The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia mangostana Peel Extract against Pathogenicity of Listeria monocytogenes in Female BALB/c Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Total Phenolic Content
2.3. Total Flavonoids
2.4. DPPH (2,2–diphenyl–1–picrylhydrazyl) Radical Scavenging Activity
2.5. ABTS [2,4,6–tri(2–pyridyl)–s–triazine] Radical Scavenging Activity
2.6. Ferric Reducing Antioxidant Power (FRAP)
2.7. Synthesis of Ag-NPs using the Peel of G. Mangostana
2.8. Listeria monocytogenes Preparation and Culture
2.9. Experimental Protocol
2.10. Oxidative Stress
2.11. Enzymatic Antioxidant Status
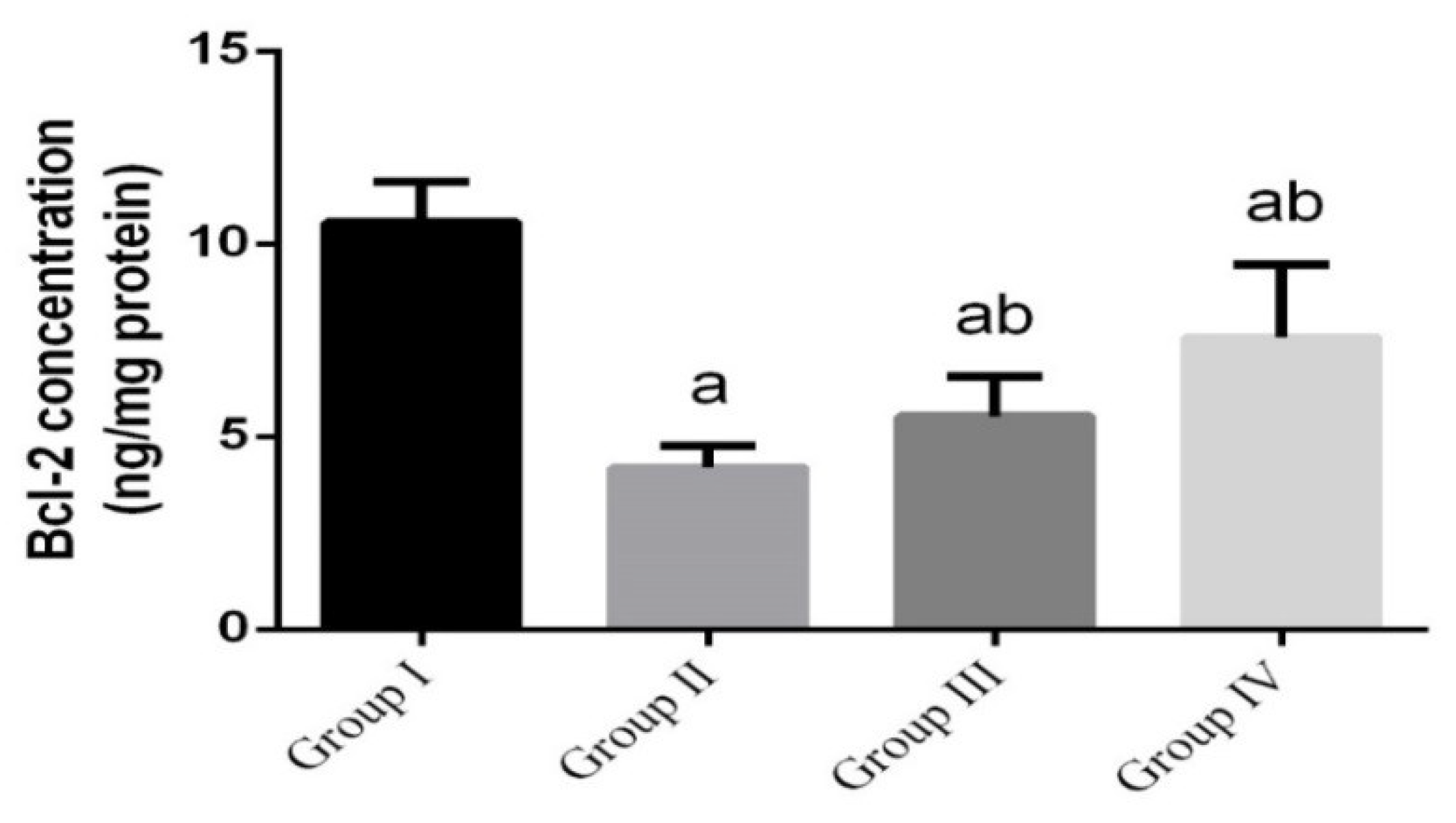
2.12. Determination of Apoptotic Markers in Intestinal Tissue
2.13. Real-Time PCR
| Name | Sense (5′---3′) | Antisense (5′---3′) |
| TNF-α | AGAACTCAGCGAGGACACCAA | GCTTGGTGGTTTGCTACGAC |
| IL-1β | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC |
| GAPDH | GCATCTTCTTGTGCAGTGCC | GATGGTGATGGGTTTCCCGT |
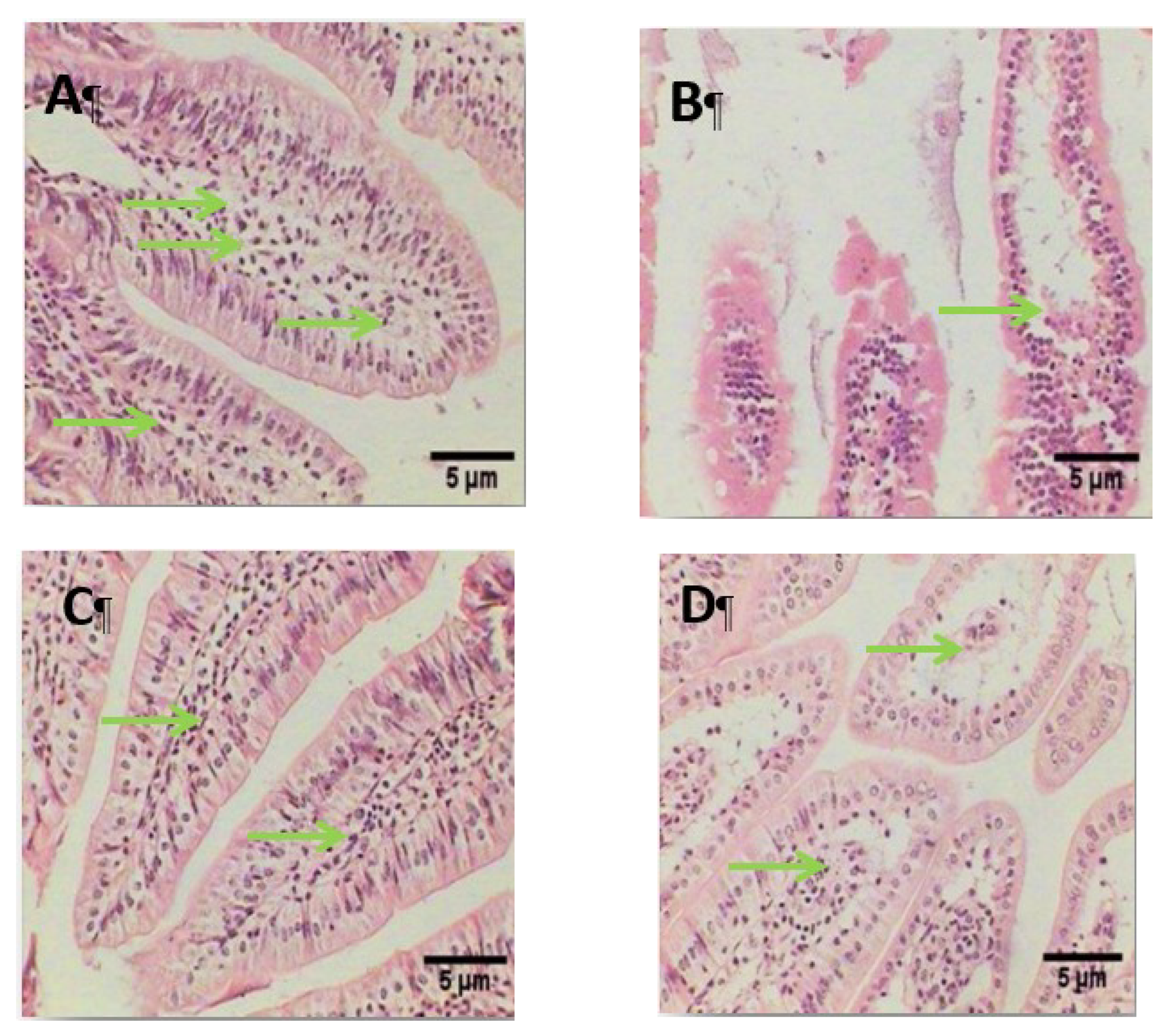
2.14. Histopathological Examination
2.15. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denbow, M. Gastrointestinal anatomy and physiology. In Sturkie’s Avian Physiology, 6th ed.; Academic Press: San Diego, CA, USA, 2015; pp. 337–366. [Google Scholar]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Khumsupan, P.; Gritsanapan, W. Anti-acne activity of Garcinia mangostana L.: A review. Plant Sci. Today 2014, 1, 147–150. [Google Scholar] [CrossRef]
- Hitchins, A.D. Listeria monocytogenes—Detection by chemiluminescent DNA hybridization. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 494–500. [Google Scholar]
- Lin, H.; Gao, D.; Hu, M.M.; Zhang, M.; Wu, X.-X.; Feng, L.; Xu, W.-H.; Yang, Q.; Zhong, X.; Wei, J.; et al. MARCH3 attenuates IL-1β–triggered inflammation by mediating K48-linked polyubiquitination and degradation of IL-1RI. Proc. Natl. Acad. Sci. USA 2018, 115, 12483–12488. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Dtsch. Arztebl. Int. 2016, 113, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Littmann, E.R.; Carter, R.A.; Kim, S.G.; Morjaria, S.M.; Ling, L.; Gyaltshen, Y.; Fontana, E.; Taur, Y.; Leiner, I.M.; et al. Commensal microbes provide first line defense against Listeria monocytogenes infection. J. Exp. Med. 2017, 214, 1973–1989. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Moneim, A.E.; Al-Quraishy, S. Indigofera oblongifolia ameliorates lead acetate-induced testicular oxidative damage and apoptosis in a rat model. Biol. Trace Elem. Res. 2016, 173, 354–361. [Google Scholar] [CrossRef]
- Nazre, M. New evidence on the origin of mangosteen (Garcinia mangostana L.) based on morphology and ITS sequence. Genet. Resour. Crop Evol. 2014, 61, 1147–1158. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Ayman, E.L.; Hassan, S.M.; Osman, H.E.H. Mangosteen (Garcinia mangostana L.). In Nonvitamin and Nonmineral Nutritional Supplements, 1st ed.; Nabavi, S., Silva, A.S., Eds.; Academic Press: London, UK; San Diego, CA, USA, 2019; pp. 313–319. [Google Scholar]
- Rajakannu, S.; Shankar, S.; Perumal, S.; Subramanian, S.; Dhakshinamoorthy, G.P. Biosynthesis of silver nanoparticles using Garcinia mangostana fruit extract and their antibacterial, antioxidant activity. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 944–952. [Google Scholar]
- Tsai, S.Y.; Chung, P.C.; Owaga, E.E.; Tsai, I.J.; Wang, P.Y.; Tsai, J.I.; Yeh, T.S.; Hsieh, R.H.; Hsieh, R.H. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab. 2016, 13, 88. [Google Scholar] [CrossRef]
- El-Khadragy, M.; Alolayan, E.M.; Metwally, D.M.; El-Din, M.F.S.; Alobud, S.S.; Alsultan, N.I.; Alsaif, S.S.; Awad, M.A.; Abdel Moneim, A.E. Clinical efficacy associated with enhanced antioxidant enzyme activities of silver nanoparticles biosynthesized using moringa oleifera leaf extract, against cutaneous leishmaniasis in a murine model of leishmania major. Int. J. Environ. Res. Public Health 2018, 15, 1037. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Ustundag, C.B.; Kaya, C.; Kaya, F.; Rafailovich, M. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int. J. Nanomed. 2011, 6, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Ch, W.; Hn, M.; Ej, H.; Ky, P. Improved production of caffeic acid derivatives in suspension cultures of Echinacea purpurea by medium replenishment strategy. Arch. Pharm. Res. 2007, 30, 945–949. [Google Scholar]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Akillioglu, H.G.; Karakaya, S. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci. Biotechnol. 2010, 19, 633–639. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Antioxidant potential of artemisia argentea l’hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Angelakopoulos, H.; Loock, K.; Sisul, D.M.; Jensen, E.R.; Miller, J.F.; Hohmann, E.L. Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: A dose escalation study of oral inoculation. Infect. Immun. 2002, 70, 3592–3601. [Google Scholar] [CrossRef]
- Lecuit, M.; Dramsi, S.; Gottardi, C.; Fedor-Chaiken, M.; Gumbiner, B.; Cossart, P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999, 18, 3956–3963. [Google Scholar] [CrossRef]
- Golnazarian, C.A.; Donnelly, C.W.; Pintauro, S.J.; Howard, D.B. Comparison of Infectious Dose of Listeria monocytogenes F5817 as Determined for Normal Versus Compromised C57B1/6J Mice. J. Food Prot. 1989, 52, 696–701. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.D.; Antonopoulos, D.A.; Wagner, B.; Duhamel, G.E.; Keresztes, I.; Ross, D.A.; Young, V.B.; Altier, C. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect. Immun. 2009, 77, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Narendra, K.; Uday, M. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Martemyanov, V.V.; Vorontsova, Y.L.; Rantala, M.J.; Gryzanova, E.V.; Glupov, V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 1–5. [Google Scholar] [CrossRef]
- Zhu, H.; Santo, A.; Jia, Z.; Li, Y.R. GPx4 in bacterial infection and polymicrobial sepsis: Involvement of ferroptosis and pyroptosis. React. Oxyg. Species 2019, 7, 154. [Google Scholar] [CrossRef]
- Seveau, S. Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Subcell. Biochem. 2014, 80, 161–195. [Google Scholar]
- Taher, M.; Susanti, D.; Rezali, M.F.; Zohri, F.S.A.; Ichwan, S.J.A.; Alkhamaiseh, S.I.; Ahmad, F. Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk. f. Asian Pac. J. Trop. Med. 2012, 5, 136–141. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Jaisupa, N.; Moongkarndi, P.; Lomarat, P.; Samer, J.; Tunrungtavee, V.; Muangpaisan, W.; Mangmool, S. Mangosteen peel extract exhibits cellular antioxidant activity by induction of catalase and heme oxygenase-1 mRNA expression. J. Food Biochem. 2018, 42, e12511. [Google Scholar] [CrossRef]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Moneim, A.E.A. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 2004, 172, 4866–4874. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Conte, M.P.; Ranaldi, S.; Penta, M.; Valenti, P.; Tinari, A.; Superti, F.; Seganti, L. Apoptotic death of Listeria monocytogenes-infected human macrophages induced by lactoferricin B, a bovine lactoferrin-derived peptide. Int. J. Immunopathol. Pharmacol. 2005, 18, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M. How bacteria-induced apoptosis of intestinal epithelial cells contributes to mucosal inflammation. J. Inflamm. Res. 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nikitas, G.; Deschamps, C.; Disson, O.; Niault, T.; Cossart, P.; Lecuit, M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 2011, 208, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Jindarat, S. Xanthones from mangosteen (Garcinia mangostana): Multi-targeting pharmacological properties. J. Med. Assoc. Thai. 2014, 97, S196–S201. [Google Scholar]
- Markus, S.; Martin, J.L. The Opportunistic Pathogen Listeria monocytogenes: Pathogenicity and Interaction with the Mucosal Immune System. Int. J. Inflamm. 2010, 2010, 704321. [Google Scholar]
- Sukhadeo, B.B.; Trinad, C. Molecular mechanisms of bacterial infection via the gut. Curr. Top. Microbiol. Immunol. 2009, 337, 173–195. [Google Scholar]
- Hatice, Y. Apoptosis and Infections. In Cell Death-Autophagy, Apoptosis and Necrosis. Intech. Open. 2015. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages, Front. Cell Dev. Biol. 2019, 7, 29. [Google Scholar]
- Aizat, W.M.; Jamil, I.N.; Ahmad-Hashim, F.H.; Noor, N.M. Recent updates on metabolite composition and medicinal benefits of mangosteen plant. PeerJ 2019, 7, e6324. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, R.; Yonehara, S.; Tsutsui, H. Fas-mediated inflammatory response in Listeria monocytogenes infection. J. Immunol. 2013, 190, 4245–4254. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chitchumroonchokchai, C.; Chuang, C.C.; West, T.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. 2009, 139, 1185–1191. [Google Scholar] [CrossRef]
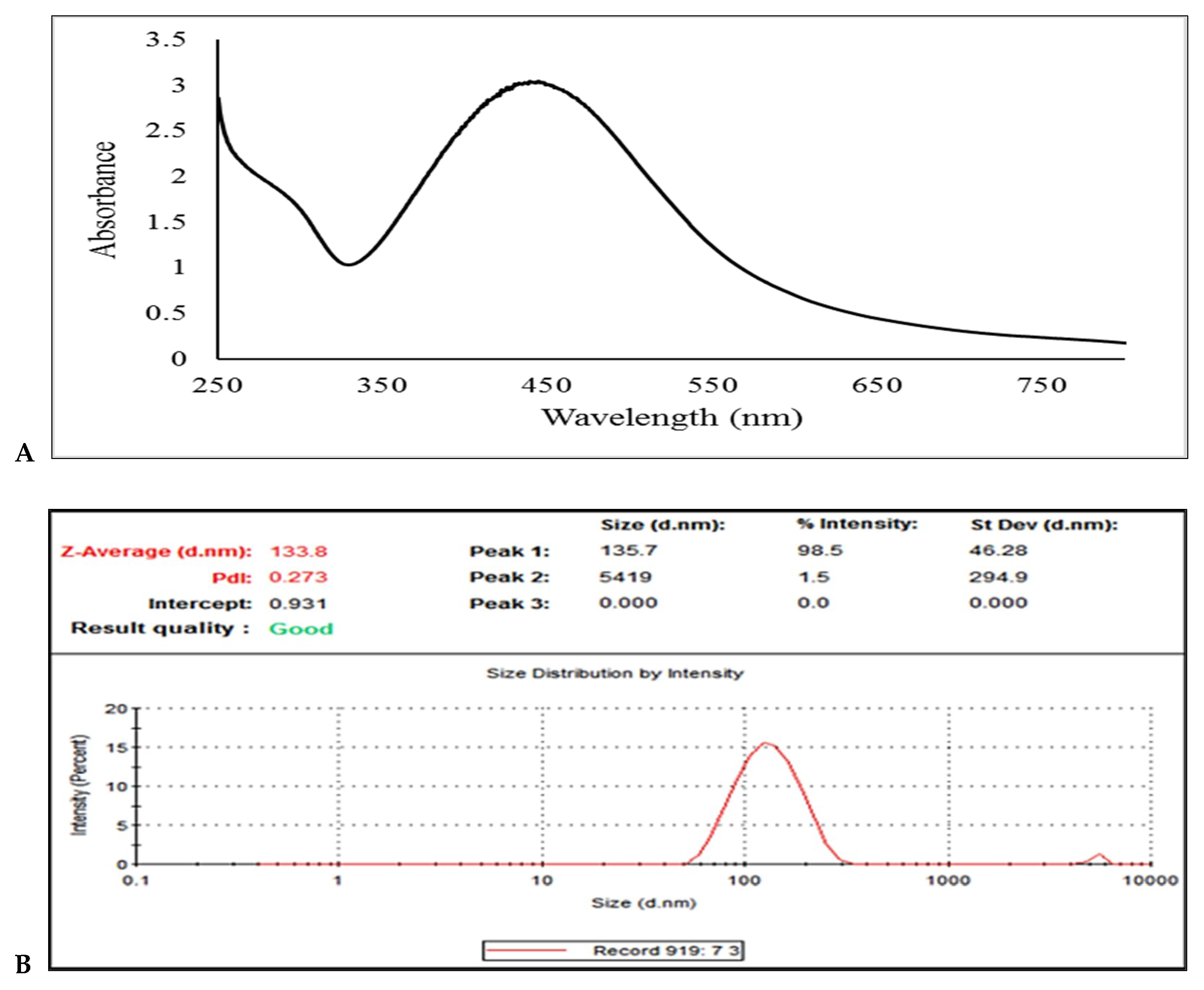




| Parameters | Mean ± SD |
|---|---|
| Total phenols (mg eq. gallic acid/g sample) | 11.453 ± 0.934 |
| Total flavonoids (mg eq. rutin/g sample) DPPH (%) | 0.725 ± 0.034 |
| DPPH (%) | 38.564 ± 1.23 |
| ABTS (_mol eq. Trolox/g sample) | 5.892 ± 0.045 |
| FRAB (_mol eq. Trolox/g sample) | 0.245 ± 0.0045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhuriji, A.F.; Majrashi, N.A.; Alomar, S.; El-Khadragy, M.F.; Awad, M.A.; Khatab, A.R.; Yehia, H.M. The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia mangostana Peel Extract against Pathogenicity of Listeria monocytogenes in Female BALB/c Mice. Animals 2020, 10, 573. https://doi.org/10.3390/ani10040573
Alkhuriji AF, Majrashi NA, Alomar S, El-Khadragy MF, Awad MA, Khatab AR, Yehia HM. The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia mangostana Peel Extract against Pathogenicity of Listeria monocytogenes in Female BALB/c Mice. Animals. 2020; 10(4):573. https://doi.org/10.3390/ani10040573
Chicago/Turabian StyleAlkhuriji, Afrah F., Nada A. Majrashi, Suliman Alomar, Manal F. El-Khadragy, Manal A. Awad, Alaa R. Khatab, and Hany M. Yehia. 2020. "The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia mangostana Peel Extract against Pathogenicity of Listeria monocytogenes in Female BALB/c Mice" Animals 10, no. 4: 573. https://doi.org/10.3390/ani10040573
APA StyleAlkhuriji, A. F., Majrashi, N. A., Alomar, S., El-Khadragy, M. F., Awad, M. A., Khatab, A. R., & Yehia, H. M. (2020). The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia mangostana Peel Extract against Pathogenicity of Listeria monocytogenes in Female BALB/c Mice. Animals, 10(4), 573. https://doi.org/10.3390/ani10040573






