Human–Wildlife Conflicts in Krakow City, Southern Poland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
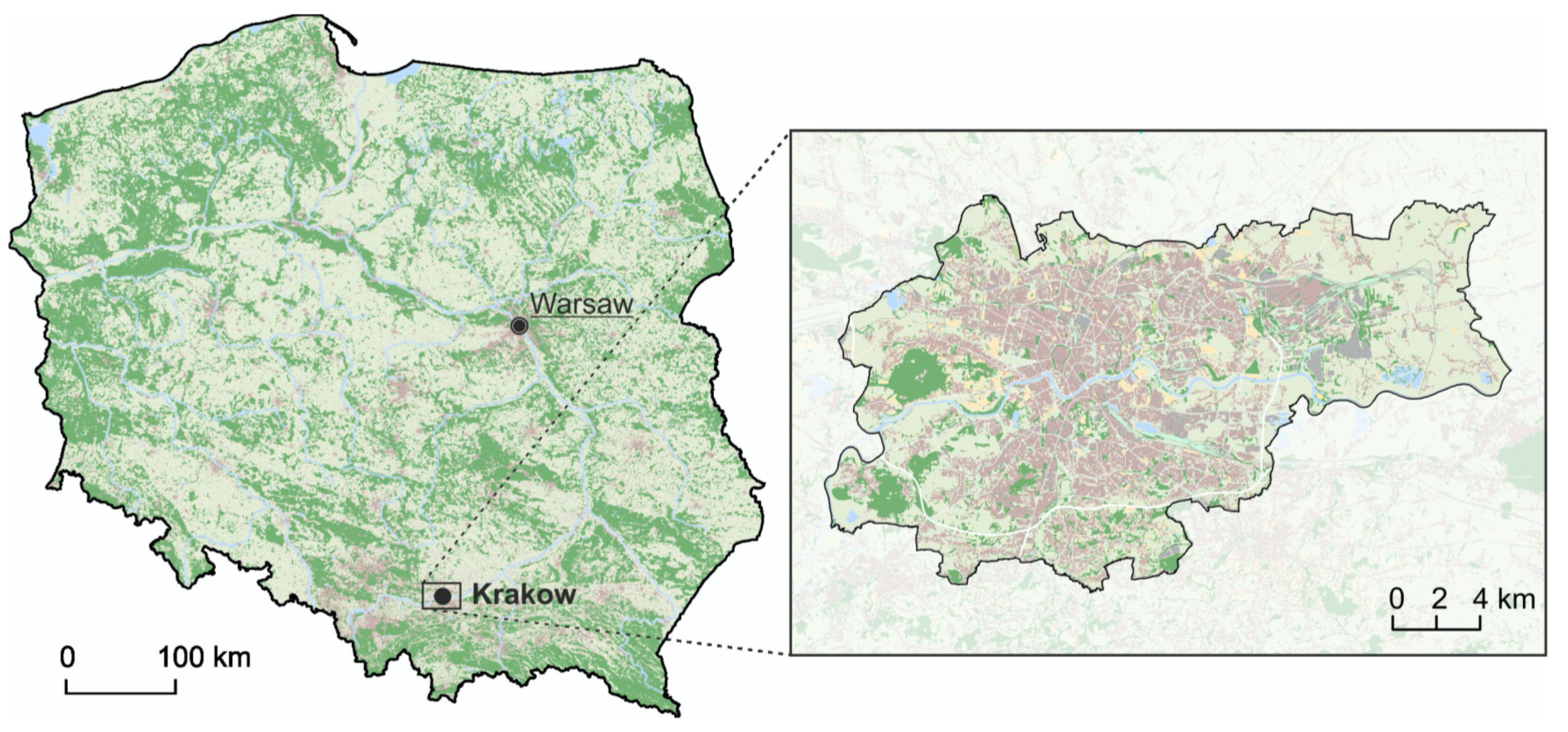
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.3.1. Temporal Pattern
2.3.2. Spatial Pattern
3. Results
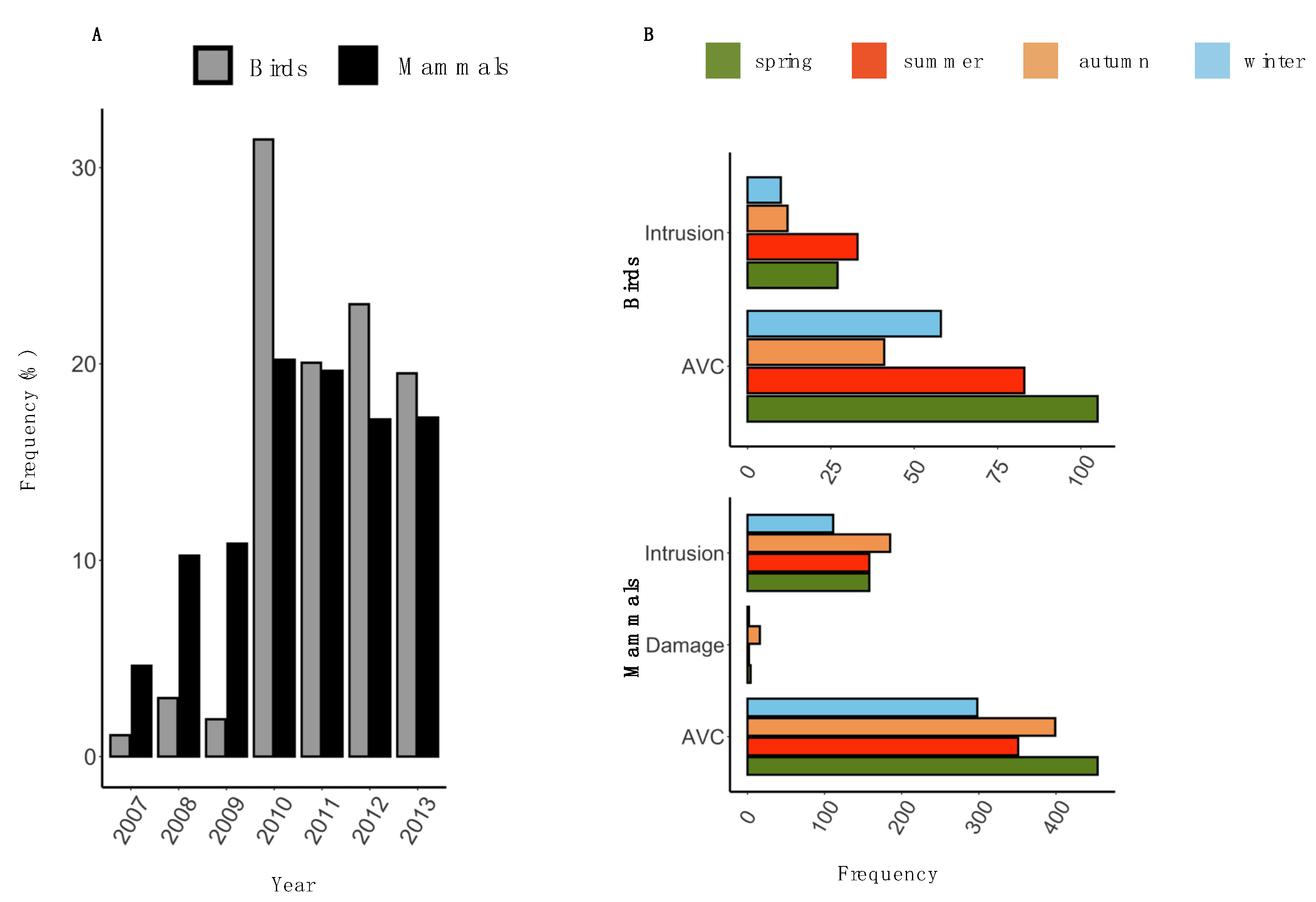
3.1. Descriptive Statistics
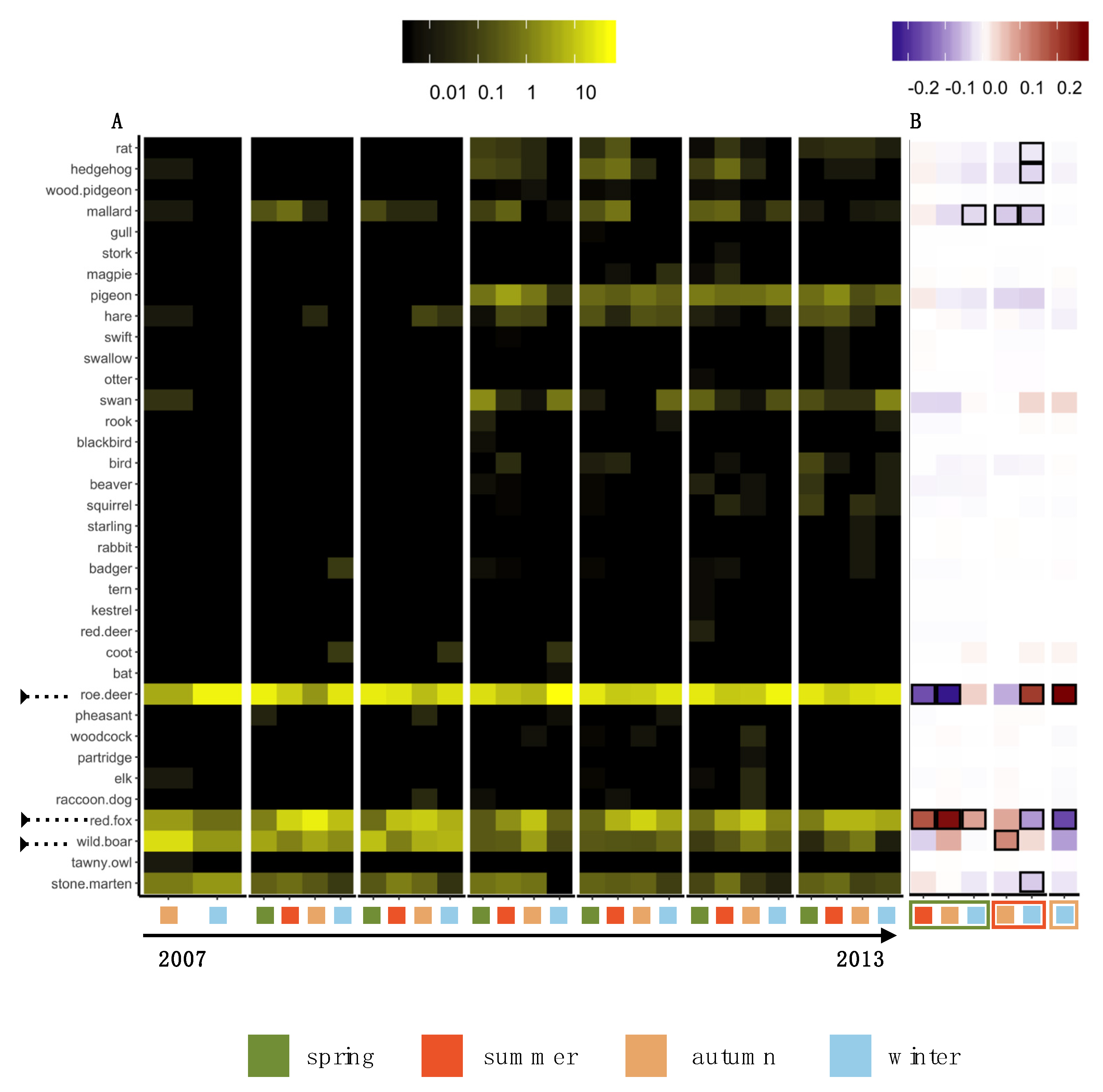
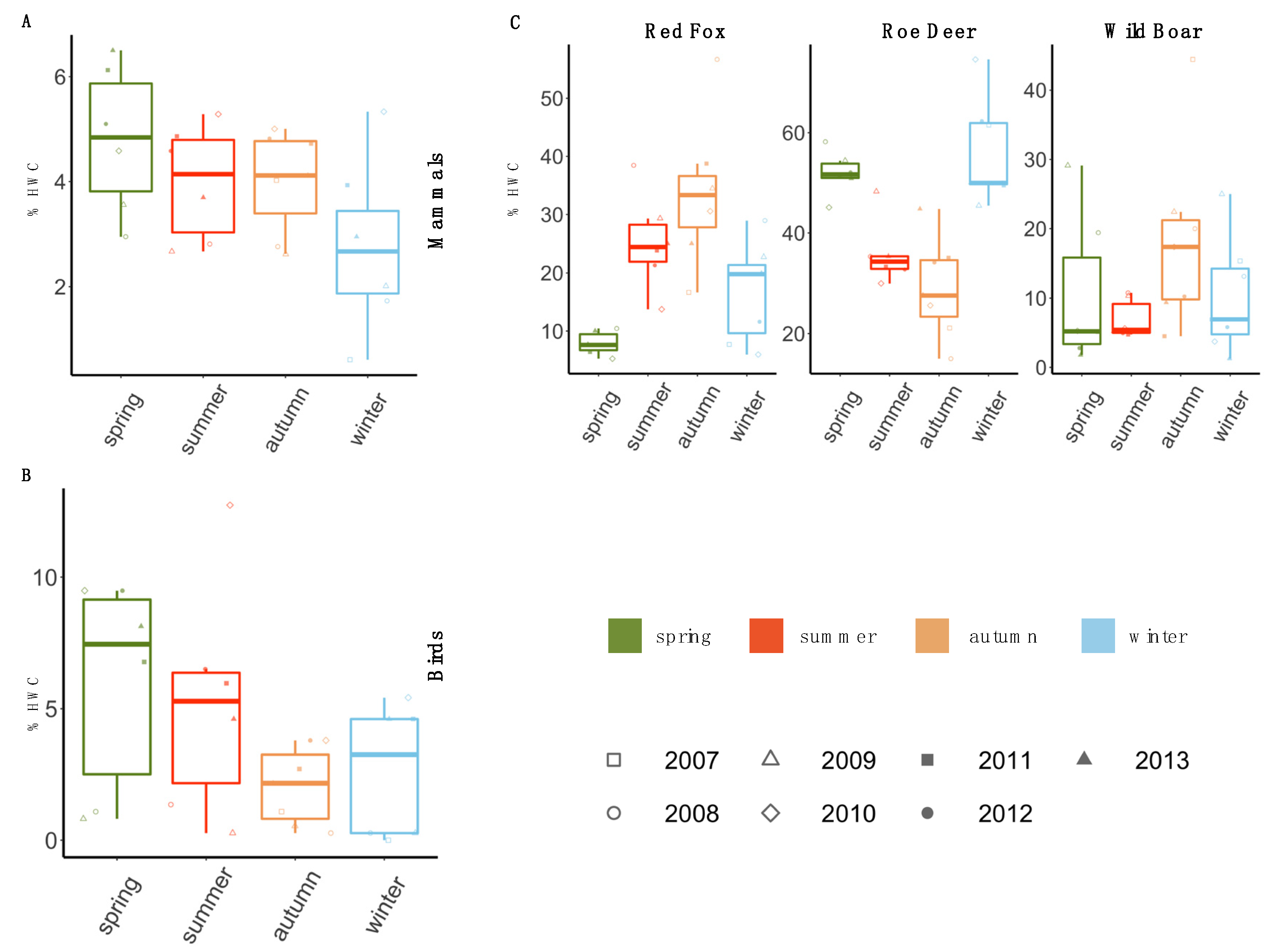
3.2. Temporal Pattern of HWC
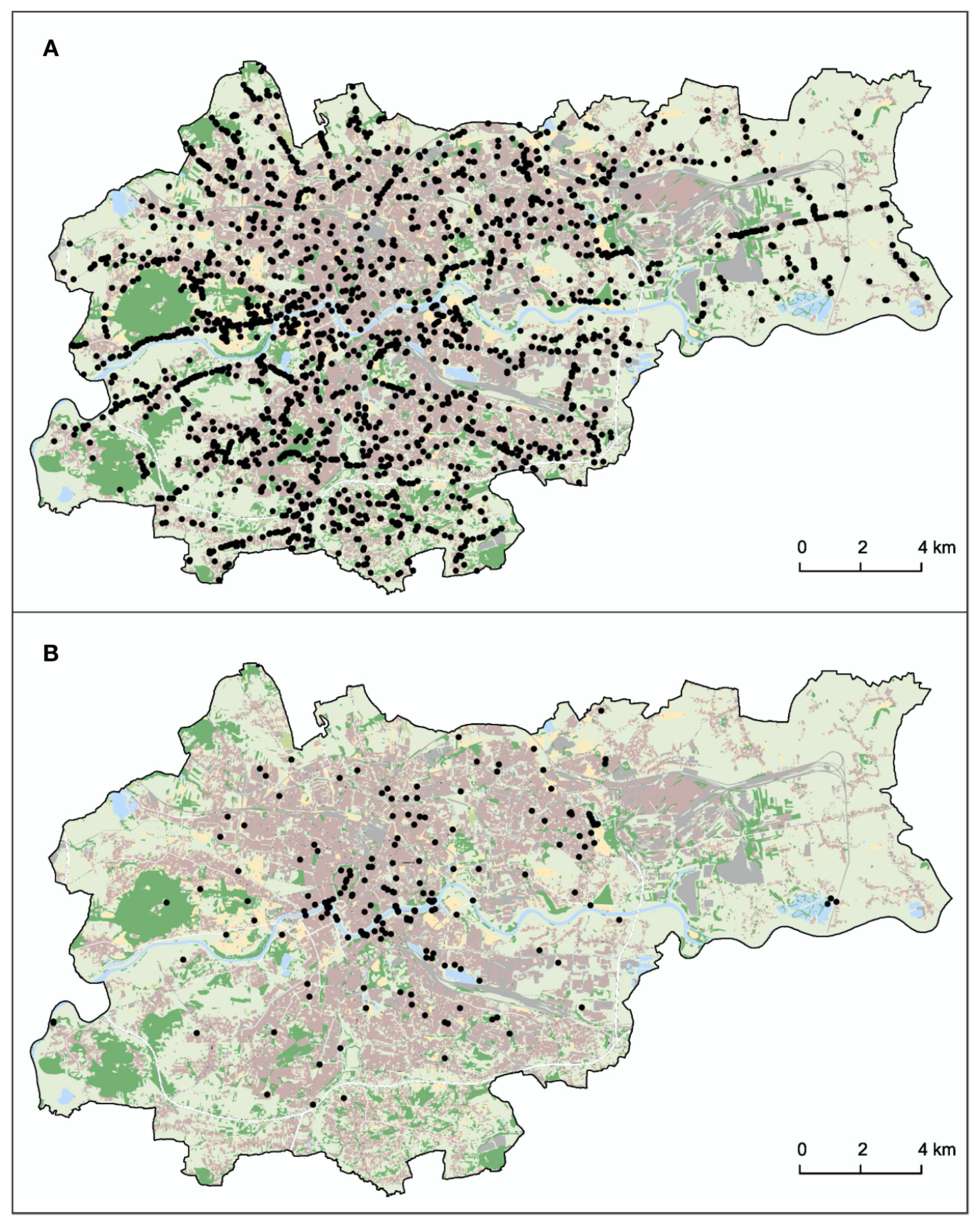
3.3. Spatial Pattern of HWC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Distefano, E. Human-Wildlife Conflict Worldwide: Collection of Case Studies, Analysis of Management Strategies and Good Practices; FAO: Rome, Italy, 2005. [Google Scholar]
- Redpath, S.M.; Gutiérrez, R.J.; Wood, K.A.; Young, J.C. Conflicts in Conservation: Navigating towards Solutions; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1-107-60346-2. [Google Scholar]
- Gutiérrez, R.J.; Wood, K.A.; Redpath, S.M.; Young, J.C. Conservation Conflicts: Future Research Challenges. In Current Trends in Wildlife Research; Wildlife Research Monographs; Mateo, R., Arroyo, B., Garcia, J.T., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 267–282. ISBN 978-3-319-27912-1. [Google Scholar]
- Adams, C.E. Urban Wildlife Management; CRC Press: New York, NY, USA, 2016; ISBN 978-1-4987-0203-4. [Google Scholar]
- McCleery, R.A.; Moorman, C.; Peterson, M.N. Urban Wildlife Conservation: Theory and Practice; Springer: New York, NY, USA; London, UK, 2014; ISBN 978-1-4899-7499-0. [Google Scholar]
- Soulsbury, C.D.; White, P.C.L. Human–wildlife interactions in urban areas: A review of conflicts, benefits and opportunities. Wildl. Res. 2015, 42, 541. [Google Scholar] [CrossRef]
- Seto, K.C.; Golden, J.S.; Alberti, M.; Turner, B.L. Sustainability in an urbanizing planet. Proc. Natl. Acad. Sci. USA 2017, 114, 8935–8938. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Urbanization. Our World Data. 2018. Available online: https://ourworldindata.org/urbanization (accessed on 3 February 2020).
- UN-Habitat, World Cities Report 2016: Urbanization and Development—Emerging Futures; UN: New York, NY USA, 2016. [CrossRef]
- McDonald, R.I.; Marcotullio, P.J.; Güneralp, B. Urbanization and Global Trends in Biodiversity and Ecosystem Services. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities; Elmqvist, T., Fragkias, M., Goodness, J., Güneralp, B., Marcotullio, P.J., McDonald, R.I., Parnell, S., Schewenius, M., Sendstad, M., Seto, K.C., et al., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Piano, E.; Souffreau, C.; Merckx, T.; Baardsen, L.F.; Backeljau, T.; Bonte, D.; Brans, K.I.; Cours, M.; Dahirel, M.; Debortoli, N.; et al. Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob. Chang. Biol. 2020, 26, 1196–1211. [Google Scholar] [CrossRef] [PubMed]
- Start, D.; Barbour, M.A.; Bonner, C. Urbanization reshapes a food web. J. Anim. Ecol. 2020, 89, 808–816. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Schipper, A.M.; Hilbers, J.P.; Meijer, J.R.; Antão, L.H.; Benítez-López, A.; de Jonge, M.M.J.; Leemans, L.H.; Scheper, E.; Alkemade, R.; Doelman, J.C.; et al. Projecting terrestrial biodiversity intactness with GLOBIO 4. Glob. Chang. Biol. 2020, 26, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Pagh, S.; Hersteinsson, P. Difference in diet and age structure of blue and white Arctic foxes (Vulpes lagopus) in the Disko Bay area, West Greenland. Polar Res. 2008, 27, 44–51. [Google Scholar] [CrossRef]
- Cervinka, T.; Sievänen, H.; Hyttinen, J.; Rittweger, J. Bone loss patterns in cortical, subcortical, and trabecular compartments during simulated microgravity. J. Appl. Physiol. 2014, 117, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Snep, R.P.; Kooijmans, J.L.; Kwak, R.G.; Foppen, R.P.; Parsons, H.; Awasthy, M.; Sierdsema, H.L.; Marzluff, J.M.; Fernandez-Juricic, E.; de Laet, J.; et al. Urban bird conservation: Presenting stakeholder-specific arguments for the development of bird-friendly cities. Urban Ecosyst. 2016, 19, 1535–1550. [Google Scholar] [CrossRef]
- Magle, S.B.; Fidino, M.; Lehrer, E.W.; Gallo, T.; Mulligan, M.P.; Ríos, M.J.; Ahlers, A.A.; Angstmann, J.; Belaire, A.; Dugelby, B.; et al. Advancing urban wildlife research through a multi-city collaboration. Front. Ecol. Environ. 2019, 17, 232–239. [Google Scholar] [CrossRef]
- Elliot, E.E.; Vallance, S.; Molles, L.E. Coexisting with coyotes (Canis latrans) in an urban environment. Urban Ecosyst. 2016, 19, 1335–1350. [Google Scholar] [CrossRef]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffmann, A.A.; Langham, G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 2008, 6, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.B.M.; Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar] [CrossRef]
- Barrett, L.P.; Stanton, L.A.; Benson-Amram, S. The cognition of ‘nuisance’ species. Anim. Behav. 2019, 147, 167–177. [Google Scholar] [CrossRef]
- Hunter, P. The human impact on biological diversity. How species adapt to urban challenges sheds light on evolution and provides clues about conservation. EMBO Rep. 2007, 8, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Poessel, S.A.; Mock, E.C.; Breck, S.W. Coyote (Canis latrans) diet in an urban environment: Variation relative to pet conflicts, housing density, and season. Can. J. Zool. 2017, 95, 287–297. [Google Scholar] [CrossRef]
- Wierzbowska, I.A.; Lesiak, M.; Zalewski, A.; Gajda, A.; Widera, E.; Okarma, H. Urban carnivores: A case study of sympatric stone marten (Martes foina) and red fox (Vulpes vulpes) in Kraków, Southern Poland. In The Martes Complex in the 21st Century: Ecology and Conservation; Zalewski, A., WierzbowskaI, A., Aubry, K.B., Birks, J.D.S., O’Mahony, D.T., Proulx, G., Eds.; Mammal Research Institute PAS: Białowieża, Poland, 2017; pp. 161–178. ISBN 978-83-932502-6-4. [Google Scholar]
- Gomes, D.J.; Wierzbowska, I.A.; Bevanger, K.; O’Mahony, D.T.; Rola, K. Diet of the European badgers (Meles meles) in urban and rural areas of Norway. Eur. J. Wildl. Res. 2019, 66, 7. [Google Scholar] [CrossRef]
- Gruber, T.; Luncz, L.; Mörchen, J.; Schuppli, C.; Kendal, R.L.; Hockings, K. Cultural change in animals: A flexible behavioural adaptation to human disturbance. Palgrave Commun. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Ciach, M.; Fröhlich, A. Habitat type, food resources, noise and light pollution explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst. 2017, 20, 547–559. [Google Scholar] [CrossRef]
- Schell, C.J.; Young, J.K.; Lonsdorf, E.V.; Santymire, R.M.; Mateo, J.M. Parental habituation to human disturbance over time reduces fear of humans in coyote offspring. Ecol. Evol. 2018, 8, 12965–12980. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.C. Mechanisms of behavioural change in urban animals: The role of microevolution and phenotypic plasticity. In Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–132. ISBN 978-3-319-43314-1. [Google Scholar]
- Lowry, H.; Lill, A.; Wong, B.B.M. Behavioural responses of wildlife to urban environments. Biol. Rev. 2013, 88, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; Lapiedra, O.; González-Lagos, C. Behavioural adjustments for a life in the city. Anim. Behav. 2013, 85, 1101–1112. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Jiménez, J. Dealing with growing forest insect pests: The role of top-down regulation. J. Appl. Ecol. 2019, 56, 2574–2576. [Google Scholar] [CrossRef]
- Braczkowski, A.; Ruzo, A.; Sanchez, F.; Castagnino, R.; Brown, C.; Guynup, S.; Winter, S.; Gandy, D.; O’Bryan, C. The ayahuasca tourism boom: An undervalued demand driver for jaguar body parts? Conserv. Sci. Pract. 2019, 1, e126. [Google Scholar] [CrossRef]
- Baruch-Mordo, S.; Evans, J.S.; Severson, J.P.; Naugle, D.E.; Maestas, J.D.; Kiesecker, J.M.; Falkowski, M.J.; Hagen, C.A.; Reese, K.P. Saving sage-grouse from the trees: A proactive solution to reducing a key threat to a candidate species. Biol. Conserv. 2013, 167, 233–241. [Google Scholar] [CrossRef]
- Abra, F.D.; Granziera, B.M.; Huijser, M.P.; de Barros, K.M.P.M.; Haddad, C.M.; Paolino, R.M. Pay or prevent? Human safety, costs to society and legal perspectives on animal-vehicle collisions in São Paulo state, Brazil. PLoS ONE 2019, 14, e0215152. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, J.; St-Laurent, M.-H. In the wrong place at the wrong time: Moose and deer movement patterns influence wildlife-vehicle collision risk. Accid. Anal. Prev. 2020, 135, 105365. [Google Scholar] [CrossRef] [PubMed]
- Saint-Andrieux, C.; Calenge, C.; Bonenfant, C. Comparison of environmental, biological and anthropogenic causes of wildlife–vehicle collisions among three large herbivore species. Popul. Ecol. 2020, 62, 64–79. [Google Scholar] [CrossRef]
- Bíl, M.; Andrášik, R.; Janoška, Z. Identification of hazardous road locations of traffic accidents by means of kernel density estimation and cluster significance evaluation. Accid. Anal. Prev. 2013, 55, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bíl, M.; Andrášik, R.; Du’a, M.; Sedoník, J. On reliable identification of factors influencing wildlife-vehicle collisions along roads. J. Environ. Manag. 2019, 237, 297–304. [Google Scholar]
- Chen, Y.; Marino, J.; Chen, Y.; Tao, Q.; Sullivan, C.D.; Shi, K.; Macdonald, D.W. Predicting Hotspots of Human-Elephant Conflict to Inform Mitigation Strategies in Xishuangbanna, Southwest China. PLoS ONE 2016, 11, e0162035. [Google Scholar] [CrossRef] [PubMed]
- Ramp, D.; Caldwell, J.; Edwards, K.A.; Warton, D.; Croft, D.B. Modelling of wildlife fatality hotspots along the Snowy Mountain Highway in New South Wales, Australia. Biol. Conserv. 2005, 126, 474–490. [Google Scholar] [CrossRef]
- Ruda, A.; Kolejka, J.; Silwal, T. GIS-Assisted Prediction and Risk Zonation of Wildlife Attacks in the Chitwan National Park in Nepal. Isprs Int. J. Geo-Inf. 2018, 7, 369. [Google Scholar] [CrossRef]
- Kansky, R.; Knight, A.T. Key factors driving attitudes towards large mammals in conflict with humans. Biol. Conserv. 2014, 179, 93–105. [Google Scholar] [CrossRef]
- Rodríguez-Morales, B.; Díaz-Varela, E.R.; Marey-Pérez, M.F. Spatiotemporal analysis of vehicle collisions involving wild boar and roe deer in NW Spain. Accid. Anal. Prev. 2013, 60, 121–133. [Google Scholar] [CrossRef]
- Soulsbury, C.D. Research resource review. Prog. Phys. Geogr. Earth Environ. 2019, 43, 727–728. [Google Scholar] [CrossRef]
- Soulsbury, C.D. Temporal patterns of human-fox interactions as revealed from internet searches. Hum. Dimens. Wildl. 2020, 25, 70–81. [Google Scholar] [CrossRef]
- Sadleir, R.M.; Linklater, W.L. Annual and seasonal patterns in wildlife road-kill and their relationship with traffic density. N. Z. J. Zool. 2016, 43, 275–291. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Schondube, J.E. Gray vs. green urbanization: Relative importance of urban features for urban bird communities. Basic Appl. Ecol. 2011, 2, 372–381. [Google Scholar] [CrossRef]
- GUS Kalendarium. Available online: https://stat.gov.pl/kalendarium (accessed on 21 April 2020).
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Chełstowska, A.; Filip, R. Krakow in Numbers 2009; The Municipality of Krakow, A City Strategy and Development Department: Krakow, Poland, 2010. [Google Scholar]
- UMK (Urzad Miasta Krakowa [Municipality of Krakow]) Raporty o Stanie Miasta—Biuletyn Informacji Publicznej Miasta Krakowa—BIP MK. Available online: https://www.bip.krakow.pl/?id=509 (accessed on 14 May 2020).
- Dubiel, D.; Rockel, B.; Naumann, M.; Dubiel, W. Diversity of COP9 signalosome structures and functional consequences. FEBS Lett. 2015, 589, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Walasz, K. Fauna. In Natural Environment of Krakow, Resources- Protection- Management; Baścik, M., Degórska, B., Eds.; Institute of Geography and Spatial Management, Jagiellonian University: Kraków, Poland, 2015; pp. 145–160. ISBN 978-83-64089-09-1. [Google Scholar]
- Podgórski, T.; Baś, G.; Jędrzejewska, B.; Sönnichsen, L.; Śnieżko, S.; Jędrzejewski, W.; Okarma, H. Spatiotemporal behavioral plasticity of wild boar (Sus scrofa) under contrasting conditions of human pressure: Primeval forest and metropolitan area. J. Mammal. 2013, 94, 109–119. [Google Scholar] [CrossRef]
- Baś, G.; Bojarska, K.; Śnieżko, S.; Król, W.; Wiesław, K.; Okarma, H. Habitat use by wild boars Sus scrofa in the city of Kraków (In Polish- Wykorzystanie siedlisk przez dziki Sus scrofa na terenie Krakowa). Chrońmy Przyr. Ojcz. 2017, 73, 354–362. [Google Scholar]
- Polish Hunting Association in Krakow. Regional Board Report on Hunting Statistics; Regional Board of Polish Hunting Association: Krakow, Poland. Available online: http://pzl.krakow.pl/gospodarka-lowiecka (accessed on 10 October 2019).
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Kabir, A.; Merrill, R.D.; Shamim, A.A.; Klemn, R.D.W.; Labrique, A.B.; Christian, P.; West, K.P.; Nasser, M. Canonical Correlation Analysis of infant’s size at birth and maternal factors: A study in rural Northwest Bangladesh. PLoS ONE 2014, 9, e94243. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Topics in constrained and unconstrained ordination. Plant Ecol. 2015, 216, 683–696. [Google Scholar] [CrossRef]
- Benjamini, Y. Discovering the false discovery rate. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 72, 405–416. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan” Community Ecology Package, Version 2. 2019. Available online: http://CRAN.R-project.org/package=vegan (accessed on 22 April 2020).
- Changelog for QGIS 2.14. Available online: https://www.qgis.org/en/site/forusers/visualchangelog214/index.html (accessed on 21 April 2020).
- Pandini, W.; Cesaris, C. Home range and habitat use of roe deer (Capreolus capreolus) reared in captivity and released in the wild. Hystrix Ital. J. Mammal. 1997, 9. [Google Scholar] [CrossRef]
- Kartografii, G.U.G. i Baza Danych Obiektów Topograficznych BDOT 10k. Available online: http://www.gugik.gov.pl/pzgik/zamow-dane/baza-danych-obiektow-topograficznych-bdot-10k (accessed on 21 April 2020).
- Moran, M. Interdisciplinarity and Political Science1. Politics 2006, 26, 73–83. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ho, D. Q1 2020 ArcGIS Release What’s New in the Q1 2020 ArcGIS Release. Arcgis Blog 2020. Available online: https://www.esri.com/arcgis-blog/products/arcgis/announcements/q1-2020-arcgis-release/ (accessed on 21 April 2020).
- Birch, C.P.D.; Oom, S.P.; Beecham, J.A. Rectangular and hexagonal grids used for observation, experiment and simulation in ecology. Ecol. Model. 2007, 206, 347–359. [Google Scholar] [CrossRef]
- Fu, W.J.; Jiang, P.K.; Zhou, G.M.; Zhao, K.L. Using Moran’s I and GIS to study the spatial pattern of forest litter carbon density in a subtropical region of southeastern China. Biogeosciences 2014, 11, 2401–2409. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J. Urban Ecology; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-76097-3. [Google Scholar]
- Torres, L.G.; Nieukirk, S.L.; Lemos, L.; Chandler, T.E. Drone Up! Quantifying Whale Behavior From a New Perspective Improves Observational Capacity. Front. Mar. Sci. 2018, 5, 319. [Google Scholar] [CrossRef]
- Hensley, C.B.; Trisos, C.H.; Warren, P.S.; MacFarland, J.; Blumenshine, S.; Reece, J.; Katti, M. Effects of Urbanization on Native Bird Species in Three Southwestern US Cities. Front. Ecol. Evol. 2019, 7, 71. [Google Scholar] [CrossRef]
- Fuller, R.A.; Irvine, K.N.; Davies, Z.; Armsworth, P.R.; Gaston, K.J. Interactions between people and birds in urban landscapes. In Urban Bird Ecology and Conservation. Studies in Avian Biology; Lepczyk, C.A., Warren, P.S., Eds.; University of California Press: Berkeley, CA, USA, 2012; pp. 249–266. [Google Scholar]
- Alfano, A. Not Just Sparrows and Pigeons: Cities Harbor 20 Percent of World’s Bird Species. 2014 All about Birds. 2014. Available online: https://www.allaboutbirds.org/news/not-just-sparrows-and-pigeons-cities-harbor-20-percent-of-worlds-bird-species/ (accessed on 21 May 2020).
- BirdLife International. Response to the Post-2020 Global Biodiversity Framework Discussion Document. 2019. Available online: https://www.cbd.int/api/v2013/documents/72B01150-A9BD-D116-0CA7-53F37995C8D6/attachments/birdlife.pdf (accessed on 28 April 2020).
- Lesiak, M. (“KABAN” Maciej Lesiak, Krakow, Poland). Personal Observation, 2013.
- Tryjanowski, P.; Morelli, F.; Skórka, P.; Goławski, A.; Indykiewicz, P.; Møller, A.P.; Mitrus, C.; Wysocki, D.; Zduniak, P. Who started first? Bird species visiting novel birdfeeders. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Gehrt, S.D. Wildlife Population Dynamics in Urban Landscapes. In Urban Wildlife Conservation: Theory and Practice; McCleery, R.A., Moorman, C.E., Peterson, M.N., Eds.; Springer: Boston, MA, USA, 2014; pp. 117–147. ISBN 978-1-4899-7500-3. [Google Scholar]
- Włodarczyk, R.; Minias, P. Non-adaptive territory selection by a bird with exceptionally long parental care. PeerJ 2016, 4, e1852. [Google Scholar] [CrossRef] [PubMed]
- Rytwinski, T.; Fahrig, L. Why are some animal populations unaffected or positively affected by roads? Oecologia 2013, 173, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Su, Y.; Xu, W.; Cai, W.; Wang, X.; Lu, F.; Ouyang, Z. The effect of habitat changes along the urbanization gradient for breeding birds: An example from the Xiong’An New Area. PeerJ 2019, 7, e7961. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Varela, E.R.; Vazquez-Gonzalez, I.; Marey-Pérez, M.F.; Álvarez-López, C.J. Assessing methods of mitigating wildlife–vehicle collisions by accident characterization and spatial analysis. Transp. Res. Part Transp. Environ. 2011, 16, 281–287. [Google Scholar] [CrossRef]
- Johansson, A. Territory Establishment and Antler Cycle in Male Roe Deer. Ethology 1996, 102, 549–559. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; Iossa, G.; Baker, P.J.; White, P.C.L.; Harris, S. Behavioral and spatial analysis of extraterritorial movements in red foxes (Vulpes vulpes). J. Mammal. 2011, 92, 190–199. [Google Scholar] [CrossRef]
- Kamieniarz, R.; Szymański, M.; Panek, M. Factors determining the activity of field roe deer (In Polish- Czynniki determinujące aktywność saren polnych). Sylwan 2018, 162, 258–264. [Google Scholar]
- Malmsten, A.; Jansson, G.; Lundeheim, N.; Dalin, A.-M. The reproductive pattern and potential of free ranging female wild boars (Sus scrofa) in Sweden. Acta Vet. Scand. 2017, 59, 52. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.C.; Huang, L.; Xie, Q.; Tiwari, R.C. A modified version of Moran’s I. Int. J. Health Geogr. 2010, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Conover, M.R. Resolving Human-Wildlife Conflicts: The Science of Wildlife Damage Management; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-429-14416-5. [Google Scholar]
- Redpath, S.M.; Sutherland, W.J. The value of ecological information in conservation conflict. Confl. Conserv. Navig. Solut. 2015, 35–48. [Google Scholar]
| Species | Moran’s Index | Expected Index | Variance | Z-Score | p-Value |
|---|---|---|---|---|---|
| Red fox | 0.107638 | −0.000160 | 0.000054 | 14.641317 | 0.000001 |
| Roe deer | 0.126715 | −0.000160 | 0.000054 | 17.249950 | 0.000001 |
| Wild boar | 0.180016 | −0.000160 | 0.000053 | 24.686691 | 0.000001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basak, S.M.; Wierzbowska, I.A.; Gajda, A.; Czarnoleski, M.; Lesiak, M.; Widera, E. Human–Wildlife Conflicts in Krakow City, Southern Poland. Animals 2020, 10, 1014. https://doi.org/10.3390/ani10061014
Basak SM, Wierzbowska IA, Gajda A, Czarnoleski M, Lesiak M, Widera E. Human–Wildlife Conflicts in Krakow City, Southern Poland. Animals. 2020; 10(6):1014. https://doi.org/10.3390/ani10061014
Chicago/Turabian StyleBasak, Sayantani M., Izabela A. Wierzbowska, Agnieszka Gajda, Marcin Czarnoleski, Maciej Lesiak, and Elzbieta Widera. 2020. "Human–Wildlife Conflicts in Krakow City, Southern Poland" Animals 10, no. 6: 1014. https://doi.org/10.3390/ani10061014
APA StyleBasak, S. M., Wierzbowska, I. A., Gajda, A., Czarnoleski, M., Lesiak, M., & Widera, E. (2020). Human–Wildlife Conflicts in Krakow City, Southern Poland. Animals, 10(6), 1014. https://doi.org/10.3390/ani10061014





