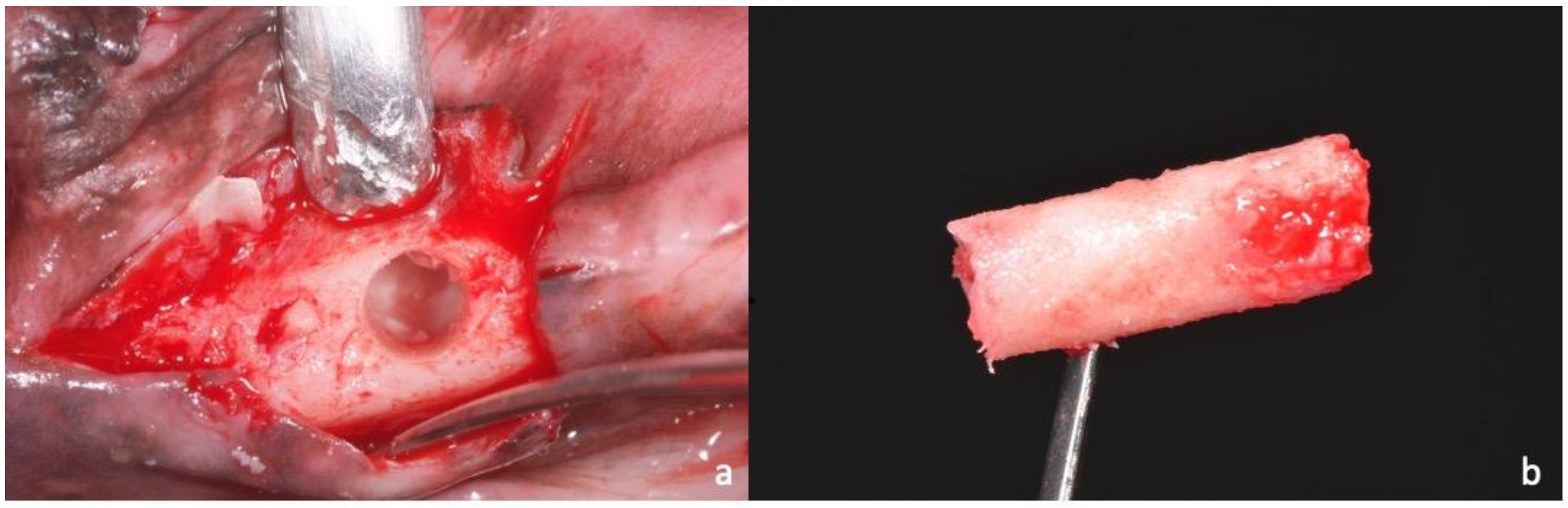
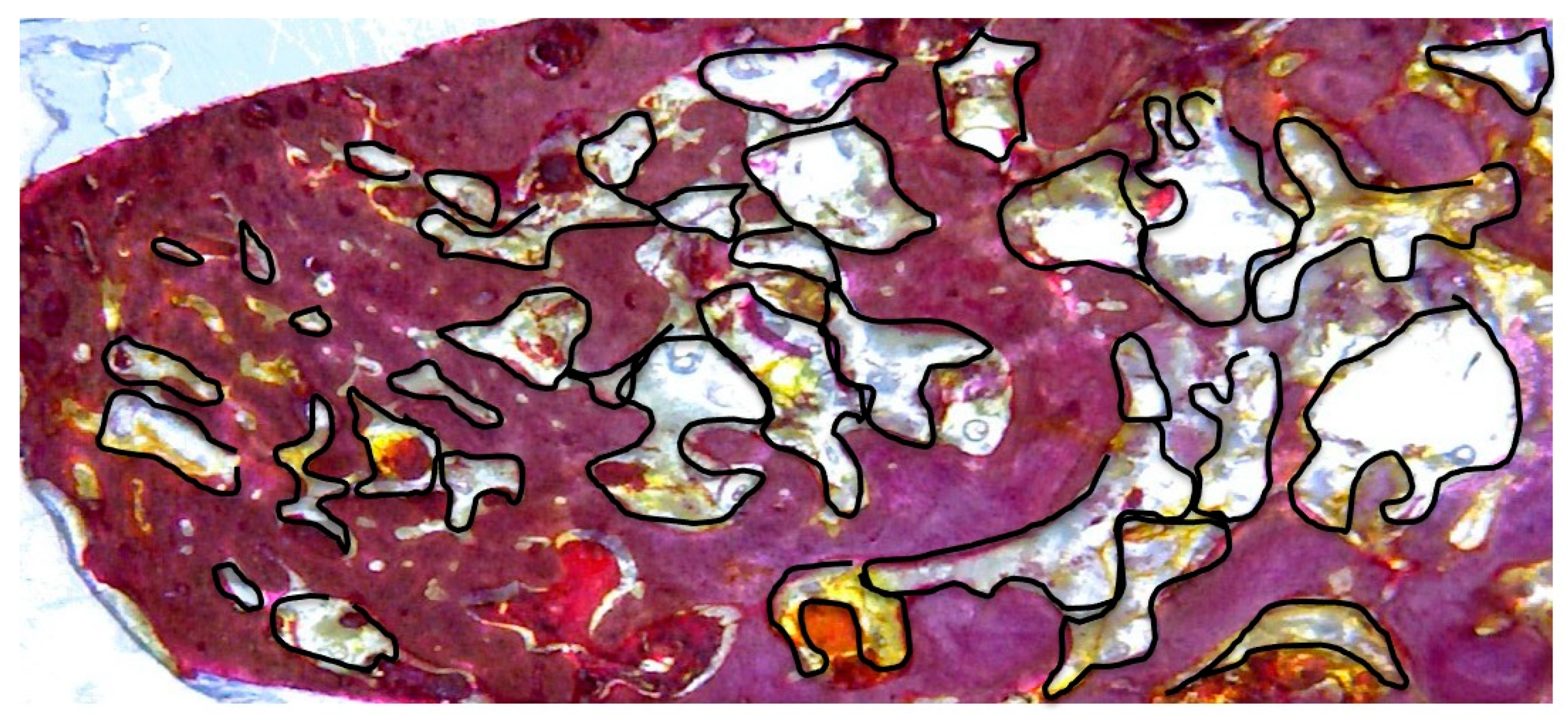
2.1. Sample Preparation, Histology, and Histomorphometry
2.2. Statistical Analysis
3. Results
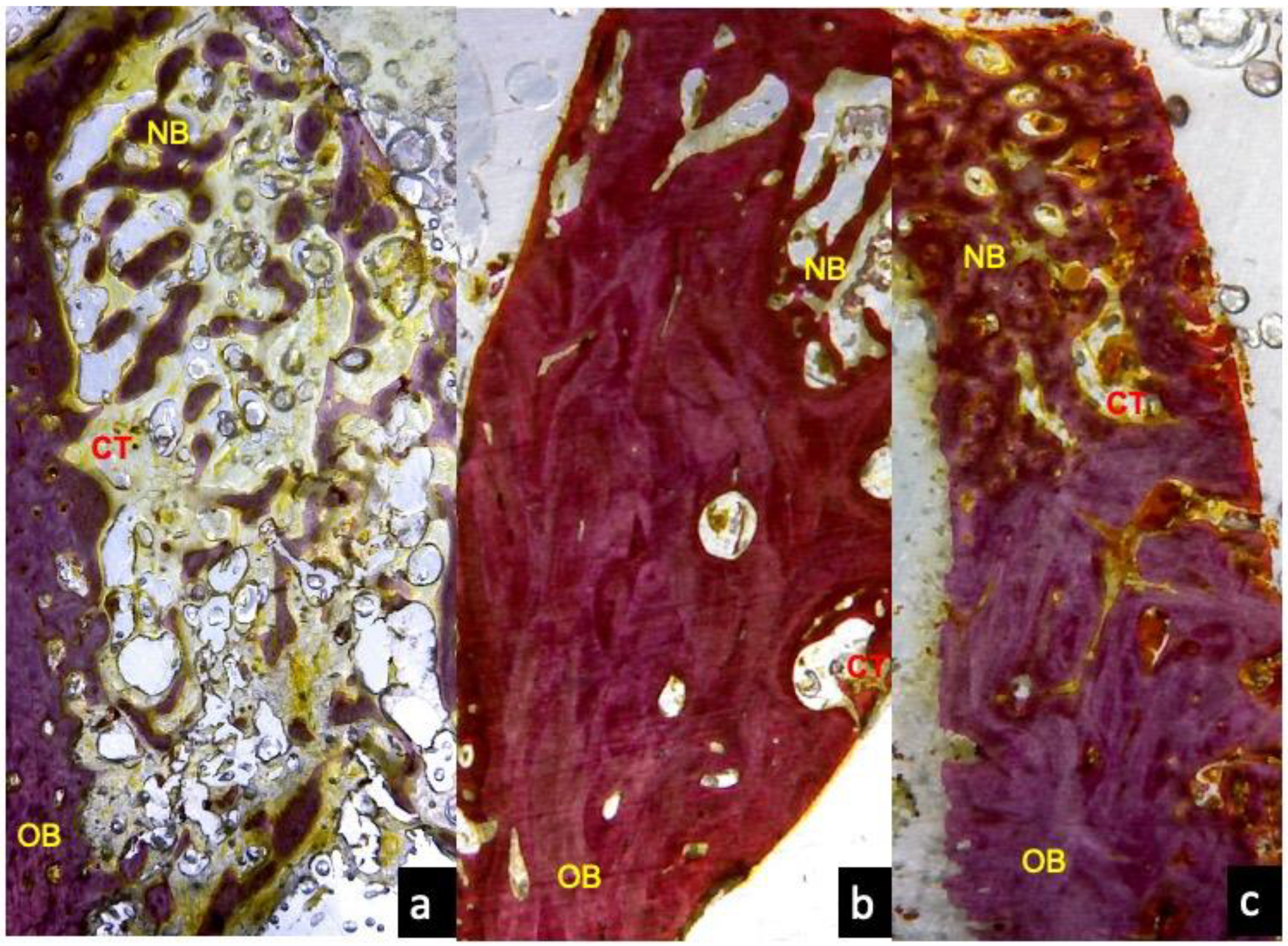
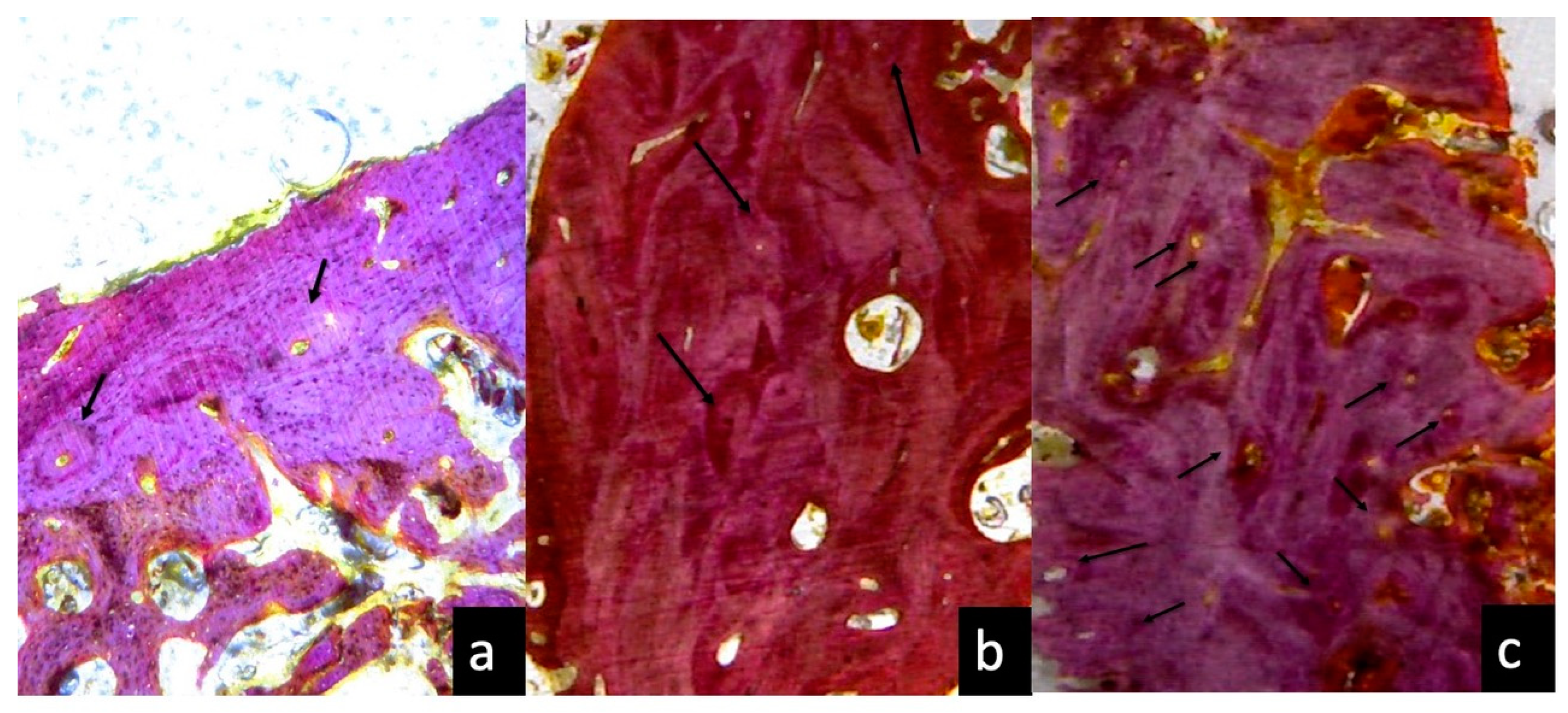
3.1. One Month Optical Microscopy
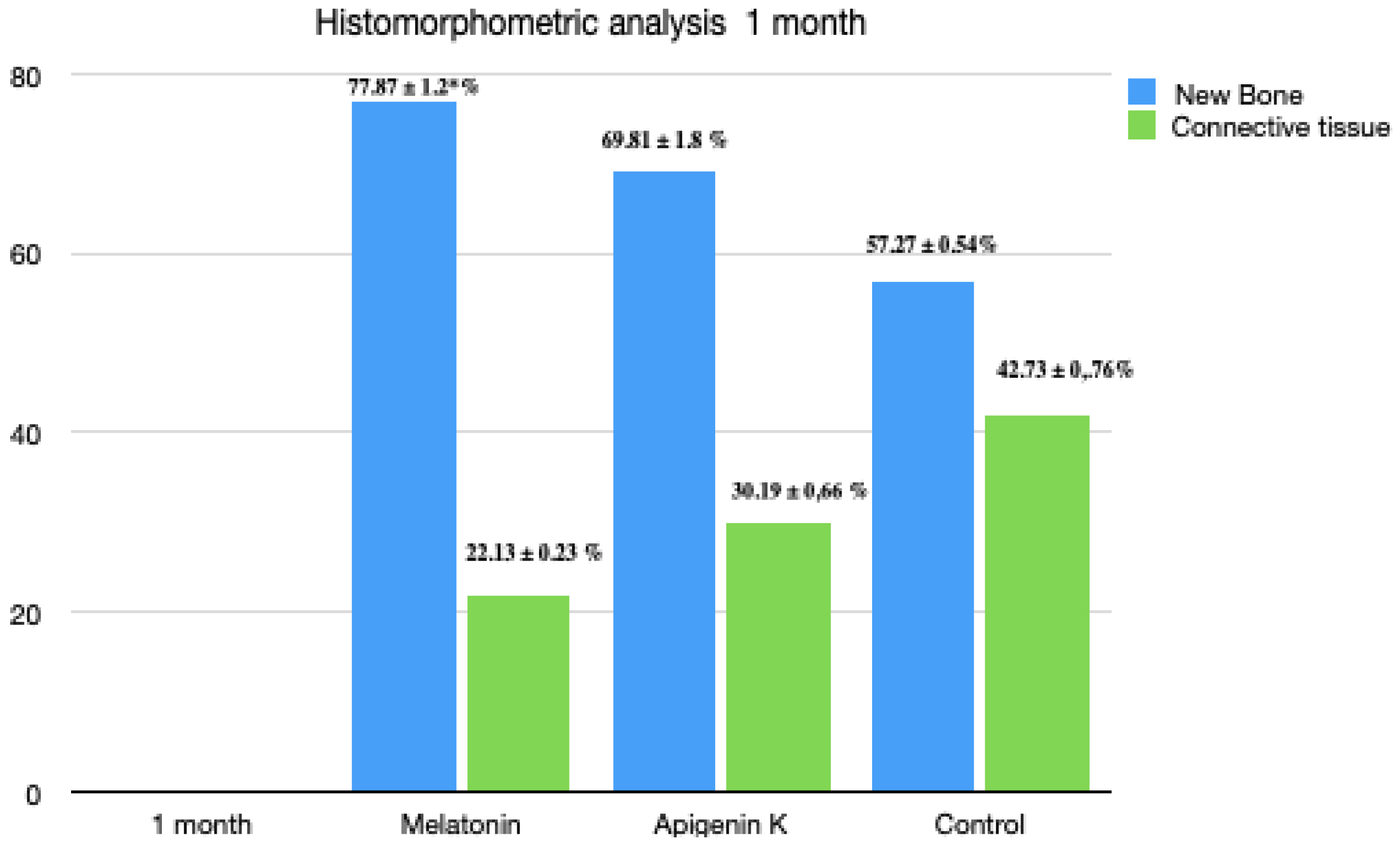
3.2. One Month Histomorphometric Analysis
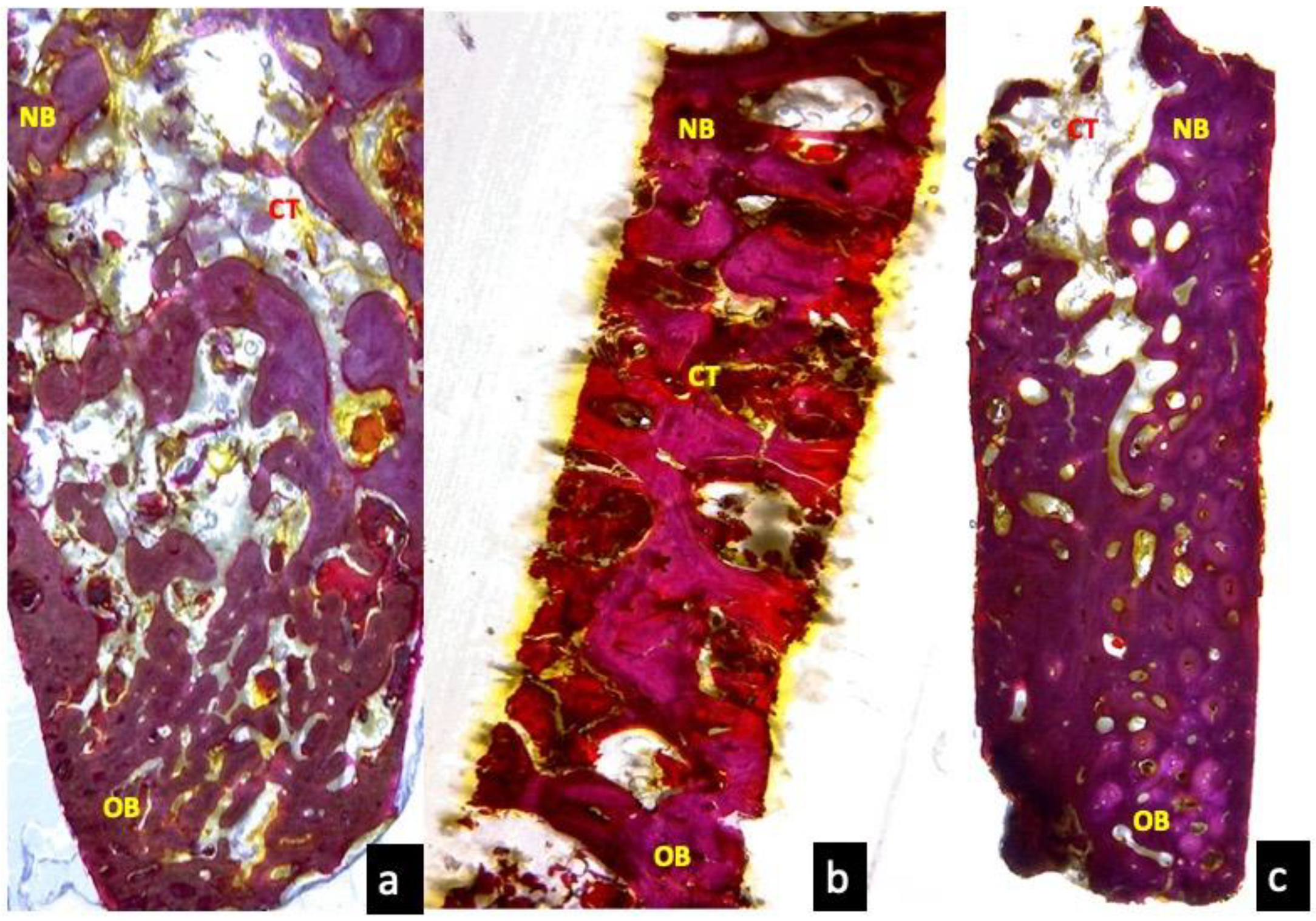
3.3. Two Months Optical Microscopy
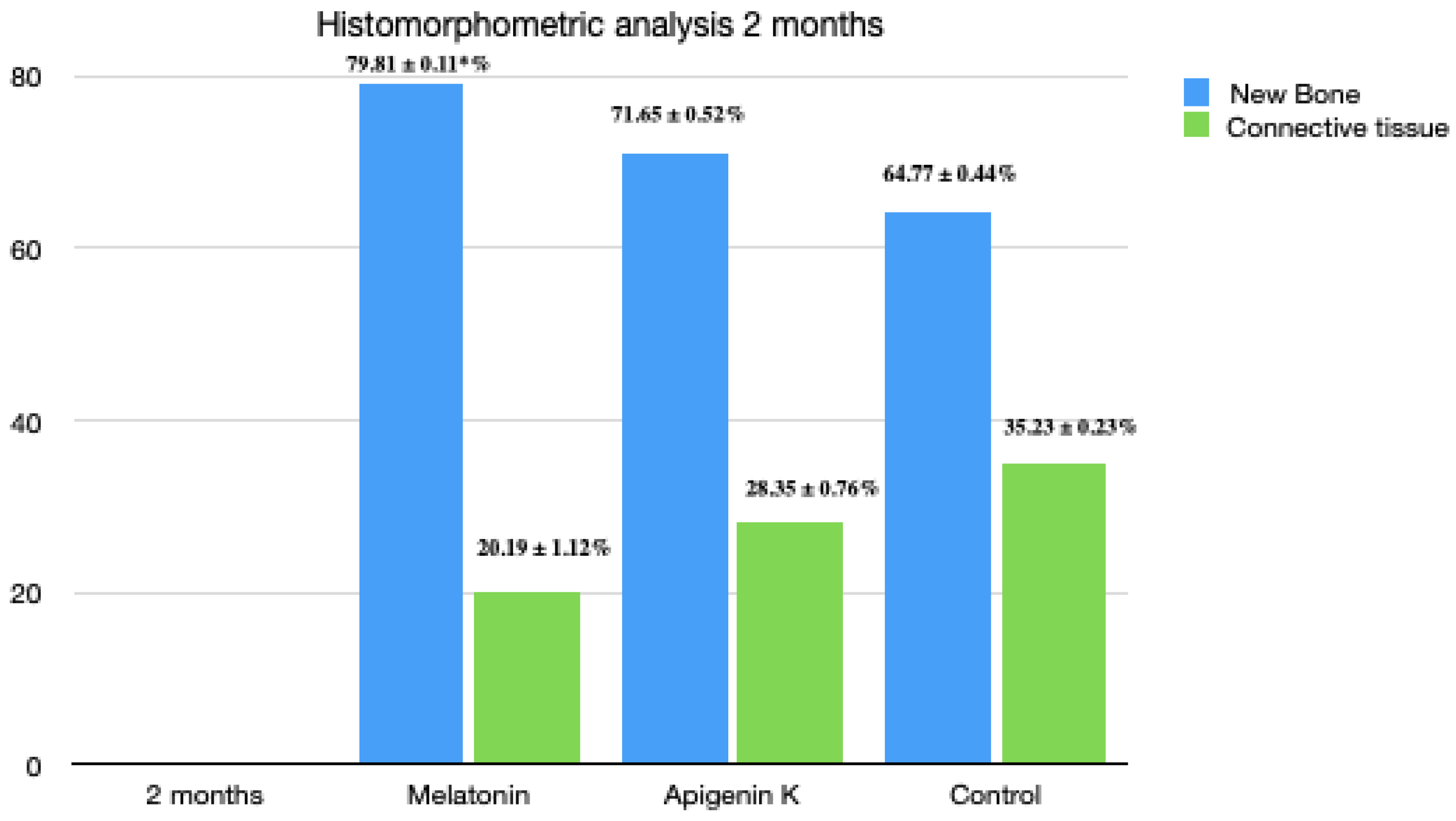
3.4. Two Months Histomorphometric Analysis
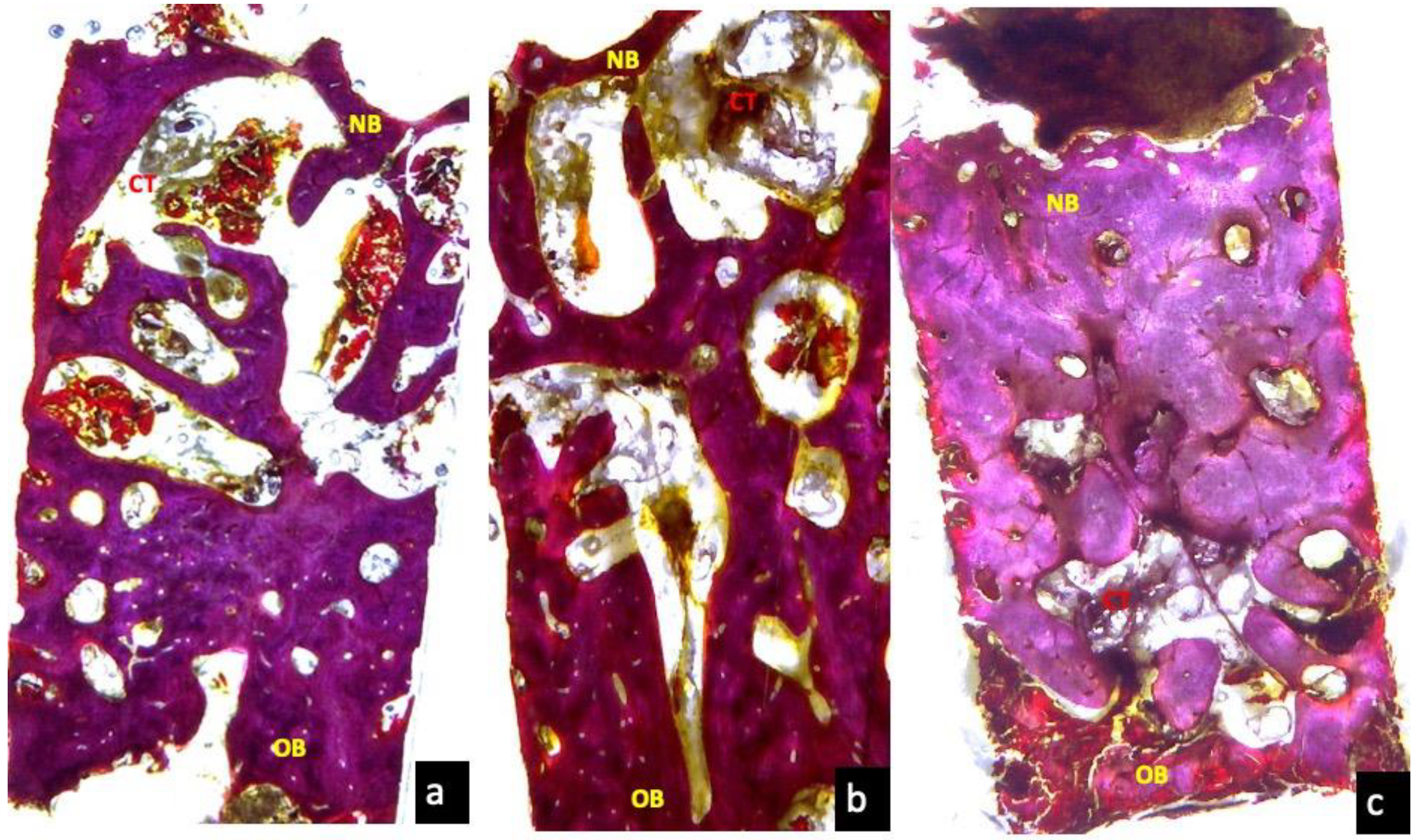
3.5. Three Months Optical Microscopy
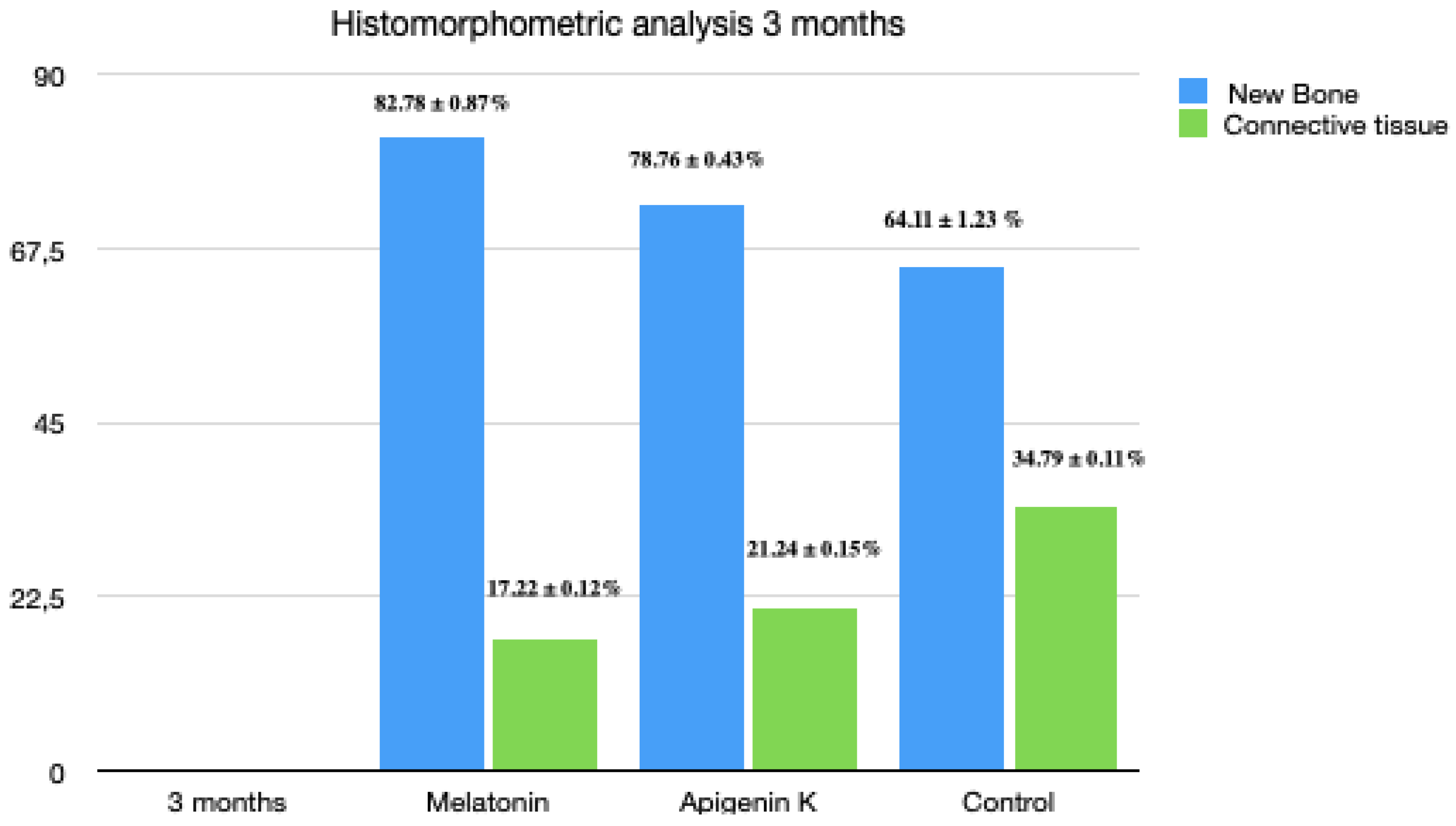
3.6. Three Months Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Araújo, M.G.; Sukekava, F.; Wennström, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Ridge alterations following tooth extraction with and without flap elevation: An experimental study in the dog. Clin. Oral Implant. Res. 2009, 20, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Januário, A.L.; Duarte, W.R.; Barriviera, M.; Mesti, J.C.; Araújo, M.G.; Lindhe, J. Dimension of the facial bone wall in the anterior maxilla: A cone-beam computed tomography study. Clin. Oral Implant. Res. 2011, 22, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Hard-tissue alterations following immediate implant placement in extraction sites. J. Clin. Periodontol. 2004, 31, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue engineering of bone: Search for a better scaffold. Orthod. Craniofacial Res. 2005, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef]
- Misawa, M.; Lindhe, J.; Araújo, M.G. The alveolar process following single-tooth extraction: A study of maxillary incisor and premolar sites in man. Clin. Oral Implant. Res. 2016, 27, 884–889. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Cegarra Del Pino, P.; Sapoznikov, L.; Delgado Ruiz, R.A.; Fernández-Domínguez, M.; Gehrke, S.A. A new procedure for processing extracted teeth for immediate grafting in post-extraction sockets. An experimental study in American Fox Hound dogs. Ann Anat. 2018, 217, 14–23, Erratum in: Ann Anat. 2018, 10, 218:213. [Google Scholar] [CrossRef]
- Maté Sánchez de Val, J.E.; Calvo-Guirado, J.L.; Gómez-Moreno, G.; Gehrke, S.; Mazón, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part B: A comparative study with biphasic synthetic biomaterials. Clin. Oral Implant. Res. 2018, 29, 1077–1084. [Google Scholar]
- Acuña-Castroviejo, D.; Reiter, R.J.; Menéndez-Peláez, A. Characterization of high-affinity melatonin binding sites in purified cell nuclei of rat liver. J. Pineal Res. 1994, 16, 100–112. [Google Scholar] [CrossRef]
- Hirose, T.; Smith, R.J.; Jetten, A.M. ROR gamma: The third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem. Biophys. Res. Commun. 1994, 205, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Hooft van Huijsduijnen, R.; Staple, J.K.; DeLamarter, J.F.; Becker-André, M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol. Endocrinol. 1994, 8, 757–770. [Google Scholar] [PubMed]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.A.; Mautalen, C. Melatonin effects bone: Experimental facts and clinical perspectives. J. Pineal Res. 2003, 34, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; Escames, G.; Leon, J.; Coto, A.; Sbihi, Y.; Osuna, A.; Acuña-Castroviejo, D. Calreticulin–melatonin. An unexpected relationship. Eur. J. Biochem. 2003, 270, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Somei, M.; Seki, A.; Reiter, R.J.; Hattori, A. Novel bromomelatonin derivatives as potentially effective drugs to treat bone diseases. J. Pineal Res. 2008, 45, 229–234. [Google Scholar] [CrossRef]
- Roth, J.A.; Kim, B.G.; Lin, W.L.; Cho, M.I. Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. 1999, 274, 22041–22047. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; Barone, A.; Cutando, A.; Alcaraz-Baños, M.; Chivas, F.; López-Marí, L.; Guardia, J. Melatonin plus porcine bone on discrete calcium deposit implant surface stimulates osteointegration in dental implants. J. Pineal Res. 2009, 47, 164–172. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Gómez-Moreno, G.; López-Marí, L.; Guardia, J.; Martínez-González, J.M.; Barone, A.; Tresguerres, I.F.; Paredes, S.D.; Fuentes-Breto, L. Actions of melatonin mixed with collagenized porcine bone versus porcine bone only on osteointegration of dental implants. J. Pineal Res. 2010, 48, 194–203. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Ramírez-Fernández, M.P.; Gómez-Moreno, G.; Maté-Sánchez, J.E.; Delgado-Ruiz, R.; Guardia, J.; López-Marí, L.; Barone, A.; Ortiz-Ruiz, A.J.; Martínez-González, J.M.; et al. Melatonin stimulates the growth of new bone around implants in the tibia of rabbits. J. Pineal Res. 2010, 49, 356–363. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derives: A never ending interaction of melatonin with reactive oxygen and reactive nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, F.; He, H.W. Melatonin effects on hard tissues: Bone and tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Acuña-Castroviejo, D.; Reiter, R.J. Melatonin: Potential functions in the oral cavity. J. Periodontology 2007, 78, 1094–1102. [Google Scholar] [CrossRef]
- Cutando, A.; Aneiros-Fernández, J.; López-Valverde, A.; Arias-Santiago, S.; Aneiros-Cachaza, J.; Reiter, R.J. A new perspective in Oral health: Potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. Arch. Oral Biol. 2011, 56, 944–950. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E.; Guerra-Librero, A.; Florido, J.; Shen, Y.Q.; Fernández-Gil, B.; Acuña-Castroviejo, D.; Escames, G. Oral Mucositis: Melatonin Gel an Effective New Treatment. Int. J. Mol. Sci. 2017, 7, 18. [Google Scholar] [CrossRef]
- Guerrero-Gironés, J.; Alcaina-Lorente, A.; Ortiz-Ruiz, C.; Ortiz-Ruiz, E.; Pecci-Lloret, M.P.; Rodríguez-Lozano, F.J.; Martínez, C.M.; Ortiz-Ruiz, A.J. Melatonin as an Agent for Direct Pulp-Capping Treatment. Int. J. Env. Res. Public Health 2020, 6, 17. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Zamfir-Chiru-Anton, A.; Stanciu, M.M.; Stoian, A.P.; Jinga, V.; Nitipir, C.; Bucur, A.; Pituru, T.S.; Arsene, A.L.; Dragoi, C.M.; et al. Clinical significance of serum melatonin in predicting the severity of oral squamous cell carcinoma. Oncol. Lett. 2020, 19, 1537–1543. [Google Scholar] [CrossRef]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, E865. [Google Scholar] [CrossRef]
- Lee, J.A.; Ha, S.K.; Cho, E.; Choi, I. Resveratrol as a Bioenhancer to Improve Anti-Inflammatory Activities of Apigenin. Nutrients 2015, 7, 9650–9661. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ukai, T.; Yoshimura, A.; Kozuka, Y.; Yoshioka, H.; Yoshinaga, Y.; Abe, Y.; Hara, Y. Green tea catechin inhibits lipopolysaccharide-induced bone resorption in vivo. J. Periodontal Res. 2010, 45, 23–30. [Google Scholar] [CrossRef]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, E288. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Martínez-Tébar, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E. Antioxidant and Photoprotective Activity of Apigenin and its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, E2148. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Tanabe, S.; Grenier, D. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal Res. 2009, 44, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, E921. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Lim, J.H.; Lim, B.O. Apigenin Attenuates Melanoma Cell Migration by Inducing Anoikis through Integrin and Focal Adhesion Kinase Inhibition. Molecules 2015, 20, 21157–21166. [Google Scholar] [CrossRef]
- Park, J.A.; Ha, S.K.; Kang, T.H.; Oh, M.S.; Cho, M.H.; Lee, S.Y.; Park, J.H.; Kim, S.Y. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci. 2008, 20, 1217–1223. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 13, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Strewler, G.J. Local and systemic control of the osteoblast. J. Clin. Investig. 2001, 107, 271–272. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Si, J.; Wang, B.; Zhang, D.; Ding, D.; Zhang, J.; Wang, H. Melatonin up-regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2-inactivated NF-κB pathway. Br. J. Pharmacol. 2020, 177, 2106–2122. [Google Scholar] [CrossRef]
- Omori, Y.; Iezzi, G.; Perrotti, V.; Piattelli, A.; Ferri, M.; Nakajima, Y.; Botticelli, D. Influence of the Buccal Bone Crest Width on Peri-Implant Hard and Soft Tissues Dimensions: A Histomorphometric Study in Humans. Implant. Dent. 2018, 27, 415–423. [Google Scholar] [CrossRef]
- Maria, S.; Samsonraj, R.M.; Munmun, F.; Glas, J.; Silvestros, M.; Kotlarczyk, M.P.; Rylands, R.; Dudakovic, A.; van Wijnen, A.J.; Enderby, L.T.; et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 2018, 64, e12465. [Google Scholar] [CrossRef]
- Martínez-Sanz, E.; Ossipov, D.A.; Hilborn, J.; Larsson, S.; Jonsson, K.B.; Varghese, O.P. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Control. Release 2011, 152, 230–240. [Google Scholar] [CrossRef]
- Ramírez-Fernández, M.P.; Calvo-Guirado, J.L.; de-Val, J.E.; Delgado-Ruiz, R.A.; Negri, B.; Pardo-Zamora, G.; Peñarrocha, D.; Barona, C.; Granero, J.M.; Alcaraz-Baños, M. Melatonin promotes angiogenesis during repair of bone defects: A radiological and histomorphometric study in rabbit tibiae. Clin. Oral Investig. 2013, 17, 147–158. [Google Scholar] [CrossRef]
- Gómez-Moreno, G.; Guardia, J.; Ferrera, M.J.; Cutando, A.; Reiter, R.J. Melatonin in diseases of the oral cavity. Oral Dis. 2010, 16, 242–247. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Radio, N.M.; Doctor, J.S.; Davis, V.L. Therapeutic treatments potentially mediated by melatonin receptors: Potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 2006, 41, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Salomó-Coll, O.; de Maté-Sánchez, J.E.; Ramírez-Fernandez, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Osseoinductive elements around immediate implants for better osteointegration: A pilot study in foxhound dogs. Clin. Oral Implant. Res. 2018, 29, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Guardia, J.; Gómez-Moreno, G.; Ferrera, M.J.; Cutando, A. Evaluation of effects of topic melatonin on implant surface at 5 and 8 weeks in Beagle dogs. Clin. Implant. Dent. Relat. Res. 2011, 13, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Minegishi, T.; Takayama, T.; Tamura, T.; Yamada, Y.; Sato, S. Effects of ipriflavone on augmented bone using a guided bone regeneration procedure. Clin. Oral Implant. Res. 2007, 18, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Guskuma, M.H.; Hochuli-Vieira, E.; Pereira, F.P.; Rangel-Garcia Junior, I.; Okamoto, R.; Okamoto, T.; Magro-Filho, O. Bone regeneration in surgically created defects filled with autogenous bone: An epifluorescence microscopy analysis in rats. J. Appl. Oral Sci. 2010, 18, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Popović, M.; Caballero-Bleda, M.; Benavente-García, O.; Castillo, J. The flavonoid apigenin delays forgetting of passive avoidance conditioning in rats. J. Psychopharmacol. 2014, 28, 498–501. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Hhan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, E35. [Google Scholar] [CrossRef]
- Songlin, P.; Ge, Z.; Yixin, H.; Xinluan, W.; Pingchung, L.; Kwoksui, L.; Ling, Q. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone 2009, 45, 534–544. [Google Scholar] [CrossRef]
- Maki, K.; Nishida, I.; Mitsutaka, K. The effect of oral ipriflavone on the rat mandible during growth. Eur. J. Orthod. 2005, 27, 27–31. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Kos-Kudla, B.; Nowak, M.; Swietochowska, E.; Marek, B.; Gorski, J.; Kajdaniuk, D.; Wolkowska, K. The relationship between bone metabolism, melatonin and other hormones in sham-operated and pinealectomized rats. Endocr. Regul. 2003, 37, 211–224. [Google Scholar]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, Anti-Inflammatory, and Anticancer Efects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Swarnkar, G.; Sharan, K.; Chakravarti, B.; Sharma, G.; Rawat, P.; Kumar, M.; Khan, F.M.; Pierroz, D.; Maurya, R.; et al. 8,8″-Biapigeninyl stimulates osteoblast functions and inhibits osteoclast and adipocyte functions: Osteoprotective action of 8,8″-biapigeninyl in ovariectomized mice. Mol. Cell Endocrinol. 2010, 29, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Lion, J.M.; Mentaverri, R.; Ricupero, D.A.; Kamel, S.; Romero, J.R.; Chattopadhyay, N. Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 2006, 14, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Apigenin Induces Apoptosis through a Mitochondria/Caspase-Pathway in Human Breast Cancer MDA-MB-453 Cells. J. Clin. Biochem. Nutr. 2009, 44, 260–265. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Mak, P.; Leung, Y.K.; Tang, W.Y.; Harwood, C.; Ho, S.M. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia 2006, 8, 896–904. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Guirado, J.L.; Fernández-Domínguez, M.; Aragoneses, J.M.; Fernández-Bodereau, E.; Garcés-Villalá, M.A.; De Carlos-Villafranca, F.; Cabello-Colás, M.; Jiménez-López, R.; Pérez Albacete-Martínez, C.; Delgado-Ruiz, R.A. RETRACTED: Experimental Study on the Influence of Apigenin K and Melatonin in Socket Preservation as Bone Stimulators: An Experimental Study in Beagle Dogs. Appl. Sci. 2020, 10, 3006. https://doi.org/10.3390/app10093006
Calvo-Guirado JL, Fernández-Domínguez M, Aragoneses JM, Fernández-Bodereau E, Garcés-Villalá MA, De Carlos-Villafranca F, Cabello-Colás M, Jiménez-López R, Pérez Albacete-Martínez C, Delgado-Ruiz RA. RETRACTED: Experimental Study on the Influence of Apigenin K and Melatonin in Socket Preservation as Bone Stimulators: An Experimental Study in Beagle Dogs. Applied Sciences. 2020; 10(9):3006. https://doi.org/10.3390/app10093006
Chicago/Turabian StyleCalvo-Guirado, José Luis, Manuel Fernández-Domínguez, Juan Manuel Aragoneses, Enrique Fernández-Bodereau, Miguel Angel Garcés-Villalá, Felix De Carlos-Villafranca, Manuel Cabello-Colás, Rocío Jiménez-López, Carlos Pérez Albacete-Martínez, and Rafael Arcesio Delgado-Ruiz. 2020. "RETRACTED: Experimental Study on the Influence of Apigenin K and Melatonin in Socket Preservation as Bone Stimulators: An Experimental Study in Beagle Dogs" Applied Sciences 10, no. 9: 3006. https://doi.org/10.3390/app10093006
APA StyleCalvo-Guirado, J. L., Fernández-Domínguez, M., Aragoneses, J. M., Fernández-Bodereau, E., Garcés-Villalá, M. A., De Carlos-Villafranca, F., Cabello-Colás, M., Jiménez-López, R., Pérez Albacete-Martínez, C., & Delgado-Ruiz, R. A. (2020). RETRACTED: Experimental Study on the Influence of Apigenin K and Melatonin in Socket Preservation as Bone Stimulators: An Experimental Study in Beagle Dogs. Applied Sciences, 10(9), 3006. https://doi.org/10.3390/app10093006







