Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals
Simple Summary
Abstract
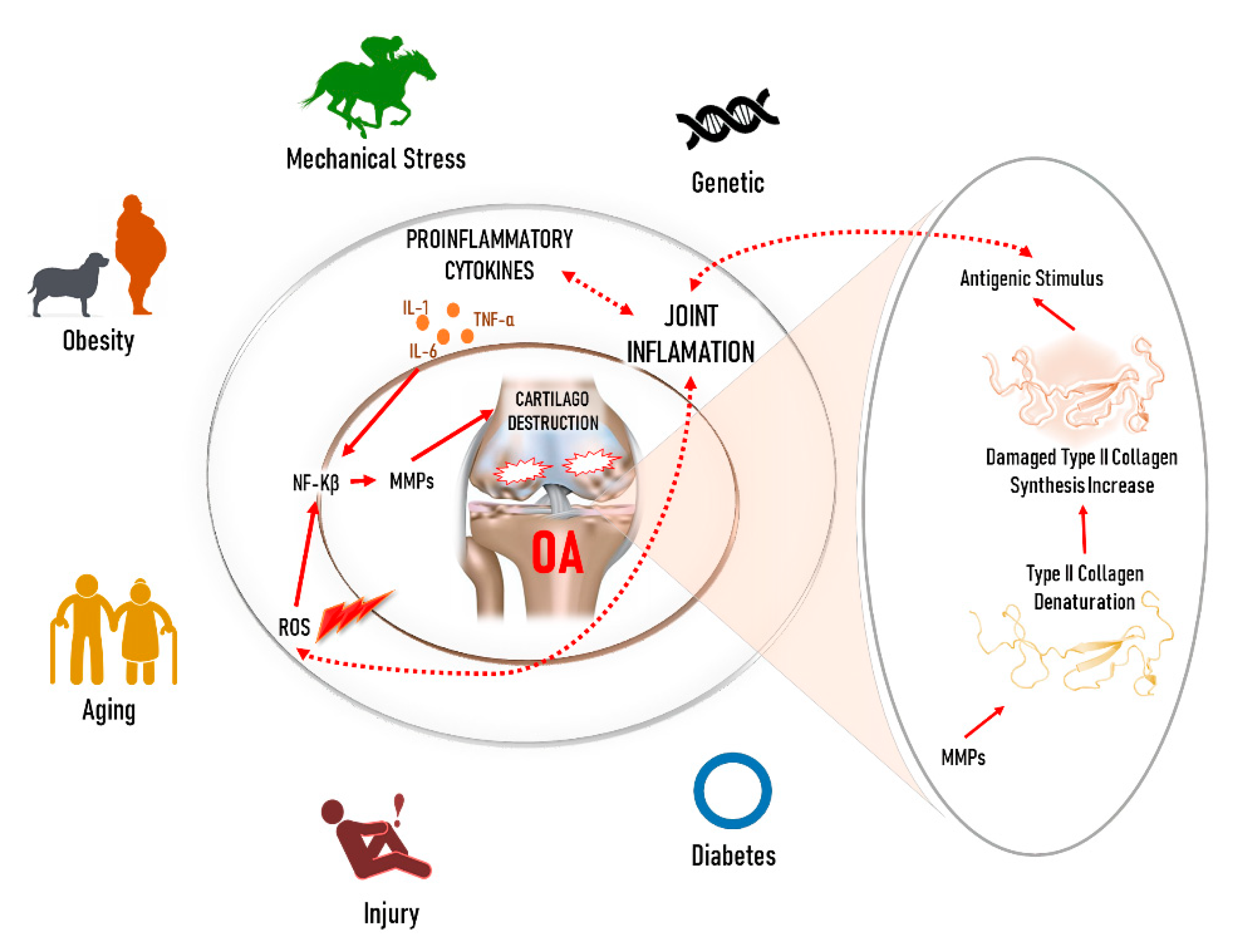
1. Introduction
2. Molecular Factors in the OA Treatment
3. Alternative Non-Surgical Treatment Approaches for OA
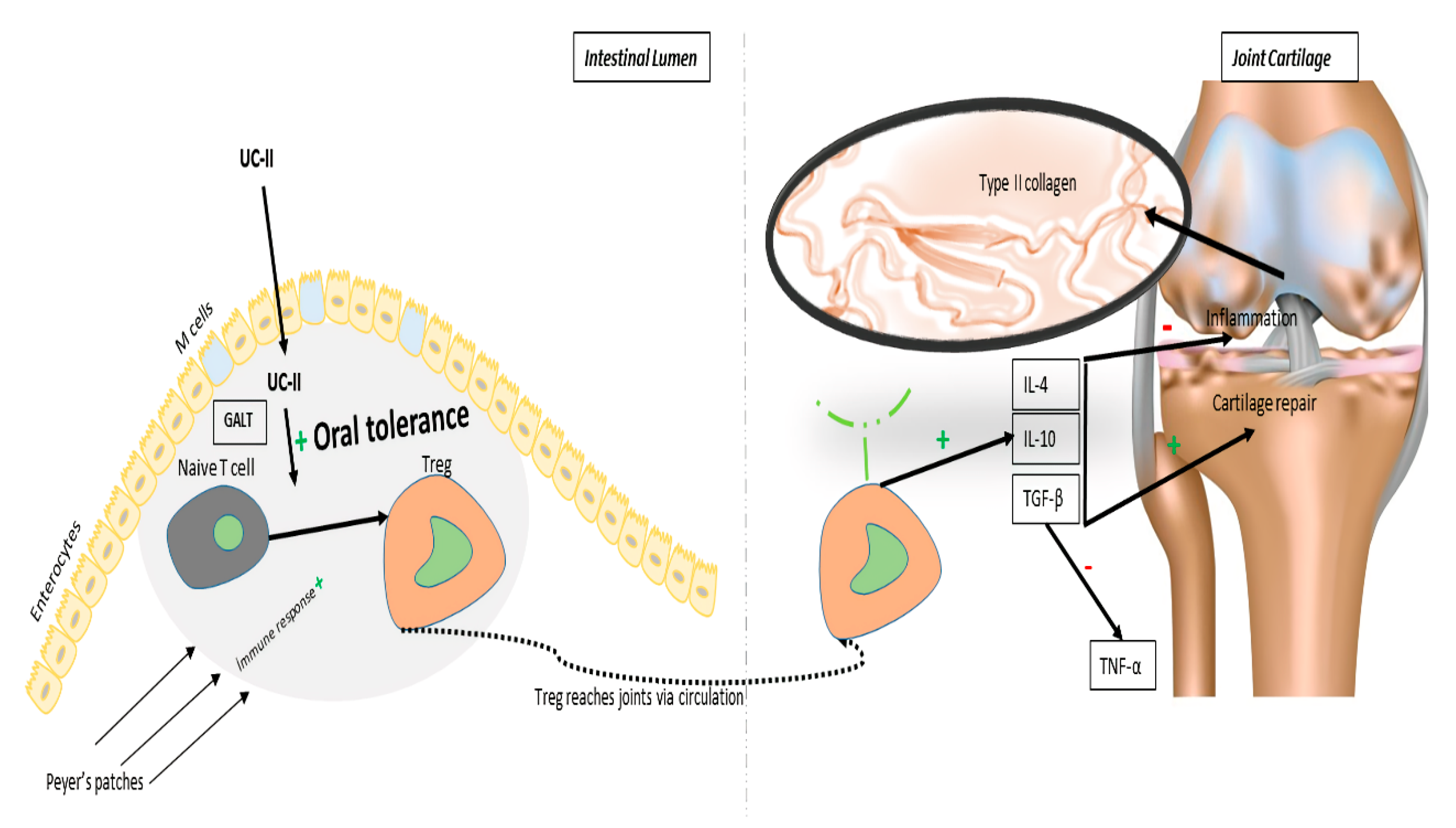
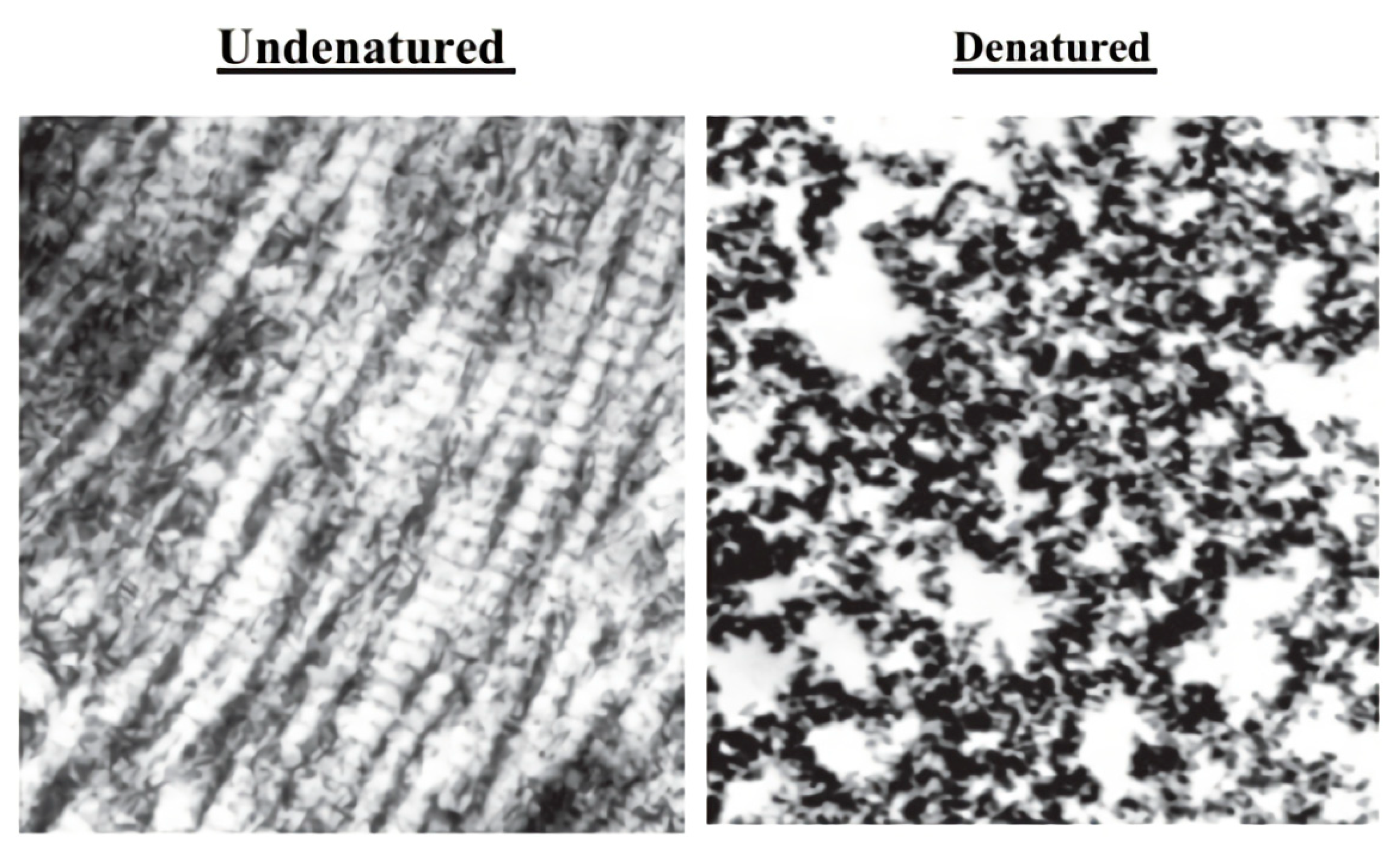
4. UC-II and its Action Mechanism
5. Basic Add-on Therapies besides UC-II
6. OA Prevalence in Dogs
UC-II Usage in Dogs
7. OA Prevalence in Horses
UC-II Usage in Horses
8. OA Prevalence in Cats
UC-II Usage in Cats
9. UC-II, Safety, Efficacy, and Adverse Effects
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbosa, J.D.; Lima, D.H.S.; Belo-Reis, A.S.; Pinheiro, C.P.; Sousa, M.G.S.; Silva, J.B.; Salvarani, F.M.; Oliveira, C.M.C. Degenerative joint disease in cattle and buffaloes in the Amazon region: A retrospective study. Pesqui. Vet. Bras. 2014, 34, 845–850. [Google Scholar] [CrossRef]
- Iagnocco, A. Chapter 14—Osteoarthritis. In Essential Applications of Musculoskeletal Ultrasound in Rheumatology; Wakefield, R.J., D’Agostino, M.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 165–180. ISBN 978-1-4377-0127-2. [Google Scholar]
- Neugebauer, V.; Han, J.S.; Adwanikar, H.; Fu, Y.; Ji, G. Techniques for assessing knee joint pain in arthritis. Mol. Pain 2007, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.I.; Soler, C. Animal models of osteoarthritis in small mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 211–221. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.M. Hind limb lameness in the young patient. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 101–123. [Google Scholar] [CrossRef]
- Ruff, K.J.; Kopp, K.J.; Von Behrens, P.; Lux, M.; Mahn, M.; Back, M. Effectiveness of NEM® brand eggshell membrane in the treatment of suboptimal joint function in dogs: A multicenter, randomized, double-blind, placebo-controlled study. Vet. Med. Res. Rep. 2016, 7, 113–121. [Google Scholar] [CrossRef]
- Cimino Brown, D. What can we learn from osteoarthritis pain in companion animals? Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 53–58. [Google Scholar]
- Schachner, E.R.; Lopez, M.J. Diagnosis, prevention, and management of canine hip dysplasia: A review. Vet. Med. Res. Rep. 2015, 6, 181–192. [Google Scholar]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE 2014, 9, e90501. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.G.P.; Meadows, J.M.; Pypendop, B.H.; Johnson, E.G. Evaluation of tramadol for treatment of osteoarthritis in geriatric cats. J. Am. Vet. Med. Assoc. 2018, 252, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Contino, E.K. Management and rehabilitation of joint disease in sport horses. Vet. Clin. N. Am. Equine Pract. 2018, 34, 345–358. [Google Scholar] [CrossRef]
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Bland, S.D. Canine osteoarthritis and treatments: A review. Vet. Sci. Dev. 2015. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Henrotin, Y.; Sanchez, C.; Balligand, M. Pharmaceutical and nutraceutical management of canine osteoarthritis: Present and future perspectives. Vet. J. Lond. Engl. 1997 2005, 170, 113–123. [Google Scholar] [CrossRef]
- Bagi, C.M.; Berryman, E.R.; Teo, S.; Lane, N.E. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthr. Cartil. 2017, 25, 2080–2090. [Google Scholar] [CrossRef]
- Aragon, C.L.; Hofmeister, E.H.; Budsberg, S.C. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2007, 230, 514–521. [Google Scholar] [CrossRef]
- Beale, B. Orthopedic clinical techniques femur fracture repair. Clin. Technol. Small Anim. Pract. 2004, 19, 134–150. [Google Scholar] [CrossRef]
- D’Altilio, M.; Peal, A.; Alvey, M.; Simms, C.; Curtsinger, A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Bagchi, M.; Bagchi, D. therapeutic efficacy and safety of undenatured type II collagen singly or in combination with glucosamine and chondroitin in arthritic dogs. Toxicol. Mech. Methods 2007, 17, 189–196. [Google Scholar] [CrossRef]
- Prabhoo, R.; Billa, G. Undenatured collagen type II for the treatment of osteoarthritis: A review. Int. J. Res. Orthop. 2018, 4, 684–689. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.-P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Collins, J.A.; Diekman, B.O.; Loeser, R.F. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 101. [Google Scholar] [CrossRef]
- Li, Y.-S.; Xiao, W.; Luo, W. Cellular aging towards osteoarthritis. Mech. Ageing Dev. 2017, 162, 80–84. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Yuan, G.-H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K. Immunologic intervention in the pathogenesis of osteoarthritis. Arthritis Rheum. 2003, 48, 602–611. [Google Scholar] [CrossRef]
- Poole, A.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61, ii78–ii81. [Google Scholar] [CrossRef]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in Arthritis: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M.; Amin, A.R.; Clancy, R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr. Rheumatol. Rep. 2001, 3, 535–541. [Google Scholar] [CrossRef]
- Amin, A.R.; Dave, M.; Attur, M.; Abramson, S.B. COX-2, NO, and cartilage damage and repair. Curr. Rheumatol. Rep. 2000, 2, 447–453. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res Ther. 2003, 5, 54. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Nagase, H.; Kashiwagi, M. Aggrecanases and cartilage matrix degradation. Arthritis Res. Ther. 2003, 5, 94. [Google Scholar] [CrossRef]
- Poole, A.R. An introduction to the pathophysiology of osteoarthritis. Front. Biosci. J. Virtual Libr. 1999, 4, D662–D670. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Caron, J.P.; Evans, C.; Robbins, P.D.; Georgescu, H.I.; Jovanovic, D.; Fernandes, J.C.; Martel-Pelletier, J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997, 40, 1012–1019. [Google Scholar] [CrossRef]
- Cicero, A.F.; Laghi, L. Activity and potential role of licofelone in the management of osteoarthritis. Clin. Interv. Aging 2007, 2, 73–79. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Boileau, C.; Boily, M.; Brunet, J.; Mineau, F.; Geng, C.; Reboul, P.; Laufer, S.; Lajeunesse, D.; Martel-Pelletier, J. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res. Ther. 2005, 7, R1091–R1102. [Google Scholar] [CrossRef]
- Jovanovic, D.V.; Fernandes, J.C.; Martel-Pelletier, J.; Jolicoeur, F.C.; Reboul, P.; Laufer, S.; Tries, S.; Pelletier, J.P. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: Suppression of collagenase 1 and interleukin-1beta synthesis. Arthritis Rheum. 2001, 44, 2320–2330. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Jafarzadeh, S.R.; Felson, D.T. Updated estimates suggest a much higher prevalence of arthritis in United States adults than previous ones. Arthritis Rheumatol. 2018, 70, 185–192. [Google Scholar] [CrossRef]
- Bhathal, A.; Spryszak, M.; Louizos, C.; Frankel, G. Glucosamine and chondroitin use in canines for osteoarthritis: A review. Open Vet. J. 2017, 7, 36. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef]
- Sahin, K.; Perez Ojalvo, S.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ozercan, I.H.; Sylla, S.; Koca, S.S.; Yilmaz, I.; et al. Effect of inositol -stabilized arginine silicate on arthritis in a rat model. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 125, 242–251. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Martel-Pelletier, J.; Raynauld, J.-P. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: Today and tomorrow. Arthritis Res. Ther. 2006, 8, 206. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Bagchi, D.; Misner, B.; Bagchi, M.; Kothari, S.C.; Downs, B.W.; Fafard, R.D.; Preuss, H.G. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: A mechanistic exploration. Int. J. Clin. Pharmacol. Res. 2002, 22, 101–110. [Google Scholar]
- Lerman, R.H.; Chang, J.-L.; Konda, V.; Desai, A.; Montalto, M.B. Nutritional approach for relief of joint discomfort: A 12-week, open-case series and illustrative case report. Integr. Med. 2015, 14, 10. [Google Scholar]
- Tong, T.; Zhao, W.; Wu, Y.-Q.; Chang, Y.; Wang, Q.-T.; Zhang, L.-L.; Wei, W. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010, 59, 369–377. [Google Scholar] [CrossRef]
- Nagler-Anderson, C.; Bober, L.A.; Robinson, M.E.; Siskind, G.W.; Thorbecke, G.J. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc. Natl. Acad. Sci. USA 1986, 83, 7443–7446. [Google Scholar] [CrossRef]
- Asnagli, H.; Martire, D.; Belmonte, N.; Quentin, J.; Bastian, H.; Boucard-Jourdin, M.; Fall, P.B.; Mausset-Bonnefont, A.-L.; Mantello-Moreau, A.; Rouquier, S.; et al. Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis. Arthritis Res. Ther. 2014, 16, R115. [Google Scholar] [CrossRef]
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000, 2, 379–385. [Google Scholar] [CrossRef]
- Marshall, S.; Bacote, V.; Traxinger, R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991, 266, 4706–4712. [Google Scholar]
- Simon, R.R.; Marks, V.; Leeds, A.R.; Anderson, J.W. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab. Res. Rev. 2011, 27, 14–27. [Google Scholar] [CrossRef]
- Patti, M.E.; Virkamäki, A.; Landaker, E.J.; Kahn, C.R.; Yki-Järvinen, H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 1999, 48, 1562–1571. [Google Scholar] [CrossRef]
- Rossetti, L.; Hawkins, M.; Chen, W.; Gindi, J.; Barzilai, N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J. Clin. Investig. 1995, 96, 132–140. [Google Scholar] [CrossRef]
- Ando, A.; Hagiwara, Y.; Chimoto, E.; Hatori, K.; Onoda, Y.; Itoi, E. Intra-articular injection of hyaluronan diminishes loss of chondrocytes in a rat immobilized-knee model. Tohoku J. Exp. Med. 2008, 215, 321–331. [Google Scholar] [CrossRef]
- Rausch-Derra, L.; Huebner, M.; Wofford, J.; Rhodes, L. A prospective, randomized, masked, placebo-controlled multisite clinical study of grapiprant, an EP4 Prostaglandin Receptor Antagonist (PRA), in dogs with osteoarthritis. J. Vet. Intern. Med. 2016, 30, 756–763. [Google Scholar] [CrossRef]
- Bergh, M.S.; Budsberg, S.C. The coxib NSAIDs: Potential clinical and pharmacologic importance in veterinary medicine. J. Vet. Intern. Med. 2005, 19, 633–643. [Google Scholar] [CrossRef]
- KuKanich, B.; Bidgood, T.; Knesl, O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet. Anaesth. Analg. 2012, 39, 69–90. [Google Scholar] [CrossRef]
- Ho, K.Y.; Gwee, K.A.; Cheng, Y.K.; Yoon, K.H.; Hee, H.T.; Omar, A.R. Nonsteroidal anti-inflammatory drugs in chronic pain: Implications of new data for clinical practice. J. Pain Res. 2018, 11, 1937–1948. [Google Scholar] [CrossRef]
- Jerosch, J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef]
- Copeland, R.J.; Bullen, J.W.; Hart, G.W. Cross-talk between GlcNAcylation and phosphorylation: Roles in insulin resistance and glucose toxicity. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E17–E28. [Google Scholar] [CrossRef]
- Zhou, P.-H.; Liu, S.-Q.; Peng, H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 1643–1648. [Google Scholar] [CrossRef]
- Peal, A.; D’Altilio, M.; Simms, C.; Alvey, M.; Gupta, R.C.; Goad, J.T.; Canerdy, T.D.; Bagchi, M.; Bagchi, D. Therapeutic efficacy and safety of undenatured type-II collagen (UC-II) alone or in combination with (-)-hydroxycitric acid and chromemate in arthritic dogs. J. Vet. Pharmacol. Ther. 2007, 30, 275–278. [Google Scholar] [CrossRef]
- Deparle, L.A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; D’Altilio, M.; Bagchi, M.; Bagchi, D. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II) in therapy of arthritic dogs. J. Vet. Pharmacol. Ther. 2005, 28, 385–390. [Google Scholar] [CrossRef]
- Bagchi, M.; Stocker, A.; Burke, R.; Wedgeford, K.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Barnett, D.; Bagchi, D. Efficacy and safety of undenatured type II collagen (UC-II) in arthritic horses. Toxicol. Lett. 2007, 172, S223. [Google Scholar] [CrossRef]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: A clinical trial. Int. J. Med. Sci. 2009, 312–321. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Skaggs, P.; Stocker, A.; Zyrkowski, G.; Burke, R.; Wegford, K.; Goad, J.T.; Rohde, K.; Barnett, D.; et al. Therapeutic efficacy of undenatured type-II collagen (UC-II) in comparison to glucosamine and chondroitin in arthritic horses. J. Vet. Pharmacol. Ther. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Bagchi, M.; Gupta, R.; Lindley, J.; Barnes, M.; Canerdy, T.; Goad, J.; Bagchi, D. Suppression of arthritic pain in dogs by undenatured type-II collagen (UC-II) treatment quantitatively assessed by ground force plate. Toxicol. Lett. 2009, 189, S231. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative therapeutic efficacy and safety of type-II collagen (uc-II), glucosamine and chondroitin in arthritic dogs: Pain evaluation by ground force plate: Arthritis treatment in dogs. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lau, F.C.; Molina, J.P.L.; Pakdaman, M.N.; Shamie, A.; Udani, J.K. Undenatured type II collagen (UC-II®) for joint support: A randomized, double-blind, placebo-controlled study in healthy volunteers. J. Int. Soc. Sports Nutr. 2013, 10, 48. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2015, 15, 14. [Google Scholar] [CrossRef]
- Blair, J.L.; Bonavaud, S. Palatability and tolerability of a novel joint supplement in the cat. Clinical/research abstracts accepted for presentation at the World Feline Congress 2017. J. Feline Med. Surg. 2017, 19, 961–969. [Google Scholar]
- Samarelli, R.; Stabile, M.; Fracassi, L. Use of UC-II (Undenaturated Type II Collagen) in Management of Osteoarthritis in Dogs: A Clinical Trial; Università degli Studi: Torino, Italy, 2018. [Google Scholar]
- Stabile, M.; Samarelli, R.; Trerotoli, P.; Fracassi, L.; Lacitignola, L.; Crovace, A.; Staffieri, F. Evaluation of the effects of undenatured type II collagen (UC-II) as compared to robenacoxib on the mobility impairment induced by osteoarthritis in dogs. Vet. Sci. 2019, 6, 72. [Google Scholar] [CrossRef]
- Mehra, A.; Anand, P.; Borate, M.; Paul, P.; Kamble, S.; Mehta, K.D.; Qamra, A.; Shah, A.; Jain, R. A non-interventional, prospective, multicentric real life Indian study to assess safety and effectiveness of un-denatured type 2 collagen in management of osteoarthritis. Int. J. Res. Orthop. 2019, 5, 315–320. [Google Scholar] [CrossRef]
- Azeem, M.A.; Patil, R. The Study of undenatured type II collagen in the knee osteoarthritis. Int. J. Orthop. 2019, 5, 4. [Google Scholar]
- Meeson, R.L.; Todhunter, R.J.; Blunn, G.; Nuki, G.; Pitsillides, A.A. Spontaneous dog osteoarthritis—A One Medicine vision. Nat. Rev. Rheumatol. 2019, 15, 273–287. [Google Scholar] [CrossRef]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar]
- Man, G.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Grandalen, J.; Lingaas, F. Arthrosis in the elbow joint of young rapidly growing dogs: A genetic investigation. J. Small Anim. Pract. 1991, 32, 460–464. [Google Scholar] [CrossRef]
- Alam, M.R.; Lee, H.B.; Kim, M.S.; Kim, N. Surgical model of osteoarthritis secondary to medial patellar luxation in dogs. Vet. Med. 2018, 53, 123–130. [Google Scholar] [CrossRef]
- Rychel, J.K. Diagnosis and treatment of osteoarthritis. Top. Companion Anim. Med. 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Mele, E. Epidemiology of osteoarthritis. Vet. Focus 2007, 17, 4–10. [Google Scholar] [CrossRef]
- Pettitt, R.A.; German, A.J. Investigation and management of canine osteoarthritis. Practice 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Smith, G.K.; Paster, E.R.; Powers, M.Y.; Lawler, D.F.; Biery, D.N.; Shofer, F.S.; McKelvie, P.J.; Kealy, R.D. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 690–693. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Goh, S.-L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.J.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Knazovicky, D.; Case, B.; Freire, M.; Innes, J.F.; Drew, A.C.; Gearing, D.P. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet. Res. 2015, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Serisier, S.; Barthelemy, N.; Balligand, M.; Henrotin, Y. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 2016, 39, 1–15. [Google Scholar] [CrossRef]
- Sieper, J.; Kary, S.; Sörensen, H.; Alten, R.; Eggens, U.; Hüge, W.; Hiepe, F.; Kühne, A.; Listing, J.; Ulbrich, N.; et al. Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial. Arthritis Rheum. 1996, 39, 41–51. [Google Scholar] [CrossRef]
- Trentham, D.E.; Dynesius-Trentham, R.A.; Orav, E.J.; Combitchi, D.; Lorenzo, C.; Sewell, K.L.; Hafler, D.A.; Weiner, H.L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993, 261, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Park, M.-J.; Cho, M.-L.; Kwok, S.-K.; Ju, J.H.; Ko, H.-J.; Park, S.-H.; Kim, H.-Y. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, O.; Moriyama, H.; Shiojima, Y. An overview of a novel, water-soluble undenatured type II collagen (NEXT-II). J. Am. Coll. Nutr. 2015, 34, 255–262. [Google Scholar] [CrossRef]
- Yoshinari, O.; Shiojima, Y.; Moriyama, H.; Shinozaki, J.; Nakane, T.; Masuda, K.; Bagchi, M. Water-soluble undenatured type II collagen ameliorates collagen-induced arthritis in mice. J. Med. Food 2013, 16, 1039–1045. [Google Scholar] [CrossRef]
- Caron, J.P. Chapter 63—Osteoarthritis. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2003; pp. 572–591. ISBN 978-0-7216-8342-3. [Google Scholar]
- Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Preventive health care and owner-reported disease prevalence of horses and ponies in Great Britain. Res. Vet. Sci. 2013, 95, 418–424. [Google Scholar] [CrossRef]
- Van Weeren, P.R.; Back, W. Musculoskeletal disease in aged horses and its management. Vet. Clin. N. Am. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef]
- Da Garcia, R.S.; de Melo, U.P.; Ferreira, C.; dos Toscano, F.S.; da Cruz, G.M. Estudo clínico e radiográfico da osteoartrite társica juvenil em potros da raça mangalarga marchador. Ciênc. Anim. Bras. 2009, 10, 254–260. [Google Scholar]
- Kawcak, C.E.; McIlwraith, C.W.; Norrdin, R.W.; Park, R.D.; James, S.P. The role of subchondral bone in joint disease: A review. Equine Vet. J. 2001, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.M.; Whitehouse, G.H.; Boyde, A. Pathology of the distal condyles of the third metacarpal and third metatarsal bones of the horse. Equine Vet. J. 1999, 31, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Neundorf, R.H.; Lowerison, M.B.; Cruz, A.M.; Thomason, J.J.; McEwen, B.J.; Hurtig, M.B. Determination of the prevalence and severity of metacarpophalangeal joint osteoarthritis in Thoroughbred racehorses via quantitative macroscopic evaluation. Am. J. Vet. Res. 2010, 71, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Penell, J.C.; Bonnett, B.N.; Olson, P.; Pringle, J. Mortality of Swedish horses with complete life insurance between 1997 and 2000: Variations with sex, age, breed and diagnosis. Vet. Rec. 2006, 158, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Klinck, M.P.; Frank, D.; Guillot, M.; Troncy, E. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can. Vet. J. 2012, 53, 1181–1186. [Google Scholar] [PubMed]
- Stamper, C. Osteoarthritis in cats: A more common disease than you might expect. FDA 2008, 23, 6–8. [Google Scholar]
- Lascelles, B.D.X. Feline degenerative joint disease. Vet. Surg. 2010, 39, 2–13. [Google Scholar] [CrossRef]
- Bennett, D.; Zainal Ariffin, S.M.; Johnston, P. Osteoarthritis in the cat: 1. how common is it and how easy to recognise? J. Feline Med. Surg. 2012, 14, 65–75. [Google Scholar] [CrossRef]
- Clarke, S.P.; Mellor, D.; Clements, D.N.; Gemmill, T.; Farrell, M.; Carmichael, S.; Bennett, D. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet. Rec. 2005, 157, 793–799. [Google Scholar] [CrossRef]
- Godfrey, D.R. Osteoarthritis in cats: A retrospective radiological study. J. Small Anim. Pract. 2005, 46, 425–429. [Google Scholar] [CrossRef]
- Hardie, E.M.; Roe, S.C.; Martin, F.R. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J. Am. Vet. Med. Assoc. 2002, 220, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, L.I.; Hazewinkel, H.W.; Meij, B.P.; Picavet, P.; Voorhout, G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet. J. Lond. Engl. 1997 2011, 187, 304–309. [Google Scholar] [CrossRef]
- Schadow, S.; Siebert, H.-C.; Lochnit, G.; Kordelle, J.; Rickert, M.; Steinmeyer, J. Collagen metabolism of human osteoarthritic articular cartilage as modulated by bovine collagen hydrolysates. PLoS ONE 2013, 8, e53955. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on biological hazards (BIOHAZ) on the safety of collagen and a processing method for the production of collagen. EFSA J. 2005, 3, 174. [Google Scholar] [CrossRef]
- Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008, 56, 7790–7795. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.P. Letter to the editor UC-II® Undenatured type II collagen: Update to analytical methods. J. Int. Soc. Sports Nutr. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Marone, P.A.; Lau, F.C.; Gupta, R.C.; Bagchi, M.; Bagchi, D. Safety and toxicological evaluation of undenatured type II collagen. Toxicol. Mech. Methods 2010, 20, 175–189. [Google Scholar] [CrossRef]
- InterHealth’s UC-II® Receives GRAS Status. Available online: https://www.newhope.com/food-amp-beverage/interhealths-uc-ii-receives-gras-status (accessed on 14 October 2019).
| Non-Pharmacological and Preventative Strategies | Pharmaceutical Therapies |
|---|---|
| Weight control | NSAIDs, corticosteroids, doxycycline, |
| Knee misalignment and knee structure protection | MMP inhibitors |
| Physical rehabilitation | IL–1 receptor antagonist (IL-1Ra) |
| Preventing from the obesity and leptin levels management | Insulin growth factor-I (IGF-I) |
| Physical activity and muscle strengthening in preventing osteoarthritis | Bone anti-resorptive agents |
| Subchondral bone edema and bone resorption | Nutraceuticals: curcumin, EGCG, ASI |
| Partial meniscectomy and osteotomy | Chondroitin sulfate, glucosamine sulfate, sodium pentosan polysulfate, |
| Tissue engineering | Intra-articular treatments: steroids, hyaluronic acid |
| No | Objective | Model | Dose and Duration | Core Findings | Conclusion | Safety | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Demonstrating the UC-II ability, whether it reduces joint pain and swelling in RA subjects. | Human | UC-II (10 mg/day) for 42 days in five female subjects (58–78 years) suffering from severe joint pain. | Reduction of pain including the stiffness was observed. | UC-II found to serve as a novel therapeutic tool in joint inflammatory conditions and symptoms of both OA and RA. | No Adverse events | [49] |
| 2 | Evaluating the clinical effectiveness and safety of UC-II in obese-arthritic dogs. | Dogs | Fifteen dogs in three groups received either UC-II (0 mg/day), (1 mg/day), or (10 mg/day) for 90 days, plus 30 days withdrawal. | Both UC-II receiving groups showed significant reductions in overall pain as well as pain during limb manipulation and lameness after physical exertion, also 10 mg showed better improvement. Additionally, no adverse effects and no major alterations were noted in the serum chemistry, suggesting that UC-II was well tolerated. | Daily treatment of arthritic dogs with UC-II, shown to ameliorate the signs and symptoms of arthritis. Relapse of pain was observed during the withdrawal period. | No Adverse events | [20] |
| 3 | Determining the therapeutic efficacy and safety of glycosylated active UC-II alone or in combination with hydroxycitric acid (HA) and chromium niacinate (CN). | Dogs | Five groups (n = 5) of 25 arthritic dogs received daily treatments; group I (Placebo control), group II (10 mg active UC-II), group III (1800 mg HA), group IV (1800 mg HA+100 lg CN), and group V (1800 mg HA+100 lg CN+ 10 mg active UC-II). The treatments were given for 120 days and followed up by a 30 days withdrawal period. | The dogs received the active UC-II alone (group II) or in combination (group V) for 90 days exhibited a noticeable decrease in overall, pain upon limb manipulation and exercise-related lameness. Maximum pain decrease was seen in groups II and V after 120 days of treatment. A relapse of pain was exhibited in all the dogs after 30 days of the withdrawal period. | Active UC-II was found to ameliorate the arthritic dogs alone or in combination with HA and CN. The supplements were found to be well tolerated and no adverse effects were noted. | No Adverse events | [68] |
| 4 | Determining the therapeutic efficacy and safety of glycosylated active UC-II alone or in combination with glucosamine-HCl and chondroitin sulfate. | Dogs | Dogs were allocated into four groups (n = 5), and orally treated daily for 120 days. Treatments were Group I (placebo control), Group II (10 mg UC-II), Group III (2000 mg glucosamine)+(1600 mg chondroitin sulfate), Group IV, UC-II (10 mg) + 2000 mg glucosamine + 1600 mg chondroitin sulfate, followed by a 30-day withdrawal period. | UC-II alone received dogs showed substantial reductions in overall pain within the first quarter of the study. Maximum decreases in pain were noted after 120 days of treatment. Glucosamine and chondroitin alleviated some pain, but in combination with UC-II (Group IV) significant decreases were provided in overall pain, pain upon limb manipulation and exercise-associated lameness. Following the withdrawal of supplements, all of the animals experienced a relapse of pain. | UC-II alone or in combination with glucosamine and chondroitin significantly alleviated the arthritis pain with daily treatment to the arthritic dogs, and these supplements were found to be well tolerated without any side effects. | No Adverse events | [69] |
| 5 | Evaluating the efficiency of pain lessening and safety of UC-II in arthritic horses. | Horses | Six groups of arthritic horses (n = 5–6). G. I (placebo control), G. II (UC-II 20 mg/day), G. III (UC-II 40 mg/day), G. IV (UC-II 80 mg/day), G. V (UC-II 120 mg/day), G. VI (UC-II 160 mg/day). A period of 150 days. | Groups IV, V, and VI of the horses exhibited significant improvements in the arthritic signs. Reduction in overall pain was at 79%, in pain upon limb manipulation was at 71%, and in pain, after physical exertion was at 68%. Horses receiving a higher dose of 120 and 160 mg of UC-II/day showed very little or no signs of arthritis. | UC-II at higher doses (80–160 mg/day) in the horses ameliorated the signs and symptoms of arthritis, which was also well-tolerated. | No Adverse events | [70] |
| 6 | Assessing the safety and efficacy of UC-II as compared to a combination of glucosamine and chondroitin (G + C) in the treatment of OA of the knee. | Human | A total of 52 subjects, half of them (n = 26) took a daily dose of 40 mg UC-II containing 10 mg of bioactive undenatured type II collagen via 2 capsules. Another half of the subjects (n = 26) took a daily dose of 1500 mg glucosamine and 1200 mg chondroitin via 4 capsules. | UC-II treatment found to be more effective when decreasing all the assessments from the baseline at 90 days. In the G + C treatment group, this effect was not observed. Specifically, although both treatments reduced the Western Ontario McMaster Osteoarthritis Index (WOMAC) score was two folds better reduced by UC-II, than the G + C treated group after 90 days. | UC-II treatment to the subjects exhibited noteworthy enhancement in daily activities, which suggested improvements for their life quality. | No Adverse events | [71] |
| 7 | Evaluating the arthritic pain reduction in the horses and comparison of its efficacy with the glucosamine and chondroitin | Horses | Five groups of moderate severity arthritic horses (n = 5–7); Group-I placebo, Group-II 320 mg UC-II, Group-III 480 mg UC-II, Group-IV 640 mg UC-II, Group-V glucosamine + chondroitin | The placebo group showed no change in arthritic conditions, whereas those receiving 320, 480, and 640 mg UC-II showed significant reductions in arthritic pain. | All supplements were tolerated well. Generally, results from this study demonstrated UC-II to be significantly more effective than the glucosamine and chondroitin supplements in arthritic horses. | No Adverse events | [72] |
| 8 | Assessing the safety and therapeutic effectiveness of UC-II in arthritic dogs | Dogs | Dogs were daily treated with either placebo or UC-II (10 mg active UC-II) for 120 days. | Substantial decreases (77%) were found in the overall pain of the dogs after the study period, inconsistent with pain reduction (83%) after limb manipulation and pain reduction after physical exercise (84%). Subchronic toxicity and primary dermal and eye irritation studies showed no adverse effects and UC-II did not induce mutagenic effects. | Study results demonstrated that UC-II significantly reduces arthritic pain and is safe. | No Adverse events | [73] |
| 9 | Determining the tolerability and safety of the therapeutic efficacy of type II collagen (UC-II) alone or in combination with glucosamine hydrochloride (GLU) and chondroitin sulphate (CHO). | Dogs | 4 groups (n = 7–10), were treated daily with; placebo (Group-I), 10 mg active UC-II (Group-II), 2000 mg GLU + 1600 mg CHO (Group-III), and UC-II + GLU + CHO (Group-IV), for 150 days. | A significant reduction in pain was noted in Groups II, III, and IV of dogs. Significant increases in peak vertical force (N/kg body wt) and impulse area (N/kg body wt), indicative of a decrease in arthritis-associated pain, were observed in Group-II (10 mg active UC-II) dogs only. None of the dogs in any group showed changes in physical, hepatic, or renal functions. | When moderately arthritic dogs treated with UC-II (10 mg), a marked reduction in arthritic pain with maximum improvement occurred by day 150. | No Adverse events | [74] |
| 10 | Assessing the efficacy and tolerability of UC-II in the moderation of the joint function/pain due to strenuous exercise in healthy subjects. | Human | 55 subjects who reported knee joint pain after joining in a standardized step mill performance test were randomized to take placebo (n = 28) or the UC-II (40 mg daily, n = 27) product for 120 days. | Subjects in the UC-II group showed significant improvements in average knee extension compared to placebo and to baseline. The UC-II cohort also revealed a significant change in average knee extension at day 90 versus baseline. | Daily supplementation with 40 mg of UC-II found to be well tolerated and led to improved knee joint extension. UC-II also showed the potential of increasing the period of pain-free strenuous exertion and lessen the joint pain from that. | No Adverse events | [75] |
| 11 | Evaluating the efficacy and safety of 150 mg of n-enriched THIAA+10 mg of UC-II in each tablet | Human | Participants took 2 tablets of nTHIAA + UC-II 2 ×/d with meals for 12 weeks. | All participants reported significant improvements in pain. The studied supplement was well tolerated, and no serious side effects occurred. | nTHIAA and UC-II were found to be safe and efficacious in participants having chronic joint pain. | No Adverse events | [50] |
| 12 | Evaluating the efficacy and safety of UC-II for knee OA pain and affiliated symptoms compared to glucosamine hydrochloride and chondroitin sulfate (GC). | Human | 191 volunteers were randomized into three groups receiving a daily dose of UC-II (40 mg), GC (1500 mg G and 1200 mg C), or placebo for 180 days. | UC-II group demonstrated a significant reduction in overall WOMAC score compared to placebo and GC. Supplementation with UC-II also resulted in significant changes for all three WOMAC subscales. Safety outcomes did not differ among the groups. | UC-II improved knee joint symptoms in knee OA subjects and was well-tolerated. | No Adverse events | [76] |
| 13 | Assessing the UC-II to prevention against the excessive articular cartilage deterioration in a partial medial meniscectomy tear (PMMT) surgery performed rat model of OA. | Rats | 20 male rats were used in this study. 10 rats received the vehicle and another 10 rats received an oral daily dose of UC-II at 0.66 mg/kg for 8 weeks. | PMMT surgery created a moderate OA at the medial tibia plateau. Immediate treatment with the UC-II protected the weight-bearing capacity of the injured leg, preserved the integrity of the cancellous bone at tibial metaphysis and limited the excessive osteophyte formation and deterioration of articular cartilage. | This study demonstrates that a clinically relevant daily dose of UC-II when applied immediately after an injury can improve the mechanical function of the injured knee and prevent excessive deterioration of articular cartilage. | No Adverse events | [17] |
| 14 | The palatability and tolerability of UC-II was studied | Cats | 33 European Shorthair cats between the ages of 24 to 72 months were given one chewable tablet containing 10 mg of UC-II, daily for 40 days. | No remarkable findings on physical examination before or after the study and no appreciable changes in body weight were noted. The consumption level rose from 58% on day 0 to 73% on day 40. After an initial acquaintance period of 2–3 weeks, the level of consumption within 5 mins rose to over 70%. | 10 mg of UC-II found to be very palatable in the cats studied and was well-tolerated based on physical examination. | No Adverse events | [77] |
| 15 | Analyzing the efficacy of UC-II alone or combined with cimicoxib, for OA treatment. | Dogs | 45 dogs: 13 cimicoxib, 20 UC-II, and 12 cimicoxib + UC-II. Cimicoxib (2 mg/kg die) and UC-II tablet /day. Study lasted for 30 days. | There was a significant reduction in LOAD scores after the study. Treatment of similar magnitude among the three groups (CIMI = 31.8%, p < 0.001; UC-II = 32.7%, p = 0.013; CIM + UC-II = 31.7%, p = 0.009). Preliminary results of the study show similar effectiveness of the 3 treatments in reducing the degree of impairment of mobility in dogs with OA. | UC-II, while not showing a synergistic effect with cimicoxib, provided a comparable clinical efficacy to the NSAIDs itself. | No Adverse events | [78] |
| 16 | This study aimed to evaluate the effects of UC-II as compared to robenacoxib in OA suffering dogs. | Dogs | 60 client-owned dogs were randomized in the R group (n = 30, robenacoxib 1 mg/kg/day for 30 days) and in the UC-II group (n = 30, UC-II 1 tablet/day for 30 days). | Based on the data obtained from the study, a significant reduction in LOAD and MOBILITY scores was recorded between T0 and T30 with a similar magnitude among the two groups (R = 31.5%, p < 0.001; UC-II = 32.7%, p = 0.013). | This study showed that UC-II and robenacoxib were able to similarly improve mobility of dogs affected by OA. | No Adverse events | [79] |
| 17 | Assessing the safety and effectiveness of un-denatured type 2 collagen in the management of OA performed in patients by 18 orthopaedicians | Human | 291 patients were enrolled and followed-up at day 30 (visit 2), day 60 (visit 3), and day 90 (visit 4). Efficacy was assessed by and WOMAC and Visual Analogue scale (VAS) on each visit. | 226 of 291 patients completed the 90 days study. Treatment with UC-II was related to a significant reduction in WOMAC and VAS scores. | UC-II was safe and efficacious in Indian patients having OA, which could be considered in the early management of OA. | No Adverse events | [80] |
| 18 | The purpose of the present study was to asses the outcome of collagen type II IN osteoarthritis of the knee joint. | Human | 100 randomly selected patients that received a daily dose of UC-II (40 mg) for 120 days. | UC-II showed a significant reduction in the overall WOMAC score, LFI, and VAS scores in 120 days of observation. The UC-II led to significant changes in the three WOMAC subscales: pain p = 0.0005; stiffness p = 0.004; physical function p = 0.004. | UC-II improved the knee joint function in knee OA. | No Adverse events | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. https://doi.org/10.3390/ani10040697
Gencoglu H, Orhan C, Sahin E, Sahin K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals. 2020; 10(4):697. https://doi.org/10.3390/ani10040697
Chicago/Turabian StyleGencoglu, Hasan, Cemal Orhan, Emre Sahin, and Kazim Sahin. 2020. "Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals" Animals 10, no. 4: 697. https://doi.org/10.3390/ani10040697
APA StyleGencoglu, H., Orhan, C., Sahin, E., & Sahin, K. (2020). Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals, 10(4), 697. https://doi.org/10.3390/ani10040697







