Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation
Abstract
:1. Introduction
2. Results
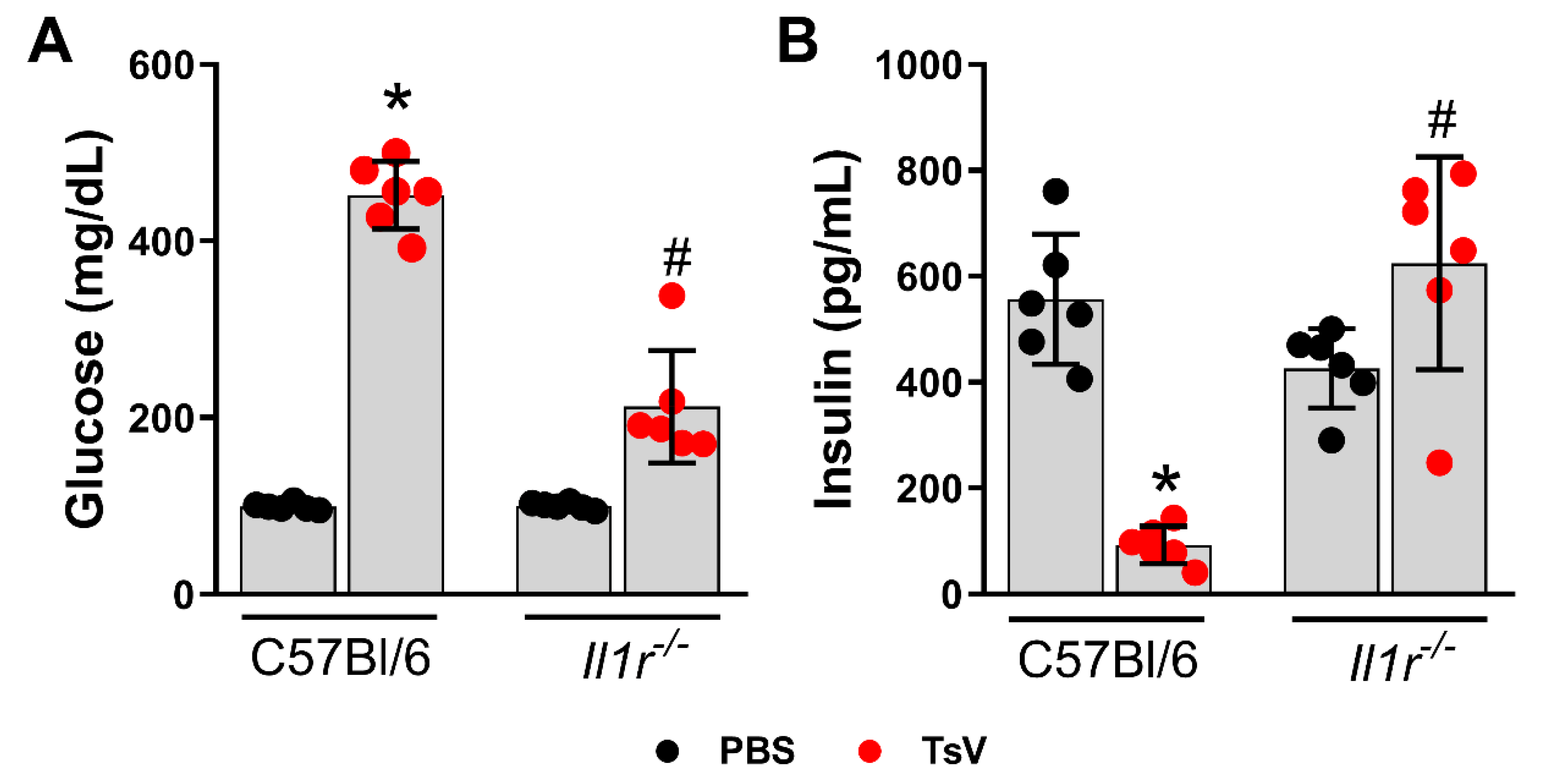
2.1. IL-1 Receptor Regulates Scorpion Venom-Induced Hyperglycemia and Insulin Levels
2.2. Nitric Oxide (NO), Chemokines, and Myeloperoxidase (MPO) are Downregulated in Il1r1−/− Animals
2.3. Inhibition of NO Production Prevents Scorpion Venom-Induced Hyperglycemia
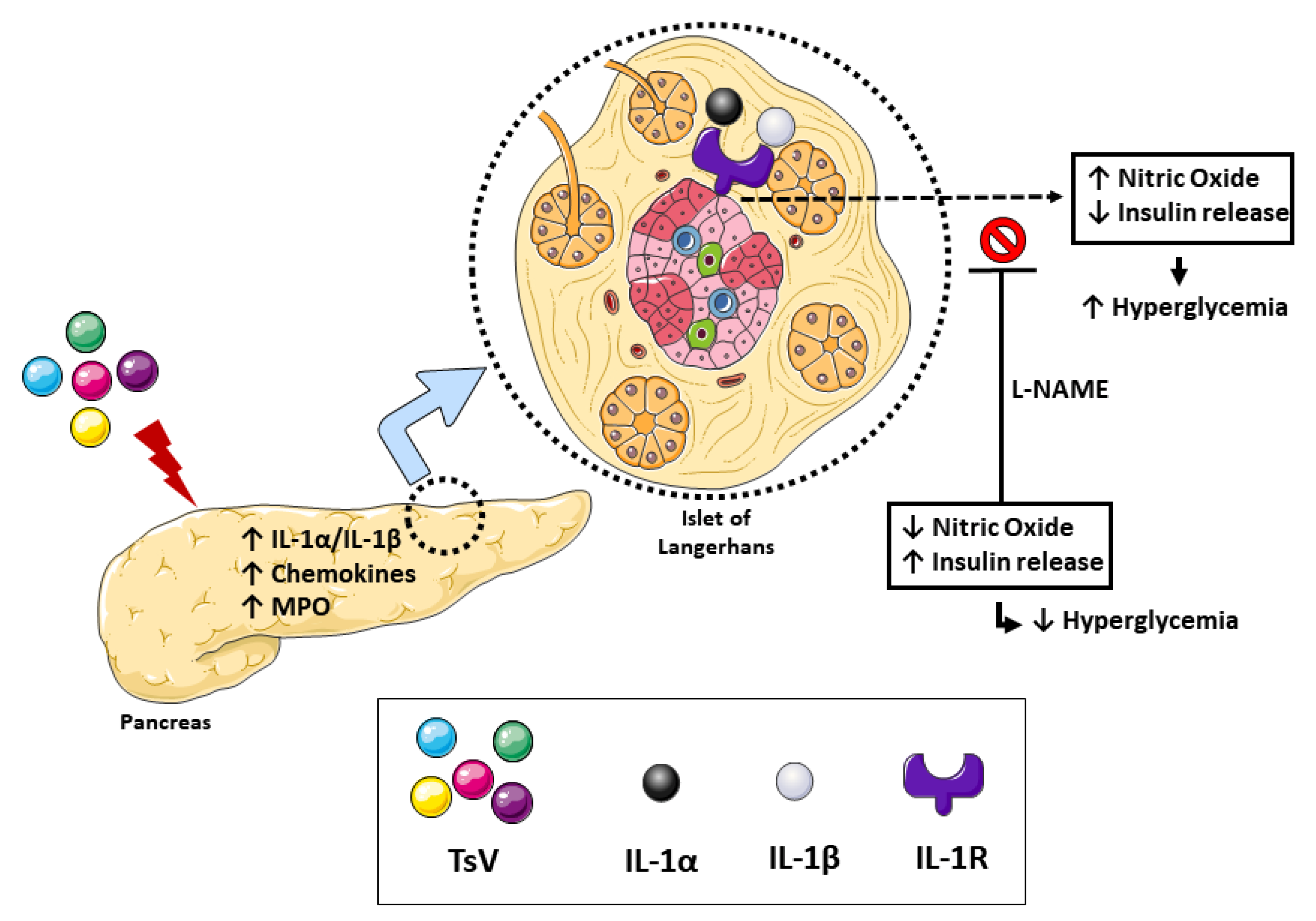
3. Discussion
4. Materials and Methods
4.1. TsV
4.2. Animals
4.3. In Vivo Experiments and Drug Treatments
4.4. Glucose Measurement
4.5. Quantification of Insulin, Cytokines, MPO, and NO
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isbister, G.K.; Bawaskar, H.S. Scorpion envenomation. N. Engl. J. Med. 2014, 371, 457–563. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, G.C.; Pinto, V.L., Jr. Scorpionism in Brazil in the years 2000 to 2012. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucca, M.B.; Cerni, F.A.; Junior, E.L.; Bordon, K.D.; Amorim, F.G.; Cordeiro, F.A.; Longhim, H.T.; Cremonez, C.M.; Oliveira, G.H.; Arantes, E.C. Tityus serrulatus venom—A lethal cocktail. Toxicon 2015, 108, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Cupo, P. Clinical update on scorpion envenoming. Rev. Soc. Bras. Med. Trop. 2015, 48, 642–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yugandhar, B.; Radha Krishna Murthy, K.; Sattar, S.A. Insulin administration in severe scorpion evenomation. J. Venom. Anim. Toxins 1999, 5, 200–219. [Google Scholar] [CrossRef]
- De Oliveira, G.H.; Cerni, F.A.; Cardoso, I.A.; Arantes, E.C.; Pucca, M.B. Tityus serrulatus envenoming in non-obese diabetic mice: A risk factor for severity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccal, K.F.; da Silva Bitencourt, C.; Secatto, A.; Sorgi, C.A.; Bordon, K.D.; Sampaio, S.V.; Arantes, E.C.; Faccioli, L.H. Tityus serrulatus venom and toxins Ts1, Ts2 and Ts6 induce macrophage activation and production of immune mediators. Toxicon 2011, 57, 1101–1108. [Google Scholar] [CrossRef]
- Zoccal, K.F.; da Silva Bitencourt, C.; Sorgi, C.A.; Bordon, K.D.; Sampaio, S.V.; Arantes, E.C.; Faccioli, L.H. Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production. Toxicon 2013, 61, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zoccal, K.F.; da Silva Bitencourt, C.; Paula-Silva, F.W.G.; Sorgi, C.A.; de Castro Figueiredo Bordon, K.; Arantes, E.C.; Faccioli, L.H. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS ONE 2014, 9, e88174. [Google Scholar] [CrossRef] [Green Version]
- Zoccal, K.F.; Paula-Silva, F.W.G.; Bitencourt, C.D.S.; Sorgi, C.A.; Bordon, K.D.C.F.; Arantes, E.C.; Faccioli, L.H. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production. Toxicon 2015, 93, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Zoccal, K.F.; Sorgi, C.A.; Hori, J.I.; Paula-Silva, F.W.G.; Arantes, E.C.; Serezani, C.H.; Zamboni, D.S.; Faccioli, L.H. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat. Commun. 2016, 7, 10760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, M.B.; Zoccal, K.F.; Gardinassi, L.G.; Faccioli, L.H. Scorpion envenomation and inflammation: Beyond neurotoxic effects. Toxicon 2019, 167, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; Gardinassi, L.G.; Sorgi, C.A.; Meirelles, A.F.G.; Bordon, K.C.F.; Glezer, I.; Cupo, P.; Matsuno, A.K.; Bollela, V.R.; Arantes, E.C.; et al. CD36 shunts eicosanoid metabolism to repress CD14 licensed interleukin-1β release and inflammation. Front. Immunol. 2018, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Zoccal, K.F.; Gardinassi, L.G.; Bordon, K.C.F.; Arantes, E.C.; Marleau, S.; Ong, H.; Faccioli, L.H. EP80317 restrains inflammation and mortality caused by scorpion envenomation in mice. Front. Pharmacol. 2019, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, M.L.; Kwon, G.; Hill, J.R.; Marshall, C.A.; Corbett, J.A. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 1996, 211, 24–32. [Google Scholar] [CrossRef]
- Moossa, A.R. Acute pancreatitis in children. Lancet 1972, 299, 638. [Google Scholar] [CrossRef]
- Cusinato, D.A.C.; Souza, A.M.; Vasconcelos, F.; Guimarães, L.F.L.; Leite, F.P.; Gregório, Z.M.O.; Giglio, J.R.; Arantes, E.C. Assessment of biochemical and hematological parameters in rats injected with Tityus serrulatus scorpion venom. Toxicon 2010, 56, 1477–1486. [Google Scholar] [CrossRef]
- Andersson, P.O.; Holst, J.; Järhult, J. Effects of adrenergic blockade on the release of insulin, glucagon and somatostatin from the pancreas in response to splanchnic nerve stimulation in cats. Acta Physiol. Scand. 1982, 116, 403–409. [Google Scholar] [CrossRef]
- Radha Krishna Murthy, K.; Haghnazari, L. The blood levels of glucagon, cortison, and insulin following the injection of venom by the scorpion (Mesobuthus tamulus concanesis, POCOCK) in dogs. J. Venom. Anim. Toxins 1999, 5, 47–55. [Google Scholar] [CrossRef]
- Corbett, J.A.; Wang, J.L.; Sweetland, M.A.; Lancaster, J.R., Jr.; McDaniel, M.L. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J. Clin. Investig. 1992, 90, 2384–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajmrle, C.; Smith, N.; Spigelman, A.F.; Dai, X.; Senior, L.; Bautista, A.; Ferdaoussi, M.; MacDonald, P.E. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight. 2016, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.R.; Goldenberg, A.; Netto, A.A.S.; Gonzalez, A.M. Comparative efficacy of Belzer or Euro-Collins solutions for pancreatic preservation during cold ischemic storage in rats. Acta Cir. Bras. 2014, 29, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, N.D.; Azmi, N.; Gopalan, H.K. The protective effects of Phaleria macrocarpa leaves methanol extract on pancreatic islets histology in streptozotocin—induced diabetic rats. Sci. Int. 2015, 27, 4219–4224. [Google Scholar]
- Karawya, F.; Metwally, E.S.M. Structural changes in pancreatic acinar cells and β-cells of rat fed with genetically modified corn. J. Exp. Clin. Anat. 2016, 15, 77–84. [Google Scholar] [CrossRef]
- Kar, E.; Sahintürk, V.; Öz, S. Early treatment with metformin decreases pancreatic damage in rats with LPS induced sepsis. Turk. J. Life Sci. 2018, 3, 194–199. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, M.B.; Elias-Oliveira, J.; Pastore, M.R.; Ramos, S.G.; Gardinassi, L.G.; Faccioli, L.H. Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation. Toxins 2020, 12, 163. https://doi.org/10.3390/toxins12030163
Reis MB, Elias-Oliveira J, Pastore MR, Ramos SG, Gardinassi LG, Faccioli LH. Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation. Toxins. 2020; 12(3):163. https://doi.org/10.3390/toxins12030163
Chicago/Turabian StyleReis, Mouzarllem B., Jefferson Elias-Oliveira, Marcella R. Pastore, Simone G. Ramos, Luiz G. Gardinassi, and Lúcia H. Faccioli. 2020. "Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation" Toxins 12, no. 3: 163. https://doi.org/10.3390/toxins12030163





