The Prevalence of Endoparasites of Free Ranging Cats (Felis catus) from Urban Habitats in Southern Poland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection and Parasitological Analysis
2.3. Diet Analysis
2.4. Statistical Analysis
3. Results
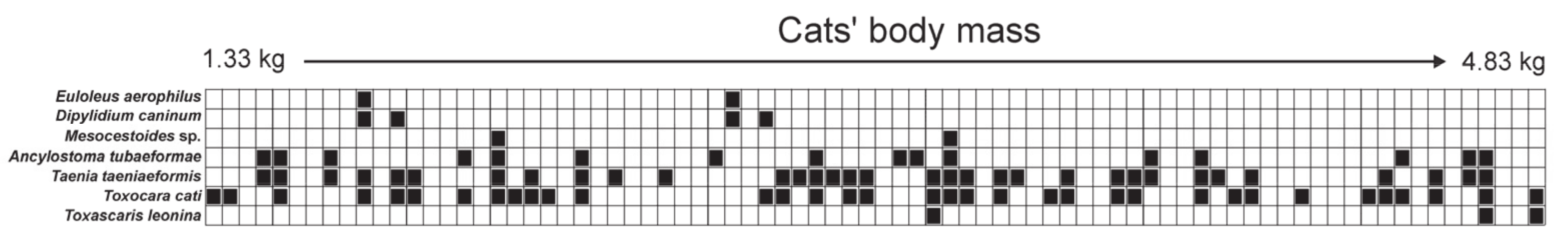
3.1. Prevalence of Endoparasites
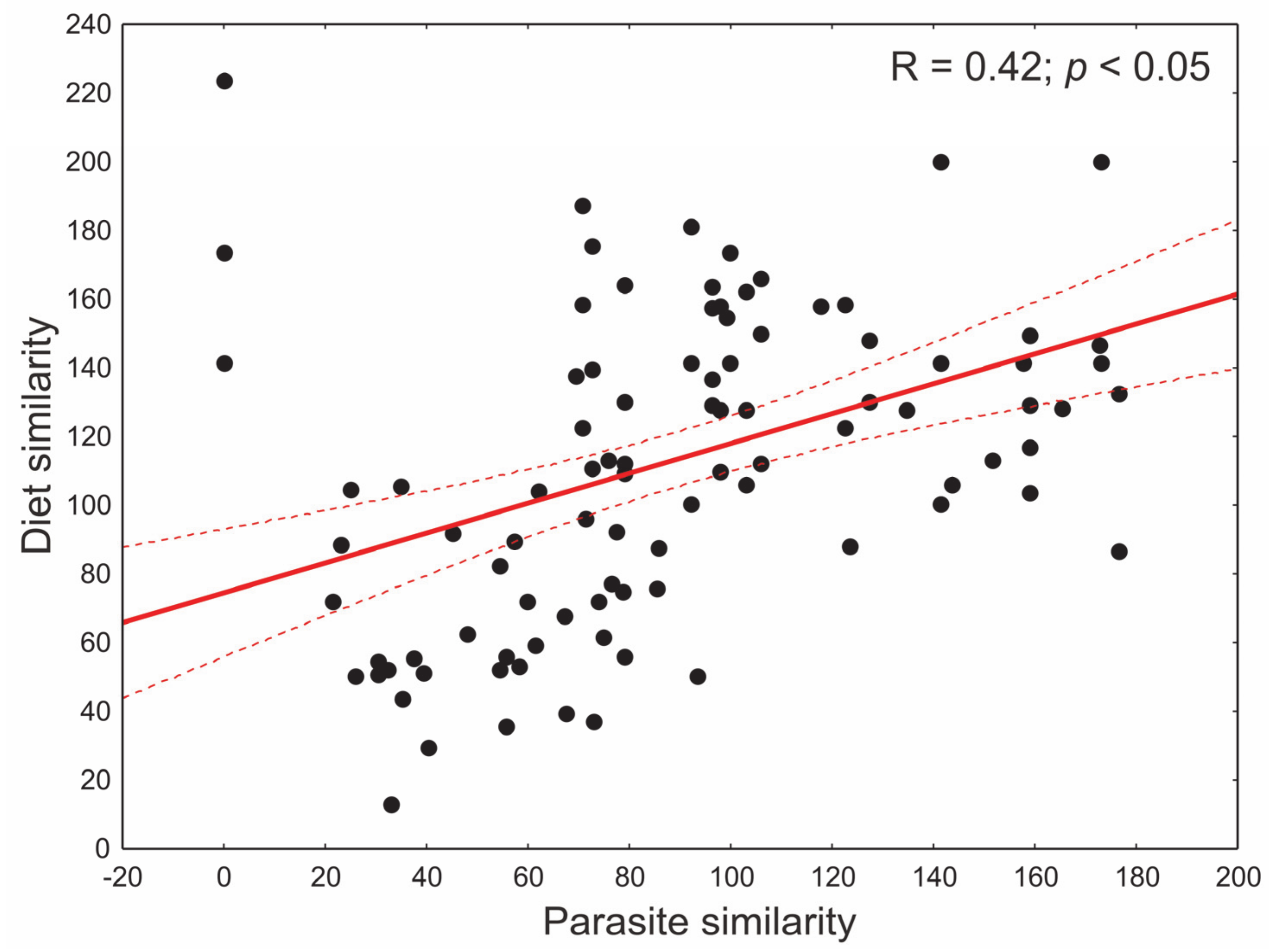
3.2. Diet Analysis
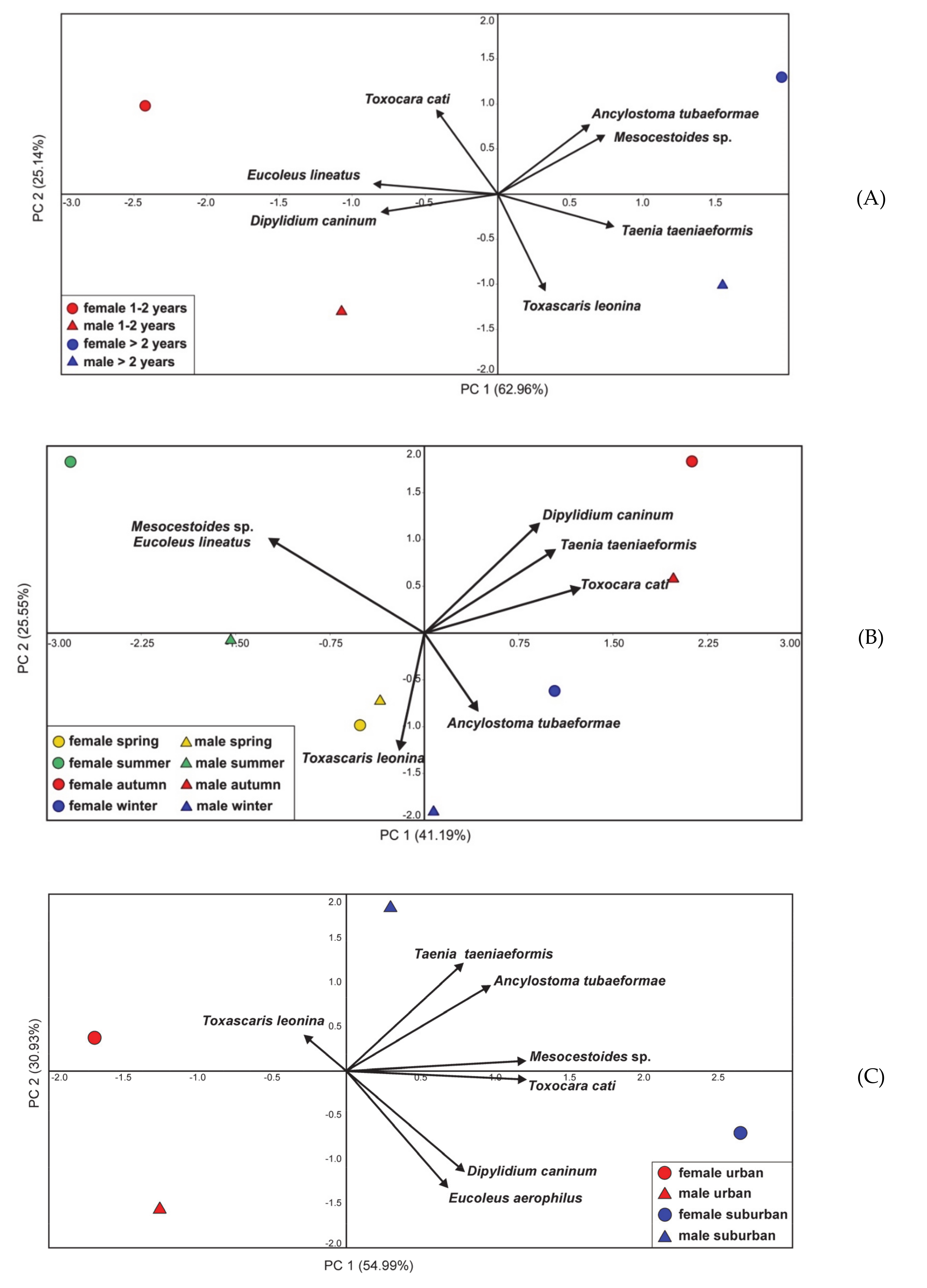
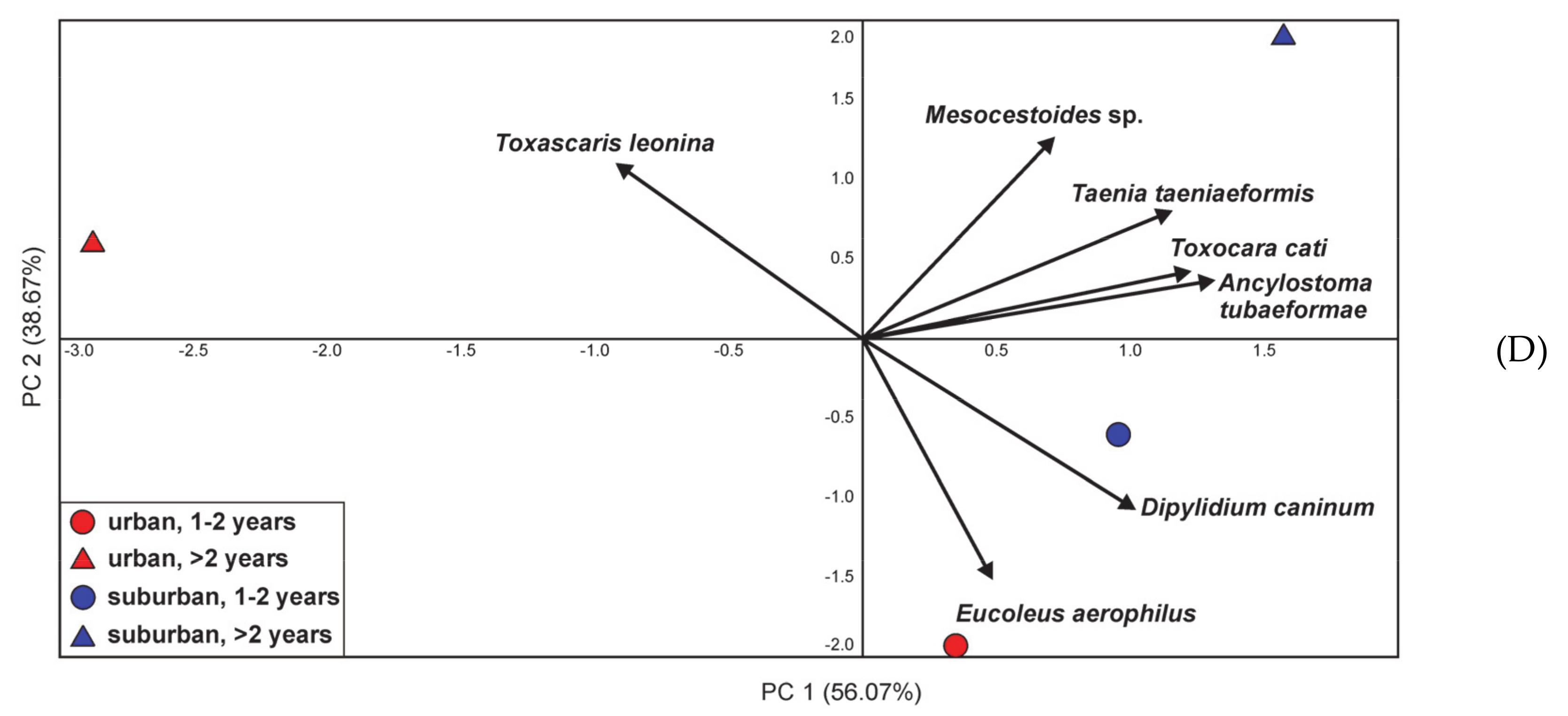
3.3. Differentiation in the Occurrence of Parasites in Cats and Its Relationship with Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.; Barber, I.; Boag, B.; Ellison, A.R.; Morgan, E.R.; Murray, K.; Pascoe, E.L.; Sait, S.M.; Wilson, A.J.; Booth, M. Global change, parasite transmission and disease control: Lessons from ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160088. [Google Scholar] [CrossRef] [PubMed]
- Koelle, K.; Pascual, M.; Yunus, M. Pathogen adaptation to seasonal forcing and climate change. Proc. R Soc. Lond. B Biol. Sci. 2005, 272, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Thieltges, D.W.; Poulin, R. Parasites and global warming: Net effects of temperature on an intertidal host–parasite system. Mar. Ecol. Prog. Ser. 2010, 415, 11–22. [Google Scholar] [CrossRef]
- Ogden, L.E. Climate change, pathogens, and people. Bioscience 2018, 68, 733–739. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Urbanization. Our World in Data. Available online: https://ourworldindata.org/urbanization (accessed on 27 February 2020).
- Symeonidou, I.; Gelasakis, A.I.; Arsenopoulos, K.; Angelou, A.; Beugnet, F.; Papadopoulos, E. Feline gastrointestinal parasitism in Greece: Emergent zoonotic species and associated risk factors. Parasit Vectors 2018, 11, 227. [Google Scholar] [CrossRef]
- Strube, C.; Neubert, A.; Springer, A.; von Samson-Himmelstjerna, G. Survey of German pet owners quantifying endoparasitic infection risk and implications for deworming recommendations. Parasit Vectors 2019, 12, 203. [Google Scholar] [CrossRef]
- Nagamori, Y.; Payton, M.E.; Looper, E.; Apple, H.; Johnson, E.M. Retrospective survey of parasitism identified in feces of client-owned cats in North America from 2007 through 2018. Vet. Parasitol. 2020, 277, 109008. [Google Scholar] [CrossRef]
- Silva-Rodriguez, E.A.; Sieving, K.E. Influence of Care of Domestic Carnivores on Their Predation on Vertebrates. Conserv. Biol. 2011, 25, 808–815. [Google Scholar] [CrossRef]
- FEDIAF Facts & Figures. Available online: http://www.fediaf.org/52-dcs-statistics (accessed on 15 December 2019).
- Friedmann, E.; Son, H. The human-companion animal bond: How humans benefit. Vet. Clin. North. Am. Small Anim. Pract. 2009, 39, 293–326. [Google Scholar] [CrossRef]
- Nyambura Njuguna, A.; Kagira, J.M.; Muturi Karanja, S.; Ngotho, M.; Mutharia, L.; Wangari Maina, N. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika region, Kenya. Biomed. Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chalkowski, K.; Wilson, A.E.; Lepczyk, C.A.; Zohdy, S. Who let the cats out? A global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus). Biol. Lett. 2019, 15, 20180840. [Google Scholar] [CrossRef] [PubMed]
- Carver, S.; Bevins, S.N.; Lappin, M.R.; Boydston, E.E.; Lyren, L.M.; Alldredge, M.; VandeWoude, S. Pathogen exposure varies widely among sympatric populations of wild and domestic felids across the United States. Ecol. Appl. 2016, 26, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.L.; Cattori, V.; Martínez, F.; López, G.; Vargas, A.; Simón, M.A.; Zorrilla, I.; Muñoz, A.; Palomares, F.; López-Bao, J.V.; et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Beugnet, F.; Bourdeau, P.; Chalvet-Monfray, K.; Cozma, V.; Farkas, R.; Guillot, J.; Rinaldi, L. Parasites of domestic owned cats in Europe: Co-infestations and risk factors. Parasit Vectors. 2014, 7, 291. [Google Scholar] [CrossRef]
- Little, S.E.; Marrinson, R. Feline Helminths: Recommendations from the Companion Animal Parasite Council. Today’s Vet. Pract. 2014, 4, 39–43. [Google Scholar]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef]
- Ilić, T.; Kulišić, Z.; Antić, N.; Radisavljević, K.; Dimitrijević, S. Prevalence of zoonotic intestinal helminths in pet dogs and cats in the Belgrade area. J. Appl. Anim. Res. 2017, 45, 204–208. [Google Scholar] [CrossRef]
- Kleine, A.; Springer, A.; Strube, C. Seasonal variation in the prevalence of Toxocara eggs on children’s playgrounds in the city of Hanover, Germany. Parasite Vector. 2017, 10, 248. [Google Scholar] [CrossRef]
- Bojar, H.; Kłapeć, T. Contamination of soil with eggs of geohelminths in recreational areas in the Lublin region of Poland. Ann. Agric. Environ. Med. 2012, 19, 267–270. [Google Scholar] [PubMed]
- Borecka, A.; Kłapec, T. Epidemiology of human toxocariasis in Poland-A review of cases 1978-2009. Ann. Agric. Environ. Med. 2015, 22, 28–31. [Google Scholar] [CrossRef]
- Szwabe, K.; Blaszkowska, J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann. Agric. Environ. Med. 2017, 24, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kornaś, S.; Wierzbowska, I.A.; Górski, P.; Okarma, H. Occurrence of internal parasites in stone martens (Martes foina) from Cracow and suburbs. Ann. Parasitol. 2013, 59, 203–205. [Google Scholar] [PubMed]
- Traversa, D.; Di Cesare, A. Diagnosis and management of lungworm infections in cats: Cornerstones, dilemmas and new avenues. J. Feline Med. Surg. 2016, 18, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Statistical Office in Kraków. Available online: https://krakow.stat.gov.pl (accessed on 16 December 2019).
- KRKNEWS.PL. Available online: https://krknews.pl/ile-kotow-mieszka-w-krakowie-zaskakujace-wyniki-badan (accessed on 2 February 2020).
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J. Alveolar echinococcosis in Europe and Poland. Threats to humans. Przegl. Epidemiol. 2016, 70, 281–288. [Google Scholar]
- Takeuchi-Storm, N.; Mejer, H.; Al-Sabi, M.N.; Olsen, C.S.; Thamsborg, S.M.; Enemark, H.L. Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration McMaster technique. Vet. Parasitol. 2015, 214, 327–332. [Google Scholar] [CrossRef]
- McDonough, S.P.; Southard, T.L. Necropsy guide for dogs, cats, and small mammals; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods & Protozoa of Domesticated Animals. In 6th Edition of Mönnig’s Veterinary Helminthology & Entomology; Lea & Febiger: Philadelphia, PA, USA, 1977. [Google Scholar]
- Pucek, Z. Klucz do Oznaczania Ssaków Polski; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1984. [Google Scholar]
- Teerink, B.J. Hair of West European Mammals: Atlas and Identification Key; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Dove, C.J.; Koch, S.L. Microscopy of feathers: A practical guide for forensic feather identification. Microsc. Chic. 2011, 59, 51. [Google Scholar]
- Newcombe, R.G. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Brower, J.C.; Kile, K.M. Seriation of an original data matrix as applied to palaeoecology. Lethaia 1988, 21, 79–93. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Lavanya, K.; Malkar, S.; Pednekar, R.; Gatne, M. Prevalence of Gastrointestinal Parasites with Special Reference to Zoonotic Parasites in Domestic Cats (Felis catus) in Mumbai, Maharashtra. J. Vet. Pub. Hlth. 2016, 14, 47–49. [Google Scholar]
- Schuster, R.K.; Thomas, K.; Sivakumar, S.; O’Donovan, D. The parasite fauna of stray domestic cats (Felis catus) in Dubai, United Arab Emirates. Parasitol. Res. 2009, 105, 125–134. [Google Scholar] [CrossRef]
- Hajipour, N.; Imani Baran, A.; Yakhchali, M.; Banan Khojasteh, S.M.; Sheikhzade Hesari, F.; Esmaeilnejad, B.; Arjmand, J. A survey study on gastrointestinal parasites of stray cats in Azarshahr, (East Azerbaijan province, Iran). J. Parasit. Dis. 2015, 40, 1255–1260. [Google Scholar] [CrossRef]
- Tun, S.; Ithoi, I.; Mahmud, R.; Samsudin, N.I.; Kek Heng, C.; Ling, L.Y. Detection of Helminth Eggs and Identification of Hookworm Species in Stray Cats, Dogs and Soil from Klang Valley, Malaysia. PLoS ONE 2015, 10, e0142231. [Google Scholar] [CrossRef]
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; Otranto, D. (). Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. Parasites Wildl. 2017, 47, 517–528. [Google Scholar] [CrossRef]
- Lee, S.H.; Ock, Y.; Choi, D.; Kwak, D. Gastrointestinal Parasite Infection in Cats in Daegu, Republic of Korea, and Efficacy of Treatment Using Topical Emodepside/Praziquantel Formulation. Korean J. Parasitol. 2019, 57, 243–248. [Google Scholar] [CrossRef]
- Gennari, S.M.; Ferreira, J.I.; Pena, H.F.; Labruna, M.B.; Azevedo, S.D. Frequency of gastrointestinal parasites in cats seen at the University of Sao Paulo Veterinary Hospital, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 423–428. [Google Scholar] [CrossRef]
- Capari, B.; Hamel, D.; Visser, M.; Winter, R.; Pfister, K.; Rehbein, S. Parasitic infections of domestic cats, Felis catus, in western Hungary. Vet. Parasitol. 2013, 192, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Todd, K.S., Jr. Coccidia of Mammals. In Parasitic Protozoa, 2nd ed.; Kreier, J., Ed.; Academic Press: New York, NY, USA, 1993; Volume 4, pp. 89–131. [Google Scholar]
- Nagamori, Y.; Payton, M.E.; Duncan-Decocq, R.; Johnson, E.M. Fecal survey of parasites in free-roaming cats in northcentral Oklahoma, United States. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; Hegglin, D.; Tanner, I.; Fischer, C.; Deplazes, P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology 2009, 136, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.C.; Rohen, M.; Epe, C.; Schnieder, T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol. Res. 2012, 111, 849–857. [Google Scholar] [CrossRef]
- Okulewicz, A.; Perec-Matysiak, A.; Buńkowska, K.; Hildebrand, J. Toxocara canis, Toxocara cati and Toxascaris leonina in wild and domestic carnivores. Helminthologia 2012, 49, 3–10. [Google Scholar] [CrossRef]
- Lucio-Forster, A.; Bowman, D.D. Prevalence of fecal-borne parasites detected by centrifugal flotation in feline samples from two shelters in upstate New York. J. Feline Med. Surg. 2011, 13, 300–303. [Google Scholar] [CrossRef]
- CFSPH Center for Food Security and Public Health. Zoonotic Hookworms. Available online: http://www.cfsph.iastate.edu (accessed on 28 February 2020).
- Khalafalla, R.E. A survey study on gastrointestinal parasites of stray cats in northern region of Nile delta, Egypt. PLoS ONE 2011, 6, e20283. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Milillo, P.; Iorio, R.; Otranto, D. Infection by Eucoleus aerophilus in dogs and cats: Is another extra-intestinal parasitic nematode of pets emerging in Italy? Res. Vet. Sci. 2009, 87, 270–272. [Google Scholar] [CrossRef]
- Crisi, P.E.; Aste, G.; Traversa, D.; Di Cesare, A.; Febo, E.; Vignoli, M.; Santori, D.; Luciani, A.; Boari, A. Single and mixed feline lungworm infections: Clinical, radiographic and therapeutic features of 26 cases (2013–2015). J. Feline Med. Surg. 2017, 19, 1017–1029. [Google Scholar] [CrossRef]
- Szczepaniak, K.; Leśniak, P.; Studzińska, M.; Roczeń-Karczmarz, M.; Demkowska-Kutrzepa, M.; Junkuszew, A.; Tomczuk, K. Occurrence of larvae of Metastrongyloidea in feaces of cats from southeastern Poland. Med. Weter. 2019, 75, 605–608. [Google Scholar] [CrossRef]
- Wells, K.; Gibson, D.I.; Clark, N.J.; Ribas, A.; Morand, S.; McCallum, H.I. Global spread of helminth parasites at the human–domestic animal–wildlife interface. Glob. Chang. Biol. 2018, 24, 3254–3265. [Google Scholar] [CrossRef] [PubMed]

| Endoparasite | N Infected, Prevalence of Infection (%) | Mean Intensity of Infection | Range of Infection | 95% C.I. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Toxocara cati | 36 (44.44) | 12.22 | 1–81 | 34.54 | 55.87 |
| Toxascaris leonina | 3 (3.70) | 1.00 | 1 | 0.96 | 11.18 |
| Ancylostoma tubaeformae | 17 (20.99) | 7.18 | 1–12 | 13.04 | 31.07 |
| Taenia taeniaeformis | 34 (41.98) | 6.12 | 1–32 | 31.27 | 53.46 |
| Dipylidium caninum | 5 (6.17) | 2.80 | 1–7 | 2.29 | 14.44 |
| Mesocestoides sp. | 2 (2.47) | 1.00 | 1 | 0.43 | 9.46 |
| Eucoleus aerophilus | 2 (2.47) | 2.50 | 1–4 | 0.43 | 9.46 |
| Mixed Endoparasite Infections | N Infected, Prevalence of Infection (%) | 95% C.I. | |
|---|---|---|---|
| Lower | Upper | ||
| T. cati + T. taeniaeformis | 24 (29.63) | 20.26 | 40.96 |
| T. cati + A. tubaeformae | 10 (12.35) | 6.40 | 21.99 |
| T. cati + T. leonina | 1 (1.23) | 0.06 | 7.63 |
| T. cati + D. caninum | 3 (3.70) | 0.96 | 11.18 |
| T. cati + Mesocestoides sp. | 2 (2.47) | 0.43 | 9.46 |
| T. cati + E. aerophilus | 1 (1.23) | 0.06 | 7.63 |
| T. cati + T. taeniaeformis + A. tubaeformae | 10 (12.35) | 6.40 | 21.99 |
| T. cati +T. leonina + T. taeniaeformis | 1 (1.23) | 0.06 | 7.63 |
| T. cati + T. leonina + A. tubaeformae + T. taeniaeformis | 1 (1.23) | 0.06 | 7.63 |
| T. taeniaeformis + A. tubaeformae | 4 (4.94) | 1.59 | 12.84 |
| Food Categories | Urban n = 26 | Suburban n = 37 | Total n = 63 | |||
|---|---|---|---|---|---|---|
| FO | %FO | FO | %FO | FO | %FO | |
| rodents | 4 | 15.4 | 11 | 29.7 | 15 | 23.8 |
| soricomorphs | - | - | 3 | 8.1 | 3 | 4.8 |
| birds | 2 | 7.7 | 3 | 8.1 | 5 | 7.9 |
| invertebrates | 2 | 7.7 | 3 | 8.1 | 5 | 7.9 |
| anthropogenic food | 20 | 76.9 | 21 | 56.8 | 41 | 65.1 |
| plant material | 12 | 46.2 | 23 | 62.2 | 35 | 55.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, I.A.; Kornaś, S.; Piontek, A.M.; Rola, K. The Prevalence of Endoparasites of Free Ranging Cats (Felis catus) from Urban Habitats in Southern Poland. Animals 2020, 10, 748. https://doi.org/10.3390/ani10040748
Wierzbowska IA, Kornaś S, Piontek AM, Rola K. The Prevalence of Endoparasites of Free Ranging Cats (Felis catus) from Urban Habitats in Southern Poland. Animals. 2020; 10(4):748. https://doi.org/10.3390/ani10040748
Chicago/Turabian StyleWierzbowska, Izabela A., Sławomir Kornaś, Aleksandra M. Piontek, and Kaja Rola. 2020. "The Prevalence of Endoparasites of Free Ranging Cats (Felis catus) from Urban Habitats in Southern Poland" Animals 10, no. 4: 748. https://doi.org/10.3390/ani10040748
APA StyleWierzbowska, I. A., Kornaś, S., Piontek, A. M., & Rola, K. (2020). The Prevalence of Endoparasites of Free Ranging Cats (Felis catus) from Urban Habitats in Southern Poland. Animals, 10(4), 748. https://doi.org/10.3390/ani10040748





