The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas
Abstract
1. Introduction
2. Materials and Methods
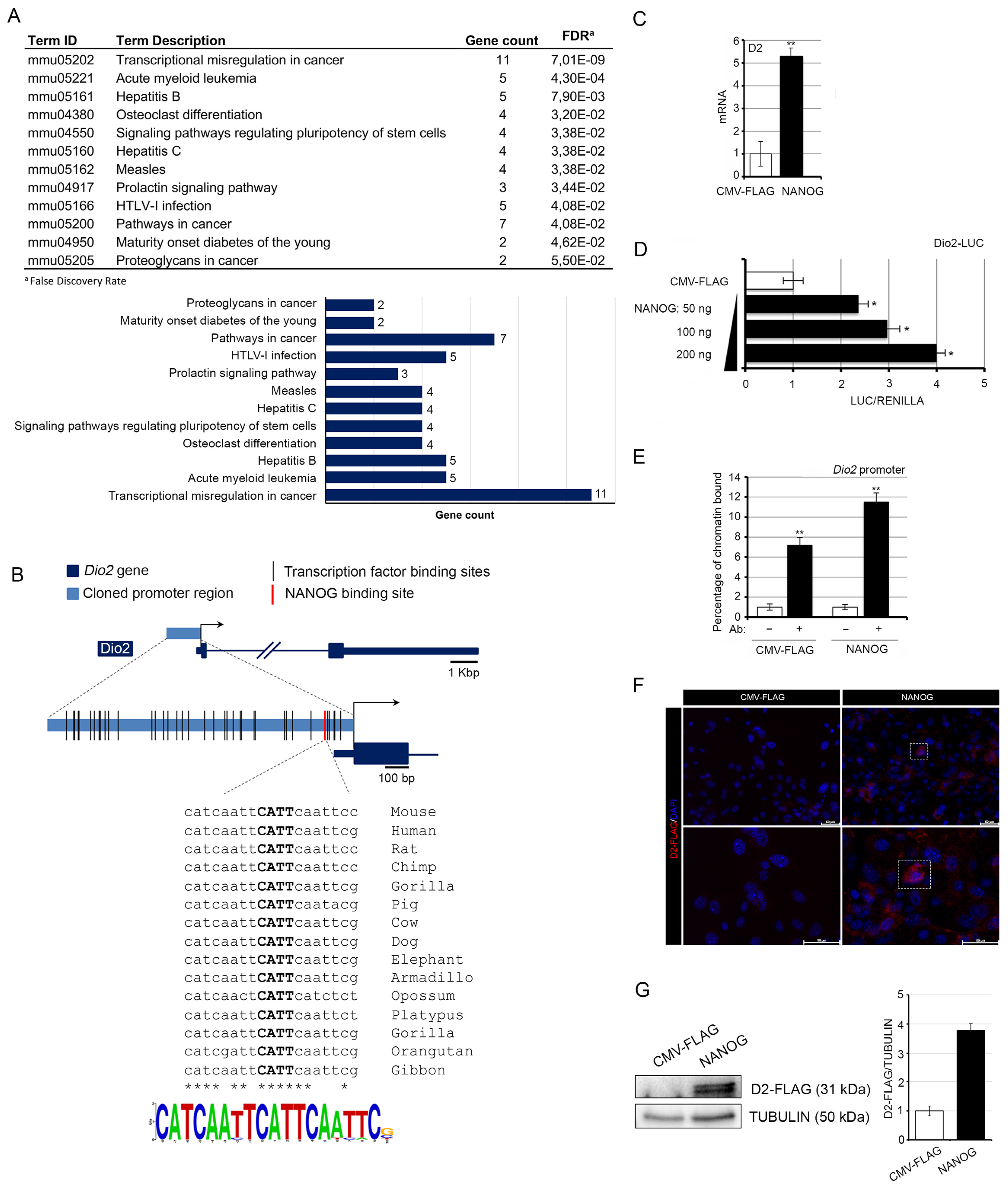
2.1. Analysis of Transition Factor Binding Sites
2.2. Cell Cultures and Transfections
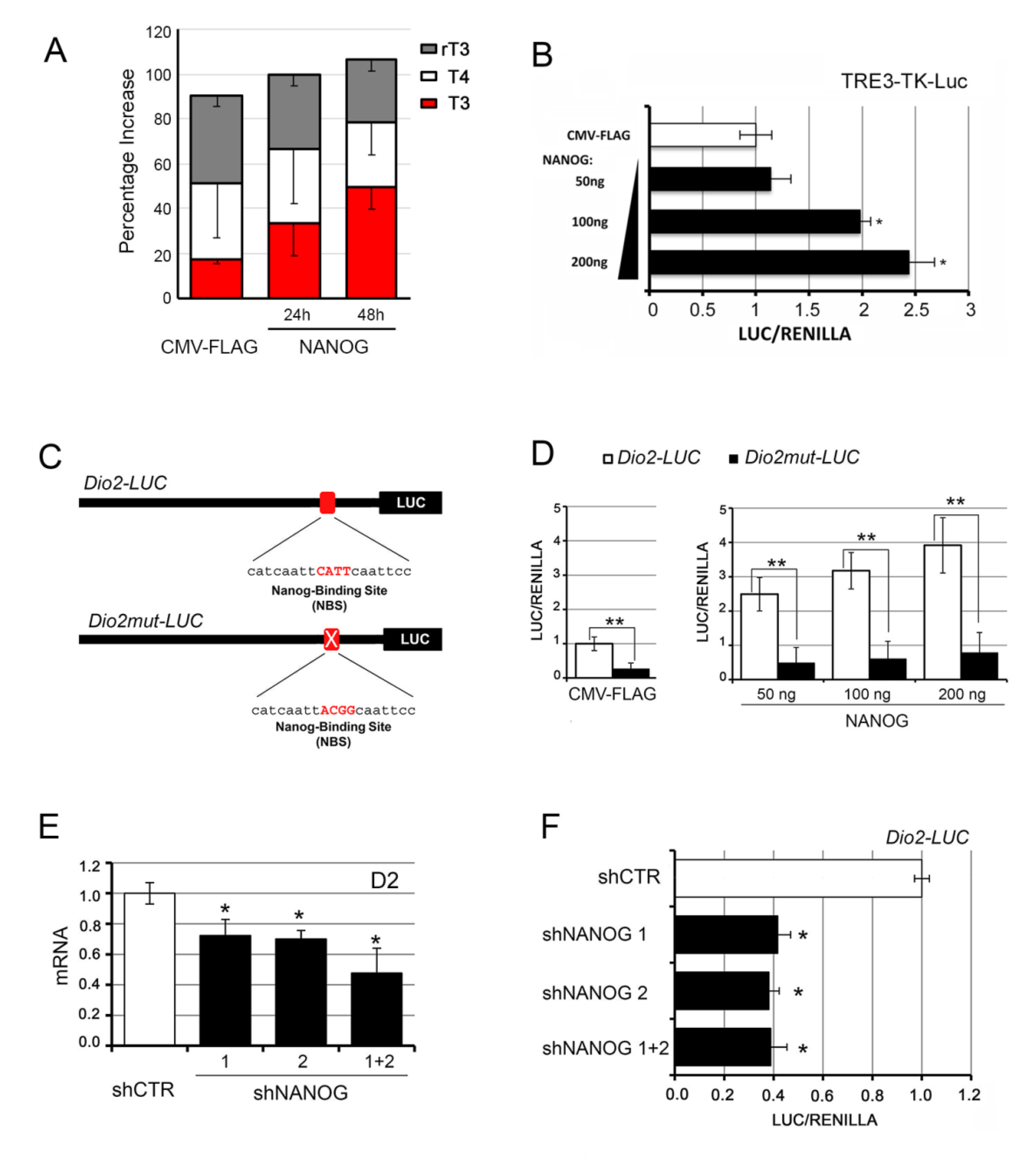
2.3. Short Hairpin RNA-Mediated Knock-Down of NANOG
2.4. Wound Scratch Assay
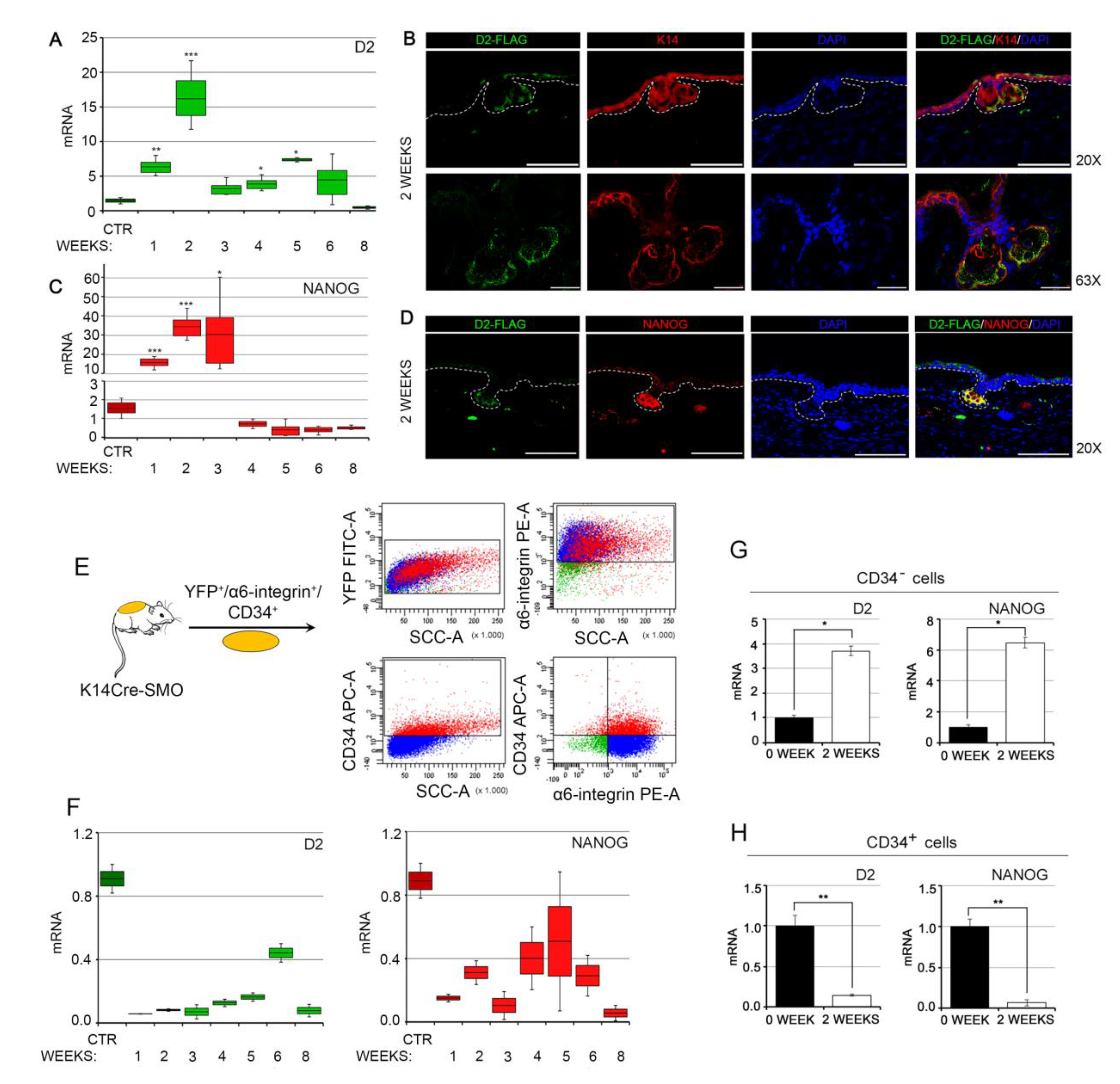
2.5. Animals, Histology, and Immunostaining
2.6. Isolation of CSCs
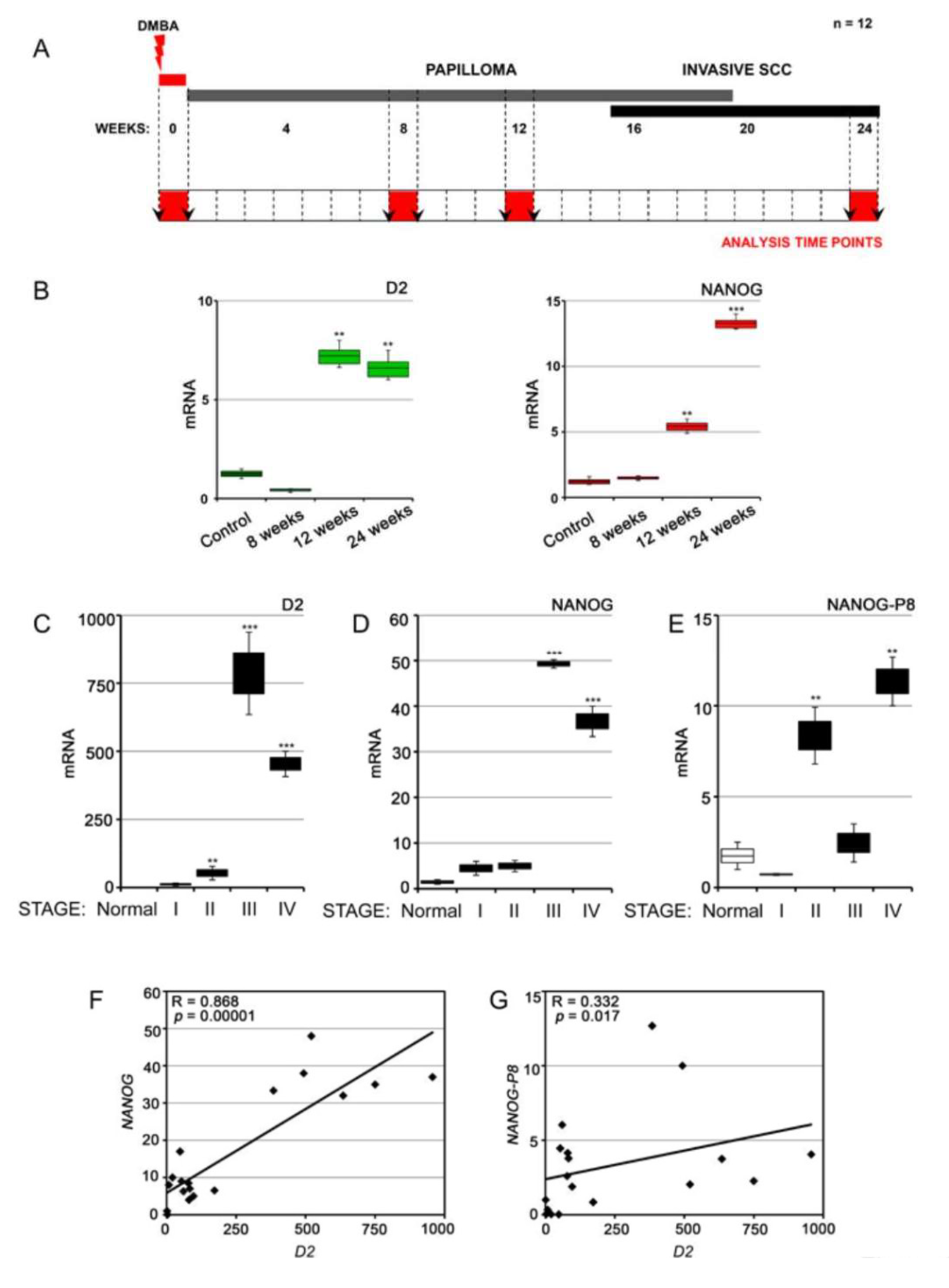
2.7. HPLC–MS Measurement of T3, T4, and rT3
2.8. Statistical Analyses
3. Results
3.1. NANOG Induces the Transcription of Type 2 Deiodinase
3.2. NANOG and Type 2 Deiodinase Colocalize in Mouse BCC and Are Expressed in the Early Phases of Tumorigenesis
3.3. NANOG and Type 2 Deiodinase Expression Is Associated with Advanced SCC Tumors in Mouse and Humans
3.4. The Intracellular Activation of TH Is Critical for the Oncogenic Effects of NANOG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, I.; Altaba, A.; Sanchez, P.; Dahmane, N. Gli and hedgehog in cancer: Tumours, embryos and stem cells. Nat. Rev. Cancer 2002, 2, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Angel, J.M.; Kiguchi, K.; DiGiovanni, J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat. Protoc. 2009, 4, 1350–1362. [Google Scholar] [CrossRef]
- Quintanilla, M.; Brown, K.; Ramsden, M.; Balmain, A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 1986, 322, 78–80. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeold, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Mancino, G.; Sibilio, A.; Luongo, C.; Di Cicco, E.; Miro, C.; Cicatiello, A.G.; Nappi, A.; Sagliocchi, S.; Ambrosio, R.; De Stefano, M.A.; et al. The thyroid hormone inactivator enzyme, Type 3 deiodinase, is essential for coordination of keratinocyte growth and differentiation. Thyroid 2020. [Google Scholar] [CrossRef]
- Dentice, M.; Luongo, C.; Huang, S.; Ambrosio, R.; Elefante, A.; Mirebeau-Prunier, D.; Zavacki, A.M.; Fenzi, G.; Grachtchouk, M.; Hutchin, M.; et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 14466–14471. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M. Hedgehog-mediated regulation of thyroid hormone action through iodothyronine deiodinases. Expert Opin. Ther. Targets 2011, 15, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Ambrosio, R.; Salzano, S.; Dlugosz, A.A.; Missero, C.; Dentice, M. The sonic hedgehog-induced type 3 deiodinase facilitates tumorigenesis of basal cell carcinoma by reducing Gli2 inactivation. Endocrinology 2014, 155, 2077–2088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dentice, M.; Antonini, D.; Salvatore, D. Type 3 deiodinase and solid tumors: An intriguing pair. Expert. Opin. Ther. Targets 2013, 17, 1369–1379. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Ambrosio, R.; De Stefano, M.A.; Mancino, G.; Porcelli, T.; Luongo, C.; Di Cicco, E.; Scalia, G.; Del Vecchio, L.; Colao, A.; et al. Reciprocal interplay between thyroid hormone and microRNA-21 regulates hedgehog pathway-driven skin tumorigenesis. J. Clin. Investig. 2016, 126, 2308–2320. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Ambrosio, R.; Dentice, M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol. Cell Endocrinol. 2017, 459, 84–89. [Google Scholar] [CrossRef]
- Miro, C.; Ambrosio, R.; De Stefano, M.A.; Di Girolamo, D.; Di Cicco, E.; Cicatiello, A.G.; Mancino, G.; Porcelli, T.; Raia, M.; Del Vecchio, L.; et al. The Concerted Action of Type 2 and Type 3 Deiodinases Regulates the Cell Cycle and Survival of Basal Cell Carcinoma Cells. Thyroid 2017, 27, 567–576. [Google Scholar] [CrossRef]
- Miro, C.; Di Cicco, E.; Ambrosio, R.; Mancino, G.; Di Girolamo, D.; Cicatiello, A.G.; Sagliocchi, S.; Nappi, A.; De Stefano, M.A.; Luongo, C.; et al. Thyroid hormone induces progression and invasiveness of squamous cell carcinomas by promoting a ZEB-1/E-cadherin switch. Nat. Commun. 2019, 10, 5410. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Hart, A.H.; Hartley, L.; Ibrahim, M.; Robb, L. Identification, cloning and expression analysis of the pluripotency promoting Nanog genes in mouse and human. Dev. Dyn. 2004, 230, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Piazzolla, D.; Abad, M.; Li, H.; Dominguez, O.; Schonthaler, H.B.; Wagner, E.F.; Serrano, M. Reprogramming activity of NANOGP8, a NANOG family member widely expressed in cancer. Oncogene 2014, 33, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.E.; Babaei-Jadidi, R.; Saadeddin, A.; Spencer-Dene, B.; Hossaini, S.; Abuzinadah, M.; Li, N.; Fadhil, W.; Ilyas, M.; Bonnet, D.; et al. Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1- and TCF-dependent mechanisms. Stem Cells 2012, 30, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.R.; Badeaux, M.; Choy, G.; Chandra, D.; Patrawala, L.; Liu, C.; Calhoun-Davis, T.; Zaehres, H.; Daley, G.Q.; Tang, D.G. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells 2009, 27, 993–1005. [Google Scholar] [CrossRef]
- Han, J.; Zhang, F.; Yu, M.; Zhao, P.; Ji, W.; Zhang, H.; Wu, B.; Wang, Y.; Niu, R. RNA interference-mediated silencing of NANOG reduces cell proliferation and induces G0/G1 cell cycle arrest in breast cancer cells. Cancer Lett. 2012, 321, 80–88. [Google Scholar] [CrossRef]
- Palla, A.R.; Piazzolla, D.; Alcazar, N.; Canamero, M.; Grana, O.; Gomez-Lopez, G.; Dominguez, O.; Duenas, M.; Paramio, J.M.; Serrano, M. The pluripotency factor NANOG promotes the formation of squamous cell carcinomas. Sci. Rep. 2015, 5, e10205. [Google Scholar] [CrossRef]
- Sheng, H.; Goich, S.; Wang, A.; Grachtchouk, M.; Lowe, L.; Mo, R.; Lin, K.; de Sauvage, F.J.; Sasaki, H.; Hui, C.-C.; et al. Dissecting the oncogenic potential of Gli2: Deletion of an NH(2)-terminal fragment alters skin tumor phenotype. Cancer Res. 2002, 62, 5308–5316. [Google Scholar]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar]
- Vasioukhin, V.; Degenstein, L.; Wise, B.; Fuchs, E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA 1999, 96, 8551–8556. [Google Scholar] [CrossRef]
- Wong, S.Y.; Seol, A.D.; So, P.L.; Ermilov, A.N.; Bichakjian, C.K.; Epstein, E.H., Jr.; Dlugosz, A.A.; Reiter, J.F. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 2009, 15, 1055–1061. [Google Scholar] [CrossRef]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.K.; Van Keymeulen, A.; Lapouge, G.; Beck, B.; Michaux, C.; Achouri, Y.; Sotiropoulou, P.A.; Blanpain, C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010, 12, 299–305. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Li, M.; Han, J.; Chen, B.; Wang, B.; Dai, J. NANOGP8 is a retrogene expressed in cancers. FEBS J. 2006, 273, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Steinsapir, J.; Bianco, A.C.; Buettner, C.; Harney, J.; Larsen, P.R. Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme’s active center. Endocrinology 2000, 141, 1127–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gereben, B.; Goncalves, C.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. Selective proteolysis of human type 2 deiodinase: A novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol. Endocrinol. 2000, 14, 1697–1708. [Google Scholar] [CrossRef]
- Jeter, C.R.; Yang, T.; Wang, J.; Chao, H.P.; Tang, D.G. Concise Review: NANOG in Cancer Stem Cells and Tumor Development: An Update and Outstanding Questions. Stem Cells 2015, 33, 2381–2390. [Google Scholar] [CrossRef]
- Kawamura, N.; Nimura, K.; Nagano, H.; Yamaguchi, S.; Nonomura, N.; Kaneda, Y. CRISPR/Cas9-mediated gene knockout of NANOG and NANOGP8 decreases the malignant potential of prostate cancer cells. Oncotarget 2015, 6, 22361–22374. [Google Scholar] [CrossRef]
- Piestun, D.; Kochupurakkal, B.S.; Jacob-Hirsch, J.; Zeligson, S.; Koudritsky, M.; Domany, E.; Amariglio, N.; Rechavi, G.; Givol, D. Nanog transforms NIH3T3 cells and targets cell-type restricted genes. Biochem. Biophys. Res. Commun. 2006, 343, 279–285. [Google Scholar] [CrossRef]
- Ji, W.; Jiang, Z. Effect of shRNA-mediated inhibition of Nanog gene expression on the behavior of human gastric cancer cells. Oncol. Lett. 2013, 6, 367–374. [Google Scholar] [CrossRef][Green Version]
- Lu, X.; Mazur, S.J.; Lin, T.; Appella, E.; Xu, Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene 2014, 33, 2655–2664. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.W.; Zhang, B.H.; Ren, Z.G.; Qiu, S.J.; Yi, Y.; Fan, J. Coexpression of stemness factors Oct4 and Nanog predict liver resection. Ann. Surg. Oncol. 2012, 19, 2877–2887. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Source | Identifier | Diluition |
|---|---|---|---|
| Anti-α-Tubulin antibody, Mouse monoclonal | Sigma-Aldrich | T8203 | 1:5000 WB |
| Anti-Cytokeratin 14, Rabbit polyclonal | Covance® | CLPRB-155P | 1:2000 IF |
| Anti-Cytokeratin 17, Rabbit polyclonal | abcam | ab53707 | 1:2000 IF |
| Anti-FLAG® M2 antibody, Mouse monoclonal | Sigma-Aldrich | F3165 | 1:1000 IF |
| 1:1000 WB | |||
| Anti-E-cadherin, Mouse monoclonal | BD Biosciences | 610181 | 1:1000 WB |
| Anti-N-cadherin, Rabbit polyclonal | Elabscience® | E-AB-32170 | 1:500 WB |
| Anti-Nanog antibody, Rabbit polyclonal | abcam | ab80892 | 1:1000 WB |
| Anti-Nanog (D73G4) XP®, Rabbit monoclonal | Cell-Signaling | #4903 | 1:2000 WB |
| Anti-ZEB1, Rabbit polyclonal | abcam | ab155249 | 1:500 WB |
| APC ANTI-MOUSE CD34 ANTIBODY 25 UG | BIOLEGEND | 119309 | 1:100 FACS |
| PE Rat Anti-Human CD49f Clone GoH3 (RUO) | BD Pharmingen™ | 555736 | 1:100 FACS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, A.; Di Cicco, E.; Miro, C.; Cicatiello, A.G.; Sagliocchi, S.; Mancino, G.; Ambrosio, R.; Luongo, C.; Di Girolamo, D.; De Stefano, M.A.; et al. The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas. Cancers 2020, 12, 715. https://doi.org/10.3390/cancers12030715
Nappi A, Di Cicco E, Miro C, Cicatiello AG, Sagliocchi S, Mancino G, Ambrosio R, Luongo C, Di Girolamo D, De Stefano MA, et al. The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas. Cancers. 2020; 12(3):715. https://doi.org/10.3390/cancers12030715
Chicago/Turabian StyleNappi, Annarita, Emery Di Cicco, Caterina Miro, Annunziata Gaetana Cicatiello, Serena Sagliocchi, Giuseppina Mancino, Raffaele Ambrosio, Cristina Luongo, Daniela Di Girolamo, Maria Angela De Stefano, and et al. 2020. "The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas" Cancers 12, no. 3: 715. https://doi.org/10.3390/cancers12030715
APA StyleNappi, A., Di Cicco, E., Miro, C., Cicatiello, A. G., Sagliocchi, S., Mancino, G., Ambrosio, R., Luongo, C., Di Girolamo, D., De Stefano, M. A., Porcelli, T., Stornaiuolo, M., & Dentice, M. (2020). The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas. Cancers, 12(3), 715. https://doi.org/10.3390/cancers12030715








