Primary Production, an Index of Climate Change in the Ocean: Satellite-Based Estimates over Two Decades
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Chlorophyll-a Data from Satellites
2.2. Primary Production Model
2.3. Photosynthesis versus Irradiance Parameters
2.4. Analyses of Primary Production
3. Results
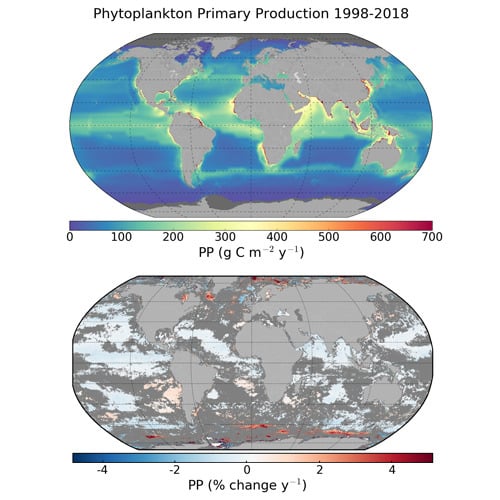
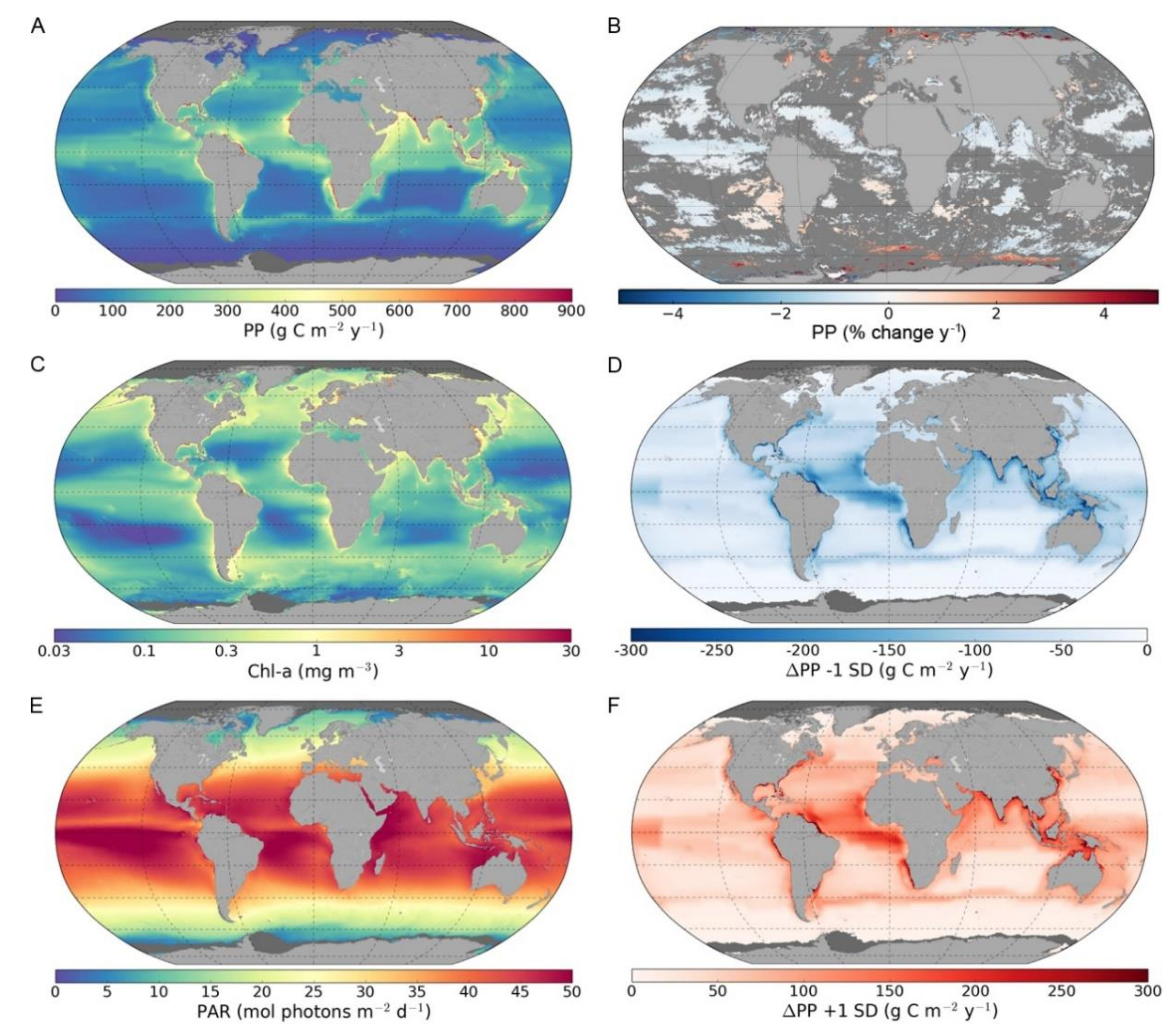
3.1. Global and Regional Annual Primary Production
3.2. Trends in Primary Production
3.3. Sensitivity of Primary Primary Production to Changes in Photosynthetic Parameters
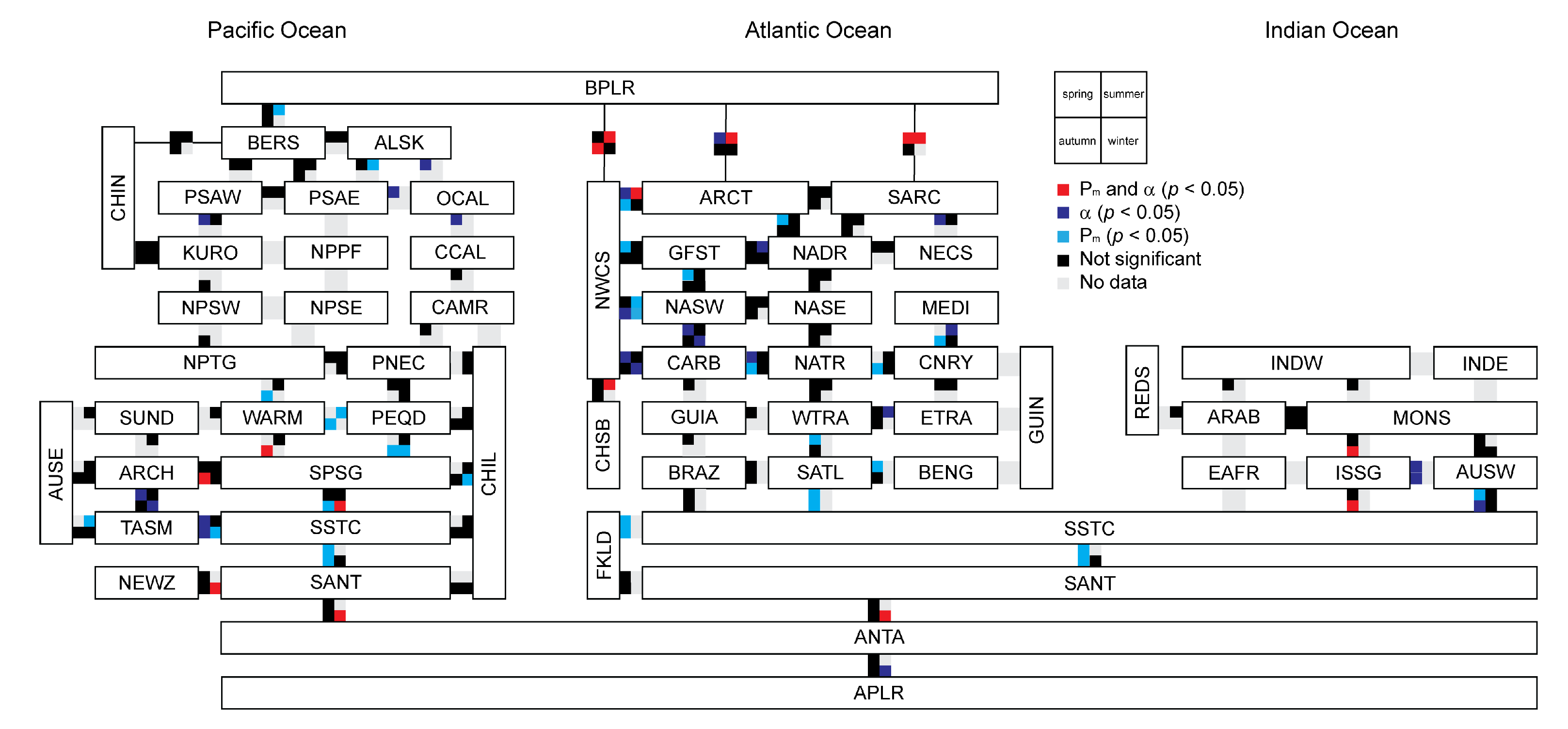
3.4. Relationship between Photosynthetic Parameters and Primary Production
3.5. Variation in Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Model of Daily Water-Column Primary Production
Appendix A.1. Phytoplankton Biomass
Appendix A.2. Irradiance Field
Appendix A.3. Daily Primary Production over the Water Column
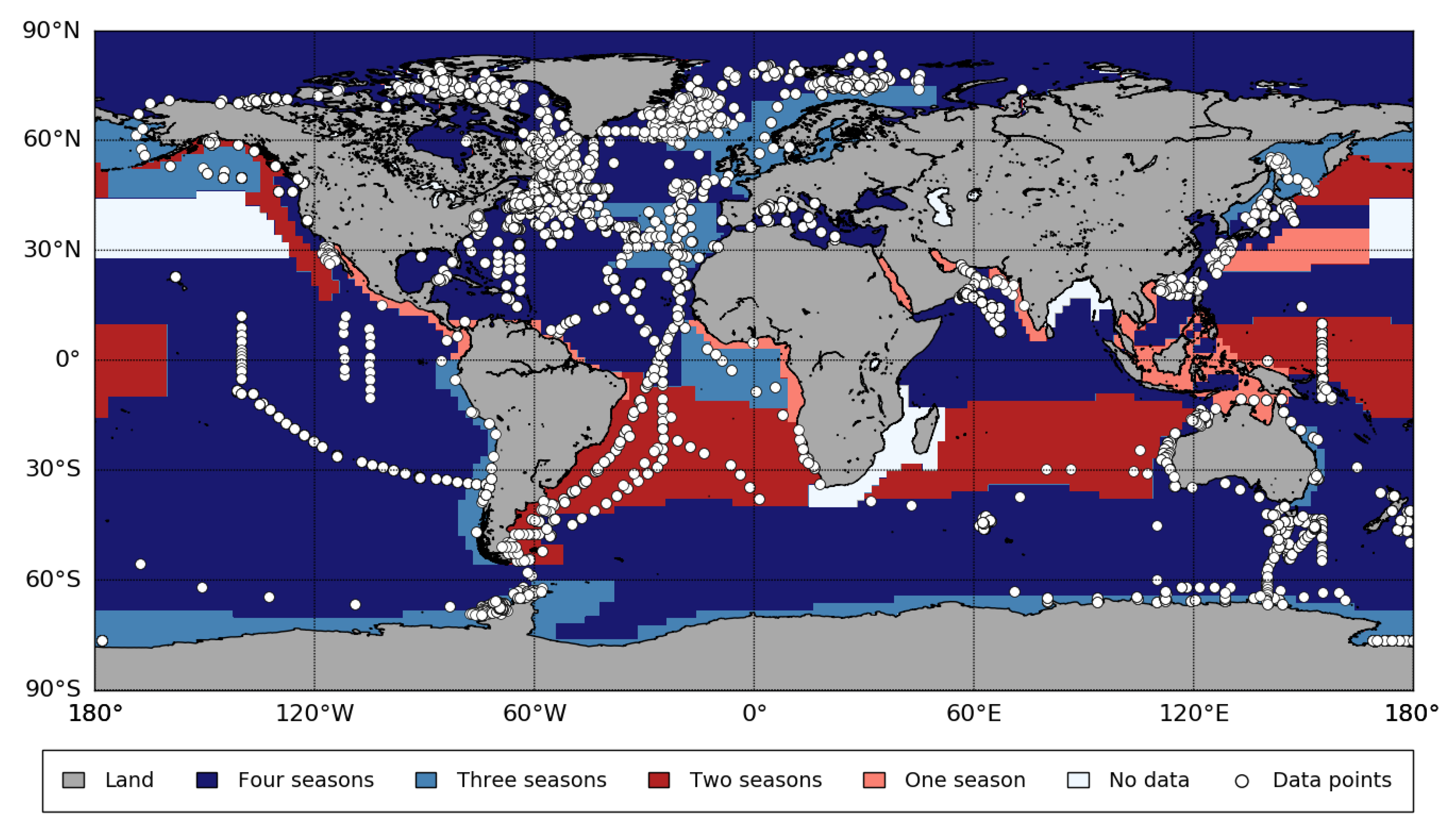
Appendix B. Biogeographic Provinces
| Number | Basin | Biome | Acronym | Province |
|---|---|---|---|---|
| 1 | Atlantic | Polar | BPLR | Boreal Polar Province |
| 2 | Atlantic | Polar | ARCT | Atlantic Arctic Province |
| 3 | Atlantic | Polar | SARC | Atlantic Subarctic Province |
| 4 | Atlantic | Westerlies | NADR | North Atlantic Drift Province |
| 5 | Atlantic | Westerlies | GFST | Gulf Stream Province |
| 6 | Atlantic | Westerlies | NASW | North Atlantic Subtropical Gyral Province (West) |
| 7 | Atlantic | Trades | NATR | North Atlantic Tropical Gyral Province |
| 8 | Atlantic | Trades | WTRA | Western Tropical Atlantic Province |
| 9 | Atlantic | Trades | ETRA | Eastern Tropical Atlantic Province |
| 10 | Atlantic | Trades | SATL | South Atlantic Gyral Province |
| 11 | Atlantic | Coastal | NECS | Northeast Atlantic Shelves Province |
| 12 | Atlantic | Coastal | CNRY | Canary Current Coastal Province |
| 13 | Atlantic | Coastal | GUIN | Guinea Current Coastal Province |
| 14 | Atlantic | Coastal | GUIA | Guianas Coastal Province |
| 15 | Atlantic | Coastal | NWCS | Northwest Atlantic Shelves Province |
| 16 | Atlantic | Westerlies | MEDI | Mediterranean Sea, Black Sea Province |
| 17 | Atlantic | Trades | CARB | Caribbean Province |
| 18 | Atlantic | Westerlies | NASE | North Atlantic Subtropical Gyral Province (East) |
| 19 | Atlantic | Coastal | CHSB | Cheasapeake Bay Province |
| 20 | Atlantic | Coastal | BRAZ | Brazil Current Coastal Province |
| 21 | Atlantic | Coastal | FKLD | Southwest Atlantic Shelves Province |
| 22 | Atlantic | Coastal | BENG | Benguela Current Coastal Province |
| 30 | Indian | Trades | MONS | Indian Monsoon Gyres Province |
| 31 | Indian | Trades | ISSG | Indian South Subtropical Gyre Province |
| 32 | Indian | Coastal | EAFR | Eastern Africa Coastal Province |
| 33 | Indian | Coastal | REDS | Red Sea, Arabian Gulf Province |
| 34 | Indian | Coastal | ARAB | Northwest Arabian Sea Upwelling Province |
| 35 | Indian | Coastal | INDE | Eastern India Coastal Province |
| 36 | Indian | Coastal | INDW | Western India Coastal Province |
| 37 | Indian | Coastal | AUSW | Australia-Indonesia Coastal Province |
| 50 | Pacific | Polar | BERS | North Epicontinental Sea Province |
| 51 | Pacific | Westerlies | PSAE | Pacific Subarctic Gyres Province (East) |
| 52 | Pacific | Westerlies | PSAW | Pacific Subarctic Gyres Province (West) |
| 53 | Pacific | Westerlies | KURO | Kuroshio Current Province |
| 54 | Pacific | Westerlies | NPPF | North Pacific Polar Front Province |
| 55 | Pacific | Westerlies | NPSE | North Pacific Subtropical Province (East) |
| 56 | Pacific | Westerlies | NPSW | North Pacific Subtropical Province (West) |
| 57 | Pacific | Westerlies | OCAL | Offshore California Current Province |
| 58 | Pacific | Westerlies | TASM | Tasman Sea Province |
| 59 | Pacific | Westerlies | SPSG | South Pacific Subtropical Gyre Province |
| 60 | Pacific | Trades | NPTG | North Pacific Tropical Gyre Province |
| 61 | Pacific | Trades | PNEC | North Pacific Equatorial Countercurrent Province |
| 62 | Pacific | Trades | PEQD | Pacific Equatorial Divergence Province |
| 63 | Pacific | Trades | WARM | Western Pacific Warm Pool Province |
| 64 | Pacific | Trades | ARCH | Archipelagic Deep Basin Province |
| 65 | Pacific | Coastal | ALSK | Alaska Coastal Downwelling Province |
| 66 | Pacific | Coastal | CCAL | California Upwelling Coastal Province |
| 67 | Pacific | Coastal | CAMR | Central American Coastal Province |
| 68 | Pacific | Coastal | CHIL | Chile–Peru Current Coastal Province |
| 69 | Pacific | Coastal | CHIN | China Sea Coastal Province |
| 70 | Pacific | Coastal | SUND | Sunda-Arafura Shelves Province |
| 71 | Pacific | Coastal | AUSE | Eastern Australian Coastal Province |
| 72 | Pacific | Coastal | NEWZ | New Zealand Coastal Province |
| 80 | Antarctic | Westerlies | SSTC | South Subtropical Convergence Province |
| 81 | Antarctic | Westerlies | SANT | Subantarctic Water Ring Province |
| 82 | Antarctic | Polar | ANTA | Antarctic Province |
| 83 | Antarctic | Polar | APLR | Austral Polar Province |
References
- Lurin, B.; Rasool, S.; Cramer, W.; Moore, B. Global terrestrial net primary production. Glob. Chang. Newsl. 1994, 19, 6–8. [Google Scholar]
- Longhurst, A.R.; Sathyendranath, S.; Platt, T.; Caverhill, C. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 1995, 17, 1245–1271. [Google Scholar] [CrossRef]
- Field, C.B. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.E.; Friedrichs, M.A.; Schmeltz, M.; Noguchi Aita, M.; Antoine, D.; Arrigo, K.R.; Asanuma, I.; Aumont, O.; Barber, R.; Behrenfeld, M.; et al. A comparison of global estimates of marine primary production from ocean color. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 741–770. [Google Scholar] [CrossRef]
- Buitenhuis, E.T.; Hashioka, T.; Quéré, C.L. Combined constraints on global ocean primary production using observations and models. Glob. Biogeochem. Cycles 2013, 27, 847–858. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef]
- Antoine, D.; André, J.M.; Morel, A. Oceanic primary production 2. Estimation at global scale from satellite (coastal zone color scanner) chlorophyll. Glob. Biogeochem. Cycles 1996, 10, 57–69. [Google Scholar] [CrossRef]
- von Schuckmann, K.; Le Traon, P.Y.; Alvarez-Fanjul, E.; Axell, L.; Balmaseda, M.; Breivik, L.A.; Brewin, R.J.; Bricaud, C.; Drevillon, M.; Drillet, Y.; et al. The Copernicus Marine Environment Monitoring Service Ocean State Report. J. Oper. Oceanogr. 2016, 9, s235–s320. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.; Ivar Korsbakken, J.; Peters, G.; Canadell, J.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.J.W.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An ocean-colour time series for use in climate studies: The experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef]
- Montes-Hugo, M.; Doney, S.C.; Ducklow, H.W.; Fraser, W.; Martinson, D.; Stammerjohn, S.E.; Schofield, O. Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 2009, 323, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R.; Van Dijken, G.L. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 2015, 136, 60–70. [Google Scholar] [CrossRef]
- Randelhoff, A.; Oziel, L.; Massicotte, P.; Bécu, G.; Galí, M.; Lacour, L.; Dumont, D.; Vladoiu, A.; Marec, C.; Bruyant, F.; et al. The evolution of light and vertical mixing across a phytoplankton ice-edge bloom. Elem. Sci. Anthr. 2019, 7, 20. [Google Scholar] [CrossRef]
- Oziel, L.; Massicotte, P.; Randelhoff, A.; Ferland, J.; Vladoiu, A.; Lacour, L.; Galindo, V.; Lambert-Girard, S.; Dumont, D.; Cuypers, Y.; et al. Environmental factors influencing the seasonal dynamics of spring algal blooms in and beneath sea ice in western Baffin Bay. Elem. Sci. Anthr. 2019, 7, 34. [Google Scholar] [CrossRef]
- Karl, D.M.; Christian, J.R.; Dore, J.E.; Hebel, D.V.; Letelier, R.M.; Tupas, L.M.; Winn, C.D. Seasonal and interannual variability in primary production and particle flux at station ALOHA. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 539–568. [Google Scholar] [CrossRef]
- Longhurst, A.R. Ecological Geography of the Sea, 2nd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2007; p. 542. [Google Scholar]
- Di Lorenzo, E.; Schneider, N.; Cobb, K.M.; Franks, P.J.; Chhak, K.; Miller, A.J.; McWilliams, J.C.; Bograd, S.J.; Arango, H.; Curchitser, E.; et al. North Pacific Gyre Oscillation links ocean climate and ecosystem change. Geophys. Res. Lett. 2008, 35, 2–7. [Google Scholar] [CrossRef]
- Martinez, E.; Antoine, D.; D’Ortenzio, F.; Gentili, B. Climate-driven basin-scale decadal oscillations of oceanic phytoplankton. Science 2009, 326, 1253–1256. [Google Scholar] [CrossRef]
- Racault, M.F.; Sathyendranath, S.; Brewin, R.J.; Raitsos, D.E.; Jackson, T.; Platt, T. Impact of El Niño variability on oceanic phytoplankton. Front. Mar. Sci. 2017, 4, 133. [Google Scholar] [CrossRef]
- Lan, K.W.; Evans, K.; Lee, M.A. Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Clim. Chang. 2013, 119, 63–77. [Google Scholar] [CrossRef]
- Taboada, F.G.; Barton, A.D.; Stock, C.A.; Dunne, J.; John, J.G. Seasonal to interannual predictability of oceanic net primary production inferred from satellite observations. Prog. Oceanogr. 2019, 170, 28–39. [Google Scholar] [CrossRef]
- Westberry, T.; Behrenfeld, M.J.; Siegel, D.A.; Boss, E. Carbon-based primary productivity modeling with vertically resolved photoacclimation. Glob. Biogeochem. Cycles 2008, 22, 1–18. [Google Scholar] [CrossRef]
- Field, C.; Barros, V.; Dokken, D.; Mach, K.; Mastrandrea, M.; Bilir, T.; Chatterjee, M.; Ebi, K.; Estrada, Y.; Genova, R.; et al. IPCC, 2014: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Rhein, M.; Rintoul, S.; Aoki, S.; Campos, E.; Chambers, D.; Feely, R.; Gulev, S.; Johnson, G.; Josey, S.; Kostianoy, A.; et al. Observations: Ocean. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T., Qin, D., Plattner, G.K., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 255–316. [Google Scholar]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Chavez, F.P.; Messié, M.; Pennington, J.T. Marine Primary Production in Relation to Climate Variability and Change. Annu. Rev. Mar. Sci. 2011, 3, 227–260. [Google Scholar] [CrossRef] [PubMed]
- Polovina, J.J.; Dunne, J.P.; Woodworth, P.A.; Howell, E.A. Projected expansion of the subtropical biome and contraction of the temperate and equatorial upwelling biomes in the North Pacific under global warming. ICES J. Mar. Sci. 2011, 68, 986–995. [Google Scholar] [CrossRef]
- Gregg, W.; Rousseaux, C.S. Global ocean primary production trends in the modern ocean color satellite record (1998–2015). Environ. Res. Lett. 2019, 14, 124011. [Google Scholar] [CrossRef]
- Saba, V.S.; Friedrichs, M.A.; Carr, M.E.; Antoine, D.; Armstrong, R.A.; Asanuma, I.; Aumont, O.; Bates, N.R.; Behrenfeld, M.J.; Bennington, V.; et al. Challenges of modeling depth-integrated marine primary productivity over multiple decades: A case study at BATS and HOT. Glob. Biogeochem. Cycles 2010, 24, 1–21. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Karl, D.; Boyd, P.; Cheung, W.; Lluch-Cota, S.; Nojiri, Y.; Schmidt, D.; Zavialov, P. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C., Barros, V., Dokken, D., Mach, K., Mastrandrea, M., Bilir, T., Chatterjee, M., Ebi, K., Estrada, Y., Genova, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- Platt, T.; Sathyendranath, S. Oceanic primary production: Estimation by remote sensing at local and regional scales. Science 1988, 241, 1613–1620. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S. Spatial structure of pelagic ecosystem processes in the global ocean. Ecosystems 1999, 2, 384–394. [Google Scholar] [CrossRef]
- Bouman, H.A.; Platt, T.; Doblin, M.; Figueiras, F.G.; Gudmundsson, K.; Gudfinnsson, H.G.; Huang, B.; Hickman, A.; Hiscock, M.; Jackson, T.; et al. Photosynthesis-irradiance parameters of marine phytoplankton: Synthesis of a global data set. Earth Syst. Sci. Data 2018, 10, 251–266. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. Remote sensing of water-column primary production. In Measurement of Primary Production from the Molecular to the Global Scale; Li, W.K.W., Maestrini, S.Y., Eds.; ICES Marine Science Symposia: Copenhagen, Denmark, 1993; Volume 197, pp. 236–243. [Google Scholar]
- Friedrichs, M.A.; Carr, M.E.; Barber, R.T.; Scardi, M.; Antoine, D.; Armstrong, R.A.; Asanuma, I.; Behrenfeld, M.J.; Buitenhuis, E.T.; Chai, F.; et al. Assessing the uncertainties of model estimates of primary productivity in the tropical Pacific Ocean. J. Mar. Syst. 2009, 76, 113–133. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 103–111. [Google Scholar]
- Côté, B.; Platt, T. Day-to-day variations in the spring-summer photosynthetic parameters of coastal marine phytoplankton. Limnol. Oceanogr. 1983, 28, 320–344. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S.; Ulloa, O.; Harrison, W.G.; Hoepffner, N.; Goes, J. Nutrient control of phytoplankton photosynthesis in the Western North Atlantic. Nature 1992, 356, 229–231. [Google Scholar] [CrossRef]
- Bouman, H.; Platt, T.; Sathyendranath, S.; Stuart, V. Dependence of light-saturated photosynthesis on temperature and community structure. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 1284–1299. [Google Scholar] [CrossRef]
- Huot, Y.; Babin, M.; Bruyant, F.; Grob, C.; Twardowski, M.S.; Claustre, H. Relationship between photosynthetic parameters and different proxies of phytoplankton biomass in the subtropical ocean. Biogeosciences 2007, 4, 853–868. [Google Scholar] [CrossRef]
- Uitz, J.; Huot, Y.; Bruyant, F.; Babin, M.; Claustre, H. Relating phytoplankton photophysiological properties to community structure on large scale. Limnol. Oceanogr. 2008, 53, 614–630. [Google Scholar]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24, GB3016. [Google Scholar] [CrossRef]
- Mélin, F. Potentiel de la Télédétection pour L’analyse des Proprietés Optiques du Système Océan-atmosphère et Application à L’estimation de la Photosynths̀e Phytoplanctonique. Ph.D. Thesis, Université Toulouse III, Toulouse, France, 1993. [Google Scholar]
- Mélin, F.; Hoepffner, N. Global Marine Primary Production: A Satellite View; Technical Report; Institute for Environment and Sustainability, ISPRA: Varese, Italy, 2004. [Google Scholar]
- Platt, T.; Sathyendranath, S. Estimators of primary production for interpretation of remotely sensed data on ocean color. J. Geophys. Res. 1993, 98, 14561–14576. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. Spectral effects in bio-optical control on the ocean system. Oceanologia 2007, 49, 5–39. [Google Scholar]
- Sathyendranath, S.; Stuart, V.; Nair, A.; Oka, K.; Nakane, T.; Bouman, H.; Forget, M.H.; Maass, H.; Platt, T. Carbon-to-chlorophyll ratio and growth rate of phytoplankton in the sea. Mar. Ecol. Prog. Ser. 2009, 383, 73–84. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T.; Žarko, K.; Dingle, J.; Jackson, T.; Brewin, R.J.W.; Franks, P.; Nón, E.M.; Kulk, G.; Bouman, H. Reconciling models of primary production and photoacclimation. Appl. Opt. 2020, submitted. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S. Biological production models as elements of coupled, atmosphere-ocean models for climate research. J. Geophys. Res. 1991, 96, 2585–2592. [Google Scholar] [CrossRef]
- Kyewalyanga, M.; Platt, T.; Sathyendranath, S. Ocean primary production calculated by spectral and broad-band models. Mar. Ecol. Prog. Ser. 1992, 85, 171–185. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Longhurst, A.; Caverhill, C.M.; Platt, T. Regionally and seasonally differentiated primary production in the North Atlantic. Deep-Sea Res. I 1995, 42, 1773–1802. [Google Scholar] [CrossRef]
- Lobanova, P.; Tilstone, G.H.; Bashmachnikov, I.; Brotas, V. Accuracy assessment of primary production models with and without photoinhibition using Ocean-Colour climate change initiative data in the North East Atlantic Ocean. Remote Sens. 2018, 10, 1116. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Jackson, T.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Clements, O.; Cipollini, P.; Danne, O.; Dingle, J.; et al. ESA Ocean Colour Climate Change Initiative (Ocean_Colour_cci): Version 4.0 Data; Technical Report; Centre for Environmental Data Analysis: Harwell, UK, 2019. [Google Scholar]
- Mélin, F.; Vantrepotte, V.; Chuprin, A.; Grant, M.; Jackson, T. Assessing the fitness-for-purpose of satellite multi-mission ocean color climate data records: A protocol applied to OC-CCI chlorophyll-a data. Remote Sens. Environ. 2017, 203, 139–151. [Google Scholar] [CrossRef]
- Bouman, H.A.; Platt, T.; Doblin, M.A.; Figueiras, F.G.; Gudmundsson, K.; Gudfinnsson, H.G.; Huang, B.; Hickman, A.; Hiscock, M.R.; Jackson, T.; et al. A global dataset of photosynthesis-irradiance parameters for marine phytoplankton. Pangaea 2017, 874087. [Google Scholar] [CrossRef]
- Thomas, W.H. On nitrogen deficiency in tropical Pacific oceanic phytoplankton: Photosynthetic parameters in poor and rich water. Limnol. Oceanogr. 1970, 15, 380–385. [Google Scholar] [CrossRef]
- Hameedi, M.J. Changes in specific photosynthetic rate of oceanic phytoplankton from the northeast Pacific Ocean. Helgoländer Wissenschaftliche Meeresuntersuchungen 1977, 30, 62–75. [Google Scholar] [CrossRef]
- Cole, B.; Cloern, J. Significance of biomass and light availability to phytoplankton productivity in San Francisco Bay. Mar. Ecol. Prog. Ser. 1984, 17, 15–24. [Google Scholar] [CrossRef]
- Harding, L.; Meeson, B.; Fisher, T. Photosynthesis patterns in Chesapeake Bay phytoplankton: Short- and long-term responses of P-l curve parameters to light. Mar. Ecol. Prog. Ser. 1985, 26, 99–111. [Google Scholar] [CrossRef]
- Harding, L.W.; Meeson, B.W.; Fisher, T.R. Phytoplankton production in two east coast estuaries: Photosynthesis-light functions and patterns of carbon assimilation in Chesapeake and Delaware Bays. Estuar. Coast. Shelf Sci. 1986, 23, 773–806. [Google Scholar] [CrossRef]
- Forbes, J.; Denman, K.; Mackas, D. Determination of photosynthetic capacity in coastal marine phytoplankton: Effects of assay irradiance and variability of photosynthetic parameters. Mar. Ecol. Prog. Ser. 1986, 32, 181–191. [Google Scholar] [CrossRef]
- Welschmeyer, N.; Goericke, R.; Strom, S.; Peterson, W. Phytoplankton growth and herbivory in the subarctic Pacific: A chemotaxonomic analysis. Limnol. Oceanogr. 1991, 36, 1631–1649. [Google Scholar] [CrossRef]
- Gallegos, C.L. Phytoplankton photosynthesis, productivity, and species composition in a eutrophic eastuary: Comparison of bloom and non-bloom assemblages. Mar. Ecol. Prog. Ser. 1992, 81, 257–267. [Google Scholar] [CrossRef]
- Vant, W.N.; Budd, R.G. Phytoplankton photosynthesis and growth in contrasting regions of Manukau harbour, New Zealand. N. Z. J. Mar. Freshw. Res. 1993, 27, 295–307. [Google Scholar] [CrossRef]
- Welschmeyer, N.A.; Strom, S.; Goericke, R.; DiTullio, G.; Belvin, M.; Petersen, W. Primary production in the subarctic Pacific Ocean: Project SUPER. Prog. Oceanogr. 1993, 32, 101–135. [Google Scholar] [CrossRef]
- Lindley, S.T.; Bidigare, R.R.; Barber, R.T. Phytoplankton photosynthesis parameters along 140 ∘W in the equatorial Pacific. Deep-Sea Res. Part II 1995, 42, 441–463. [Google Scholar] [CrossRef]
- Barber, R.T.; Sanderson, M.P.; Lindley, S.T.; Chai, F.; Newton, J.; Trees, C.C.; Foley, D.G.; Chavez, F.P. Primary productivity and its regulation in the equatorial pacific during and following the 1991-1992 El Nino. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 933–969. [Google Scholar] [CrossRef]
- Vant, W.N.; Safi, K.A. Size-fractionated phytoplankton biomass and photosynthesis in Manukau Harbour, New Zealand. N. Z. J. Mar. Freshw. Res. 1996, 30, 115–125. [Google Scholar] [CrossRef]
- Gallegos, C.L.; Vant, W.N. An incubation procedure for estimating carbon-to-chlorophyll ratios and growth-irradiance relationships of estuarine phytoplankton. Mar. Ecol. Prog. Ser. 1996, 138, 275–291. [Google Scholar] [CrossRef]
- Hawes, I.; Gall, M.; Weatherhead, M. Photosynthetic parameters in water masses in the vicinity of the Chatham rise, south pacific ocean, during late summer. N. Z. J. Mar. Freshw. Res. 1997, 31, 25–38. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Vant, W.N. Seasonal changes in factors controlling phytoplankton growth in Beatrix Bay, New Zealand. N. Z. J. Mar. Freshw. Res. 1997, 31, 237–248. [Google Scholar] [CrossRef]
- Gall, M.; Hawes, I.; Boyd, P. Predicting rates of primary production in the vicinity of the Subtropical Convergence east of New Zealand. N. Z. J. Mar. Freshw. Res. 1999, 33, 443–455. [Google Scholar] [CrossRef]
- Macedo, M.F. Annual Variation of Environmental Variables, Phytoplankton Species Composition and Photosynthetic Parameters in a Coastal Lagoon. J. Plankton Res. 2001, 23, 719–732. [Google Scholar] [CrossRef]
- Johnson, Z.; Bidigare, R.R.; Goericke, R.; Marra, J.; Trees, C.; Barber, R.T. Photosynthetic physiology and physicochemical forcing in the Arabian Sea, 1995. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 415–436. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; Gaxiola-Castro, G.; Nájera-Martínez, S.; Baumgartner, T.; Kahru, M.; Greg Mitchell, B. Phytoplankton absorption, photosynthetic parameters, and primary production off Baja California: Summer and autumn 1998. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 799–816. [Google Scholar] [CrossRef]
- Vernet, M. Production vs Irradiance data from RVIB Nathaniel B. Palmer cruise NBP0103 in the Southern Ocean in 2001 (SOGLOBEC project). Biol. Chem. Oceanogr. Data Manag. Off. 2004. [Google Scholar] [CrossRef]
- Henríquez, L.A.; Daneri, G.; Muñoz, C.A.; Montero, P.; Veas, R.; Palma, A.T. Primary production and phytoplanktonic biomass in shallow marine environments of central Chile: Effect of coastal geomorphology. Estuar. Coast. Shelf Sci. 2007, 73, 137–147. [Google Scholar] [CrossRef]
- Strom, S.L.; Macri, E.L.; Fredrickson, K.A. Light limitation of summer primary production in the coastal Gulf of Alaska: Physiological and environmental causes. Mar. Ecol. Prog. Ser. 2010, 402, 45–57. [Google Scholar] [CrossRef]
- Huot, Y. MALINA: Photosynthetic parameters. Lefe Cyber Database 2011, 30091. Available online: http://www.obs-vlfr.fr/proof/php/malina/x_datalist_1.php?xxop=malina&xxcamp=malina (accessed on 5 July 2019).
- Menden-Deuer, S. Structure-Dependent phytoplankton photosynthesis and production rates: Implications for the formation, maintenance, and decline of plankton patches. Mar. Ecol. Prog. Ser. 2012, 468, 15–30. [Google Scholar] [CrossRef]
- Vernet, M.; Wendy, A.K.; Lynn, R.Y.; Alexander, T.L.; Robin, M.R.; Langdon, B.Q.; Christian, H.F. Primary production throughout austral fall, during a time of decreasing daylength in the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2012, 452, 45–61. [Google Scholar] [CrossRef][Green Version]
- Huot, Y.; Babin, M.; Bruyant, F. Photosynthetic parameters in the Beaufort Sea in relation to the phytoplankton community structure. Biogeosciences 2013, 10, 3445–3454. [Google Scholar] [CrossRef]
- Fuentes-Lema, A.; Sobrino, C.; González, N.; Estrada, M.; Neale, P. Effect of solar UVR on the production of particulate and dissolved organic carbon from phytoplankton assemblages in the Indian Ocean. Mar. Ecol. Prog. Ser. 2015, 535, 47–61. [Google Scholar] [CrossRef]
- Kovač, Z.; Platt, T.; Sathyendranath, S.; Morović, M.; Jackson, T. Recovery of photosynthesis parameters from in situ profiles of phytoplankton production. ICES J. Mar. Sci. 2016, 73, 275–285. [Google Scholar] [CrossRef]
- Richardson, K.; Bendtsen, J.; Kragh, T.; Mousing, E.A. Constraining the distribution of photosynthetic parameters in the global ocean. Front. Mar. Sci. 2016, 3, 269. [Google Scholar] [CrossRef]
- Strom, S.L.; Fredrickson, K.A.; Bright, K.J. Spring phytoplankton in the eastern coastal Gulf of Alaska: Photosynthesis and production during high and low bloom years. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2016, 132, 107–121. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lohrenz, S.E.; Gundersen, K. Photophysiological and light absorption properties of phytoplankton communities in the river-dominated margin of the northern Gulf of Mexico. J. Geophys. Res. Ocean. 2017, 122, 4922–4938. [Google Scholar] [CrossRef]
- Endo, H.; Hattori, H.; Mishima, T.; Hashida, G.; Sasaki, H.; Nishioka, J.; Suzuki, K. Phytoplankton community responses to iron and CO2 enrichment in different biogeochemical regions of the Southern Ocean. Polar Biol. 2017, 40, 2143–2159. [Google Scholar] [CrossRef]
- Fragoso, G.M.; Poulton, A.J.; Yashayaev, I.M.; Head, E.I.J.; Purdie, D.A. Spring phytoplankton communities of the Labrador Sea (2005–2014): Pigment signatures, photophysiology and elemental ratios. Biogeosciences 2017, 14, 1235–1259. Available online: https://doi.pangaea.de/10.1594/PANGAEA.871872 (accessed on 15 March 2019).
- Fragoso, G.M.; Poulton, A.J.; Yashayaev, I.M.; Head, E.J.; Purdie, D.A. Spring phytoplankton communities of the Labrador Sea (2005–2014): Pigment signatures, photophysiology and elemental ratios. Pangaea 2017, 871872. [Google Scholar] [CrossRef]
- Endo, H.; Hattori, H.; Mishima, T.; Hashida, G.; Sasaki, H.; Nishioka, J.; Suzuki, K. Seawater carbonate chemistry and biomarker pigments and phytoplankton community composition in different biogeochemical regions of the Southern Ocean. Pangaea 2018, 888447. [Google Scholar] [CrossRef]
- Briggs, N.; Guemundsson, K.; Cetinić, I.; D’Asaro, E.; Rehm, E.; Lee, C.; Perry, M.J. A multi-method autonomous assessment of primary productivity and export efficiency in the springtime North Atlantic. Biogeosciences 2018, 15, 4515–4532. [Google Scholar] [CrossRef]
- Perry, M.J. Primary Productivity Measurements from On-Deck Bottle Incubations during R/V Knorr Cruise KN193-03 and R/V Bjarni Saemundsson Cruises B10-2008 and B4-2008 to the Subpolar North Atlantic, Iceland Basin in 2008. Biological and Chemical Oceanography Data Management Office, 2018. Available online: https://www.bco-dmo.org/dataset/746215 (accessed on 1 April 2019).
- Platt, T.; Jassby, A.D. The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. J. Phycol. 1976, 12, 421–430. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Devred, E.; Sathyendranath, S.; Lavender, S.J.; Hardman-Mountford, N.J. Model of phytoplankton absorption based on three size classes. Appl. Opt. 2011, 50, 4535–4549. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Santer, B.D.; Thorne, P.W.; Haimberger, L.; Taylor, K.E.; Wigley, T.M.L.; Lazante, J.R.; Solomon, S.; Free, M.; Gleckler, P.J.; Jones, P.D.; et al. Consistency of modelled and observed temperature trends in the tropical troposhpere. Int. J. Climatol. 2008, 28, 1703–1722. [Google Scholar] [CrossRef]
- Kao, H.Y.; Yu, J.Y. Contrasting Eastern-Pacific and Central-Pacific types of ENSO. J. Clim. 2009, 22, 615–632. [Google Scholar] [CrossRef]
- Lewis, M.; Warnock, R.; Platt, T. Absorption and photosynthesis action spectra for natural phytoplankton populations: Implications for prodution in the open ocean. Limnol. Oceanogr. 1985, 30, 794–806. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S.; Caverhill, C.M.; Lewis, M.R. Ocean primary production and available light: Further algorithms for remote sensing. Deep Sea Res. Part A Oceanogr. Res. Pap. 1988, 35, 855–879. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. Computation of aquatic primary production: Extended formalism to include effect of angular and spectral distribution of light. Limnol. Oceanogr. 1989, 34, 188–198. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S.; Ravindran, P. Primary prodution by phytoplankton: Analytic solutions for daily rates per unit area of water surface. Proc. R. Soc. B Biol. Sci. 1990, 241, 101–111. [Google Scholar]
- Sathyendranath, S.; Platt, T.; Brewin, R.J.W.; Jackson, T. Primary Production Distribution. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, J.H., Yager, L.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 635–640. [Google Scholar] [CrossRef]
- Marañón, E.; Holligan, P.M. Photosynthetic parameters of phytoplankton from 50∘N to 50∘S in the Atlantic Ocean. Mar. Ecol. Prog. Ser. 1999, 176, 191–203. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S.; Forget, M.H.; White, G.N.; Caverhill, C.; Bouman, H.; Devred, E.; Son, S. Operational mode estimation of primary production at large geographical scales. Remote Sens. Environ. 2008, 112, 3437–3448. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, B.; Lin, L.; Laws, E.A.; Wang, L.; Shang, S.; Zhang, T.; Dai, M. Photosynthetic parameters in the northern South China Sea in relation to phytoplankton community structure. J. Geophys. Res. Ocean. 2015, 120, 4187–4204. [Google Scholar] [CrossRef]
- Robinson, A.; Bouman, H.A.; Tilstone, G.H.; Sathyendranath, S. Size class dependent relationships between temperature and phytoplankton photosynthesis-irradiance parameters in the Atlantic Ocean. Front. Mar. Sci. 2018, 4, 435. [Google Scholar] [CrossRef]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Saux Picart, S.; Sathyendranath, S.; Dowell, M.; Moore, T.; Platt, T. Remote sensing of assimilation number for marine phytoplankton. Remote Sens. Environ. 2014, 146, 87–96. [Google Scholar] [CrossRef]
- Rey, F. Photosynthesis-irradiance relationships in natural phytoplankton populations of the Barents Sea. In Proceedings of the Pro Mare Symposium on Polr Marine Ecology, Trondheim, Norway, 12–16 May 1990; pp. 105–116. [Google Scholar]
- Henson, S.A.; Sarmiento, J.L.; Dunne, J.P.; Bopp, L.; Lima, I.; Doney, S.C.; John, J.; Beaulieu, C. Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences 2010, 7, 621–640. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. The spectral irradiance field at the surface and in the interior of the ocean: A model for applications in oceanography and remote sensing. J. Geophys. Res. 1988, 93, 9270–9280. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Cota, G.; Stuart, V.; Maass, H.; Platt, T. Remote sensing of phytoplankton pigments: A comparison of empirical and theoretical approaches. Int. J. Remote Sens. 2001, 22, 249–273. [Google Scholar] [CrossRef]
- Morel, A. Optical properties of pure seawater. In Optical Aspects of Oceanography; Jerlov, N.G., Nielsen, E.S., Eds.; Academic: New York, NY, USA, 1974; pp. 1–24. [Google Scholar]
- Ulloa, O.; Sathyendranath, S.; Platt, T. Effect of the particle-size distribution on the backscattering ratio in seawater. Appl. Opt. 1994, 33, 7070. [Google Scholar] [CrossRef]
- Loisel, H.; Morel, A. Light scattering and chlorophyll concentration in case 1 waters: A reexamination. Limnol. Oceanogr. 1998, 43, 847–858. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, P.; Caverhill, C.; Warnock, R.; Lewis, M. Remote sensing of oceanic primary production: Computations using a spectral model. Deep-Sea Res. I 1989, 36, 431–453. [Google Scholar] [CrossRef]

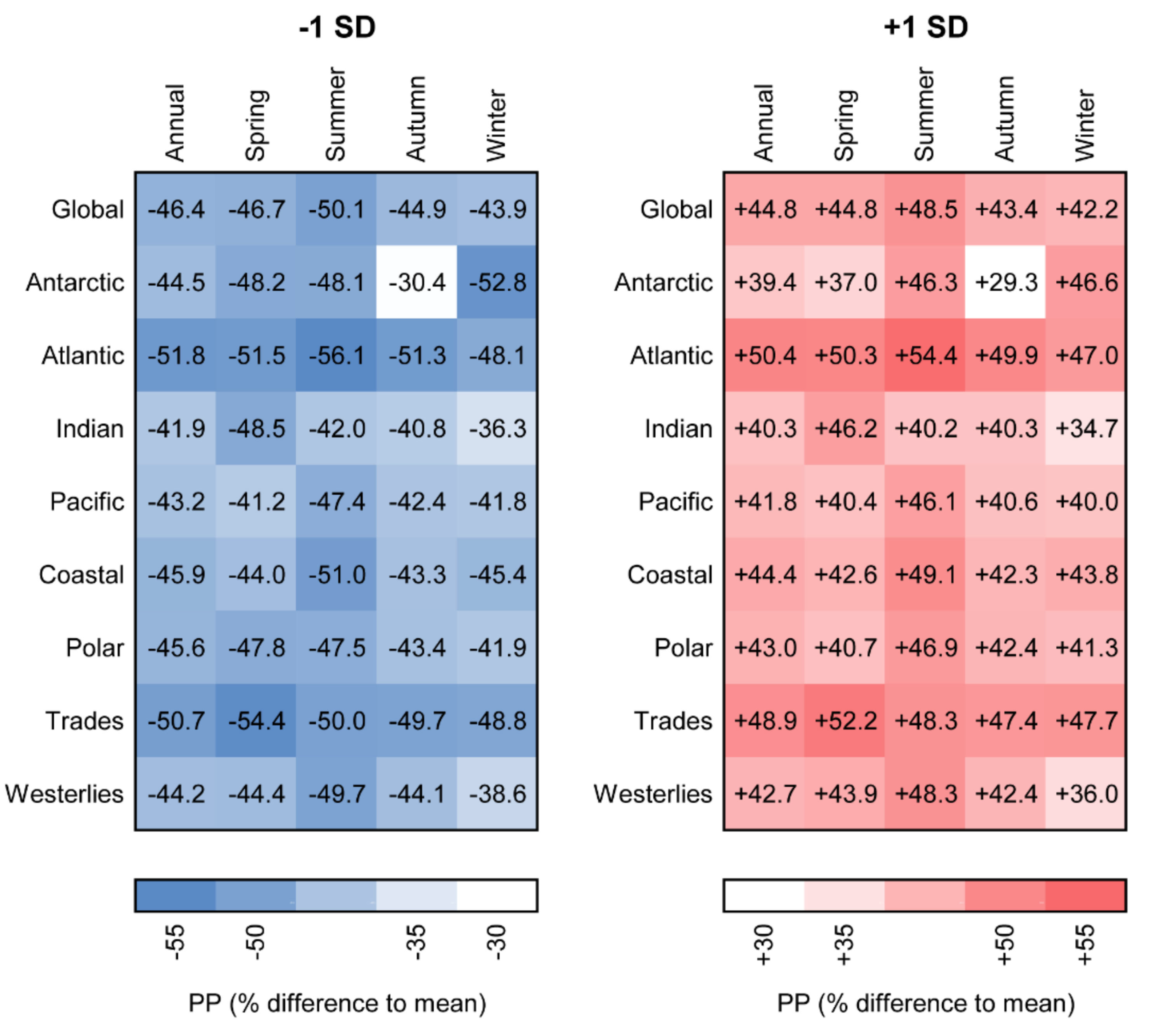
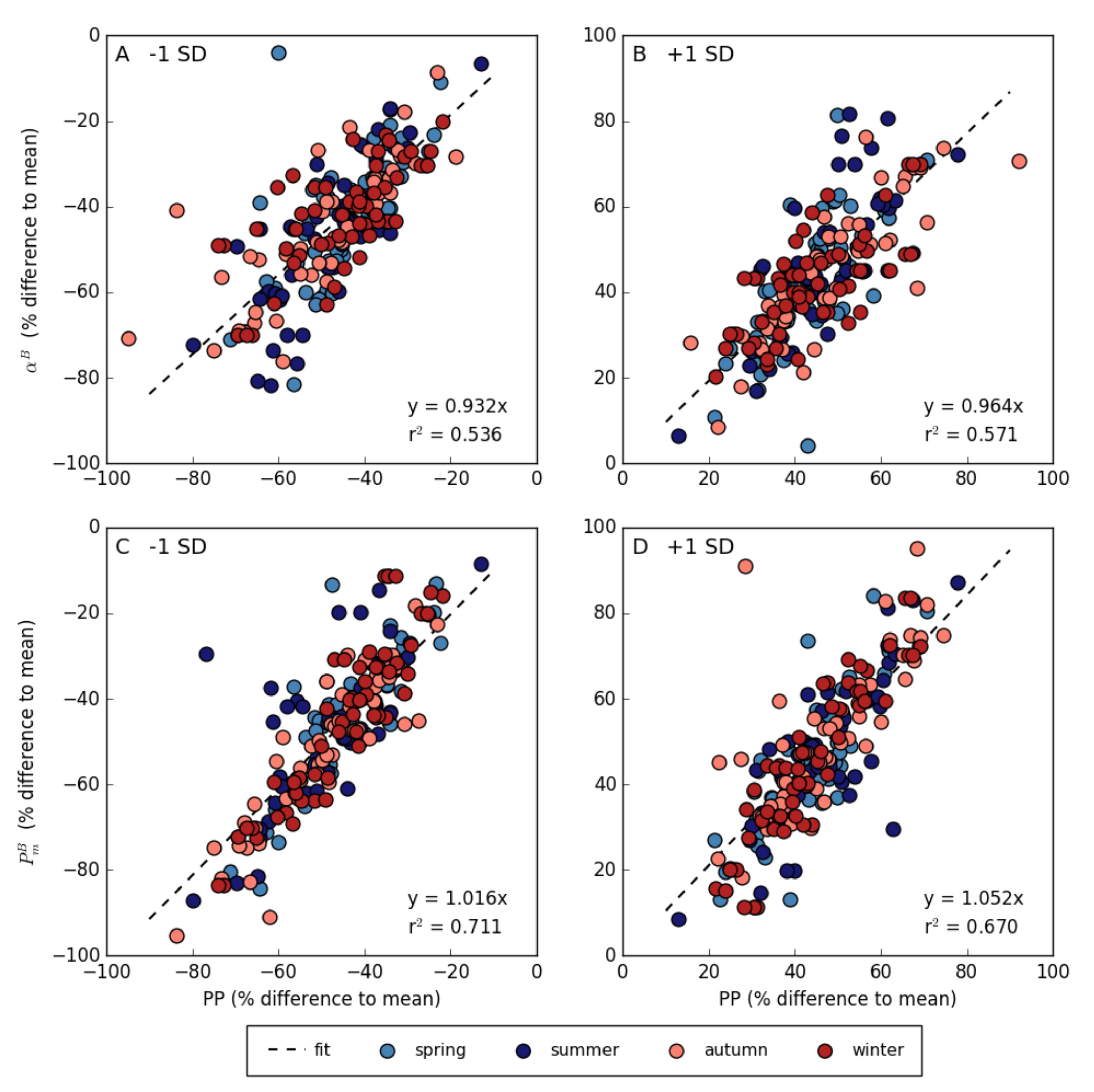
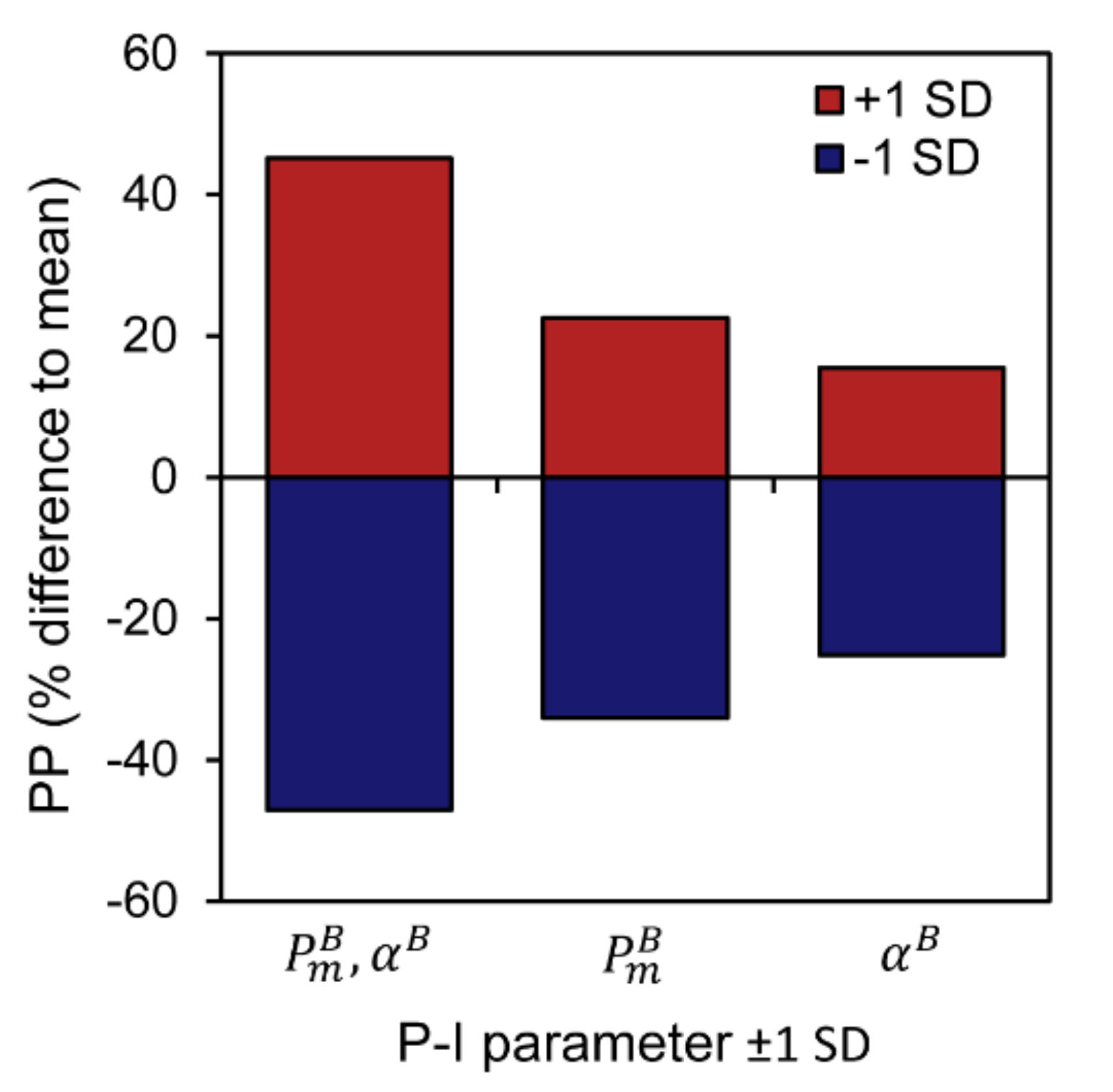
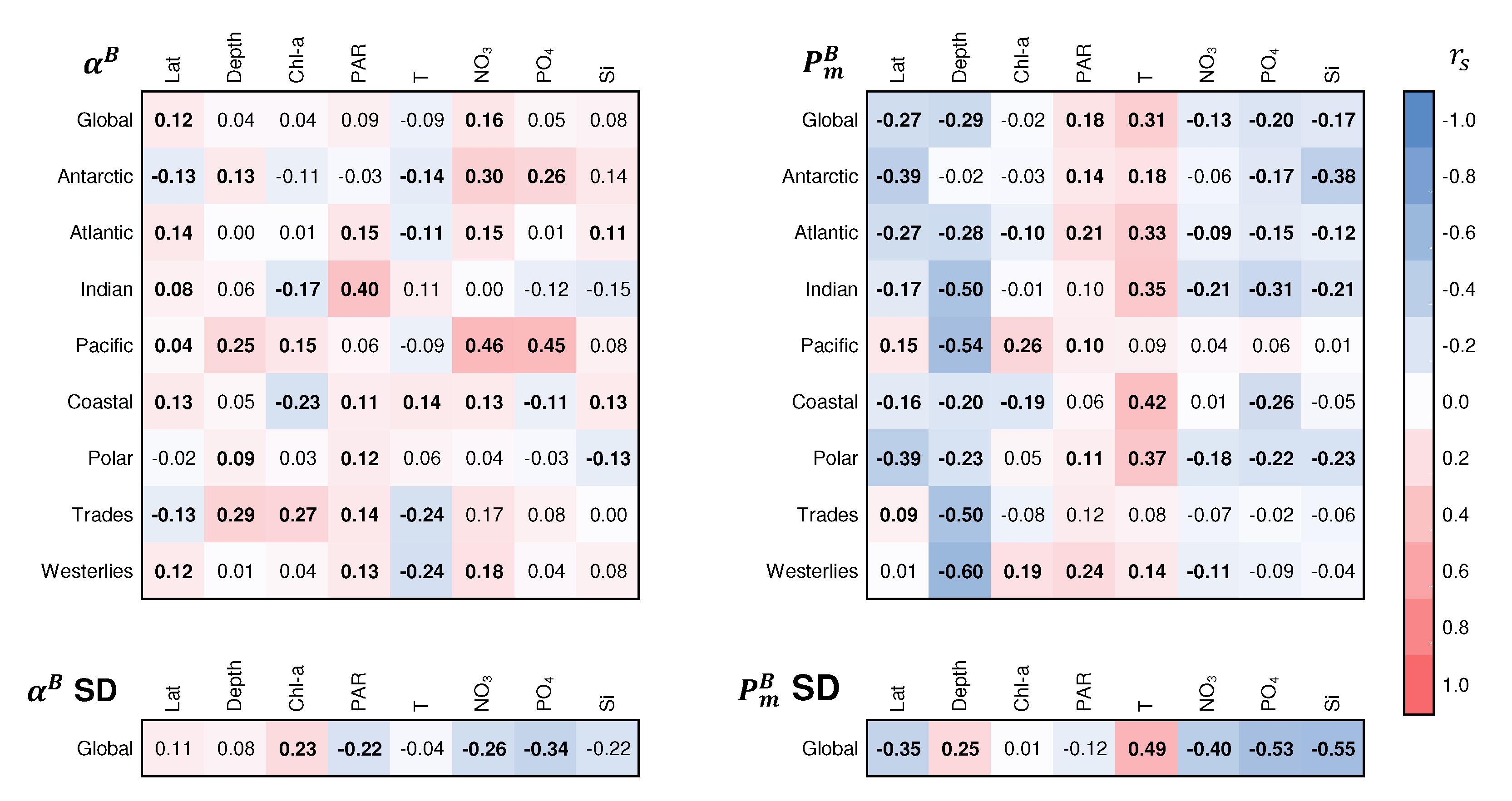
| Spring | Summer | Autumn | Winter | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BIOME | |||||||||||||||||||||||||
| /BASIN | PROV | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD |
| Coastal | |||||||||||||||||||||||||
| Atlantic | NECS | 18 | 0.020 | 0.005 | 18 | 3.25 | 0.64 | 12 | 0.022 | 0.007 | 15 | 2.83 | 1.62 | 30 | 0.021 | 0.006 | 1 | 2.22 | 30 | 0.021 | 0.006 | 34 | 3.04 | 1.18 | |
| CNRY | 1 | 0.035 | 1 | 4.00 | 33 | 0.022 | 0.006 | 34 | 3.92 | 1.56 | 11 | 0.026 | 0.005 | 34 | 4.08 | 1.70 | 1 | 0.016 | 3 | 3.30 | 1.13 | ||||
| GUIN | 1 | 0.012 | 1 | 1.50 | 48 | 0.017 | 0.008 | 50 | 1.68 | 1.04 | 20 | 0.017 | 0.006 | 19 | 1.68 | 0.62 | 48 | 0.017 | 0.008 | 50 | 1.68 | 1.04 | |||
| GUIA | 77 | 0.024 | 0.017 | 34 | 2.52 | 1.88 | 109 | 0.023 | 0.016 | 56 | 2.71 | 1.90 | 2 | 0.024 | 0.008 | 2 | 2.71 | 1.35 | 109 | 0.023 | 0.016 | 6 | 2.90 | 1.21 | |
| NWCS | 495 | 0.031 | 0.020 | 515 | 2.49 | 1.18 | 259 | 0.024 | 0.017 | 260 | 3.23 | 1.47 | 335 | 0.044 | 0.021 | 332 | 3.58 | 1.66 | 121 | 0.037 | 0.018 | 125 | 2.85 | 1.36 | |
| CHSB | 41 | 0.036 | 0.018 | 41 | 3.29 | 1.46 | 35 | 0.016 | 0.013 | 36 | 2.04 | 1.66 | 18 | 0.041 | 0.018 | 19 | 4.80 | 1.43 | 59 | 0.038 | 0.018 | 96 | 3.12 | 1.82 | |
| BRAZ | 9 | 0.029 | 0.016 | 10 | 2.22 | 1.82 | 48 | 0.017 | 0.008 | 15 | 2.69 | 1.95 | 5 | 0.014 | 0.005 | 5 | 3.63 | 2.05 | 48 | 0.017 | 0.008 | 15 | 2.69 | 1.95 | |
| FKLD | 28 | 0.017 | 0.009 | 31 | 1.68 | 1.24 | 48 | 0.017 | 0.008 | 50 | 1.68 | 1.04 | 20 | 0.017 | 0.006 | 19 | 1.68 | 0.62 | 48 | 0.017 | 0.008 | 50 | 1.68 | 1.04 | |
| BENG | 2 | 0.044 | 0.000 | 4 | 4.24 | 2.52 | 25 | 0.027 | 0.012 | 26 | 3.66 | 1.67 | 23 | 0.026 | 0.011 | 22 | 3.56 | 1.52 | 25 | 0.027 | 0.012 | 26 | 3.66 | 1.67 | |
| Indian | EAFR | 117 | 0.027 | 0.009 | 101 | 4.76 | 1.66 | 32 | 0.030 | 0.009 | 17 | 5.36 | 1.07 | 285 | 0.027 | 0.011 | 6 | 3.43 | 1.27 | 280 | 0.027 | 0.010 | 203 | 4.38 | 1.62 |
| REDS | 46 | 0.013 | 0.007 | 85 | 3.29 | 1.36 | 4 | 0.022 | 0.006 | 4 | 4.13 | 2.07 | 117 | 0.027 | 0.009 | 101 | 4.76 | 1.66 | 32 | 0.030 | 0.009 | 17 | 5.36 | 1.07 | |
| ARAB | 13 | 0.034 | 0.010 | 98 | 4.00 | 1.48 | 114 | 0.024 | 0.011 | 81 | 3.72 | 1.40 | 117 | 0.027 | 0.009 | 101 | 4.76 | 1.66 | 32 | 0.033 | 0.009 | 17 | 5.36 | 1.07 | |
| INDE | 65 | 0.042 | 0.021 | 62 | 3.39 | 2.13 | 5 | 0.034 | 0.028 | 7 | 4.10 | 1.53 | 56 | 0.034 | 0.018 | 56 | 2.97 | 1.89 | 6 | 0.039 | 0.015 | 6 | 2.50 | 1.01 | |
| INDW | 9 | 0.033 | 0.027 | 6 | 3.43 | 1.27 | 114 | 0.024 | 0.011 | 64 | 3.94 | 1.70 | 117 | 0.027 | 0.009 | 35 | 3.65 | 1.48 | 32 | 0.030 | 0.009 | 29 | 4.29 | 1.91 | |
| AUSW | 56 | 0.034 | 0.018 | 56 | 2.97 | 1.89 | 6 | 0.039 | 0.015 | 6 | 2.50 | 1.01 | 56 | 0.047 | 0.019 | 56 | 3.38 | 2.21 | 19 | 0.028 | 0.017 | 3 | 4.05 | 0.80 | |
| Pacific | ALSK | 7 | 0.029 | 0.012 | 8 | 3.91 | 0.89 | 61 | 0.026 | 0.020 | 35 | 4.67 | 1.90 | 23 | 0.023 | 0.011 | 43 | 4.53 | 1.77 | 51 | 0.025 | 0.010 | 43 | 4.53 | 1.77 |
| CCAL | 42 | 0.038 | 0.023 | 53 | 3.80 | 1.76 | 9 | 0.012 | 0.007 | 26 | 4.17 | 1.91 | 11 | 0.008 | 0.001 | 28 | 3.21 | 1.47 | 2 | 0.016 | 0.006 | 20 | 1.70 | 1.08 | |
| CAMR | 1 | 0.026 | 2 | 1.50 | 0.20 | 31 | 0.020 | 0.009 | 26 | 4.17 | 1.91 | 18 | 0.026 | 0.014 | 28 | 3.21 | 1.47 | 2 | 0.016 | 0.006 | 28 | 3.48 | 2.22 | ||
| CHIL | 8 | 0.032 | 0.011 | 7 | 2.50 | 0.93 | 79 | 0.031 | 0.012 | 78 | 2.35 | 1.36 | 33 | 0.029 | 0.010 | 89 | 2.35 | 1.35 | 4 | 0.016 | 0.016 | 4 | 2.10 | 2.12 | |
| CHIN | 25 | 0.026 | 0.007 | 17 | 5.48 | 1.72 | 31 | 0.020 | 0.009 | 22 | 3.59 | 1.99 | 18 | 0.026 | 0.014 | 10 | 4.48 | 3.23 | 2 | 0.016 | 0.006 | 2 | 4.55 | 1.34 | |
| SUND | 26 | 0.025 | 0.010 | 20 | 4.10 | 2.30 | 12 | 0.041 | 0.018 | 16 | 3.43 | 2.13 | 67 | 0.031 | 0.012 | 68 | 2.85 | 1.51 | 11 | 0.029 | 0.014 | 12 | 2.55 | 2.13 | |
| AUSE | 19 | 0.050 | 0.020 | 320 | 4.41 | 1.53 | 4 | 0.050 | 0.018 | 4 | 4.65 | 3.15 | 31 | 0.047 | 0.019 | 15 | 2.53 | 2.13 | 8 | 0.042 | 0.018 | 11 | 1.75 | 0.94 | |
| NEWZ | 8 | 0.045 | 0.013 | 9 | 4.48 | 1.46 | 23 | 0.021 | 0.011 | 22 | 4.63 | 1.51 | 9 | 0.036 | 0.009 | 8 | 3.70 | 1.42 | 2 | 0.044 | 0.003 | 2 | 3.30 | 0.28 | |
| Polar | |||||||||||||||||||||||||
| Antarctic | ANTA | 1 | 0.007 | 2 | 1.01 | 0.92 | 41 | 0.018 | 0.009 | 75 | 1.27 | 0.50 | 12 | 0.021 | 0.005 | 9 | 1.58 | 0.72 | 54 | 0.019 | 0.008 | 11 | 1.48 | 0.74 | |
| APLR | 52 | 0.035 | 0.009 | 67 | 1.66 | 0.92 | 268 | 0.029 | 0.014 | 340 | 1.89 | 1.26 | 140 | 0.026 | 0.009 | 162 | 1.86 | 0.90 | 54 | 0.019 | 0.008 | 569 | 1.86 | 1.13 | |
| Atlantic | BPLR | 141 | 0.031 | 0.016 | 154 | 2.03 | 0.83 | 542 | 0.030 | 0.017 | 572 | 1.67 | 0.99 | 189 | 0.024 | 0.013 | 192 | 1.65 | 0.84 | 21 | 0.031 | 0.019 | 21 | 1.85 | 0.78 |
| ARCT | 278 | 0.039 | 0.017 | 329 | 2.38 | 1.07 | 298 | 0.036 | 0.016 | 313 | 2.24 | 0.99 | 39 | 0.032 | 0.018 | 59 | 2.18 | 0.78 | 27 | 0.028 | 0.013 | 27 | 2.38 | 0.85 | |
| SARC | 116 | 0.044 | 0.017 | 126 | 2.74 | 1.09 | 89 | 0.041 | 0.014 | 92 | 2.59 | 1.27 | 206 | 0.043 | 0.016 | 2 | 1.40 | 0.46 | 206 | 0.043 | 0.016 | 220 | 2.66 | 1.17 | |
| Pacific | BERS | 7 | 0.029 | 0.012 | 8 | 3.26 | 1.81 | 21 | 0.024 | 0.007 | 22 | 2.85 | 1.18 | 23 | 0.023 | 0.011 | 25 | 2.41 | 1.39 | 51 | 0.025 | 0.010 | 55 | 2.71 | 1.38 |
| Trades | |||||||||||||||||||||||||
| Atlantic | NATR | 15 | 0.025 | 0.015 | 14 | 3.60 | 1.76 | 165 | 0.035 | 0.021 | 165 | 2.85 | 1.84 | 27 | 0.027 | 0.011 | 26 | 2.52 | 2.40 | 6 | 0.032 | 0.018 | 7 | 5.23 | 1.60 |
| WTRA | 16 | 0.013 | 0.007 | 16 | 3.06 | 2.18 | 109 | 0.023 | 0.016 | 6 | 2.90 | 1.21 | 32 | 0.025 | 0.019 | 34 | 2.52 | 21.88 | 109 | 0.023 | 0.016 | 56 | 2.71 | 1.90 | |
| ETRA | 4 | 0.037 | 0.026 | 4 | 2.90 | 3.23 | 62 | 0.026 | 0.016 | 61 | 3.01 | 1.79 | 6 | 0.024 | 0.014 | 6 | 2.97 | 1.06 | 52 | 0.025 | 0.016 | 51 | 3.02 | 1.76 | |
| SATL | 77 | 0.024 | 0.017 | 77 | 1.99 | 1.37 | 109 | 0.023 | 0.016 | 109 | 1.87 | 1.35 | 32 | 0.020 | 0.014 | 32 | 1.59 | 1.28 | 109 | 0.023 | 0.016 | 109 | 1.87 | 1.35 | |
| CARB | 22 | 0.010 | 0.005 | 21 | 3.20 | 1.99 | 16 | 0.023 | 0.010 | 2 | 6.25 | 0.92 | 25 | 0.028 | 0.012 | 16 | 5.73 | 1.76 | 28 | 0.022 | 0.009 | 28 | 3.48 | 2.22 | |
| Indian | MONS | 5 | 0.028 | 0.005 | 64 | 3.94 | 1.70 | 9 | 0.024 | 0.007 | 64 | 3.94 | 1.70 | 40 | 0.022 | 0.007 | 35 | 3.65 | 1.48 | 35 | 0.027 | 0.006 | 29 | 4.29 | 1.91 |
| ISSG | 10 | 0.007 | 0.003 | 10 | 1.94 | 1.61 | 14 | 0.007 | 0.003 | 6 | 2.50 | 1.01 | 4 | 0.009 | 0.002 | 26 | 2.86 | 1.31 | 14 | 0.007 | 0.003 | 3 | 4.05 | 0.80 | |
| Pacific | NPTG | 3 | 0.013 | 0.008 | 9 | 4.97 | 0.66 | 8 | 0.015 | 0.003 | 6 | 4.39 | 2.12 | 10 | 0.017 | 0.006 | 18 | 4.79 | 1.70 | 7 | 0.016 | 0.004 | 26 | 4.88 | 0.74 |
| PNEC | 2 | 0.031 | 0.011 | 9 | 3.46 | 1.49 | 12 | 0.017 | 0.006 | 15 | 3.94 | 1.85 | 11 | 0.017 | 0.004 | 27 | 3.54 | 1.74 | 27 | 0.018 | 0.005 | 3 | 1.75 | 0.77 | |
| PEQD | 11 | 0.017 | 0.004 | 18 | 4.85 | 1.58 | 27 | 0.018 | 0.005 | 6 | 3.79 | 1.04 | 27 | 0.018 | 0.005 | 8 | 4.76 | 0.97 | 16 | 0.019 | 0.005 | 17 | 4.39 | 1.33 | |
| WARM | 163 | 0.030 | 0.015 | 160 | 2.23 | 1.31 | 220 | 0.031 | 0.016 | 221 | 2.16 | 1.28 | 220 | 0.031 | 0.016 | 221 | 2.16 | 1.28 | 57 | 0.033 | 0.020 | 61 | 1.97 | 1.19 | |
| ARCH | 67 | 0.031 | 0.012 | 68 | 2.85 | 1.51 | 11 | 0.029 | 0.014 | 12 | 2.55 | 2.13 | 26 | 0.025 | 0.010 | 20 | 4.10 | 2.30 | 19 | 0.028 | 0.017 | 22 | 2.81 | 1.92 | |
| Westerlies | |||||||||||||||||||||||||
| Antarctic | SSTC | 18 | 0.021 | 0.016 | 47 | 4.12 | 2.01 | 45 | 0.034 | 0.011 | 83 | 2.25 | 1.56 | 33 | 0.029 | 0.010 | 53 | 4.42 | 1.39 | 4 | 0.016 | 0.016 | 5 | 5.30 | 1.57 |
| SANT | 3 | 0.026 | 0.002 | 28 | 1.85 | 0.42 | 41 | 0.023 | 0.006 | 136 | 1.58 | 0.53 | 10 | 0.027 | 0.003 | 18 | 1.99 | 0.54 | 55 | 0.023 | 0.006 | 242 | 1.62 | 0.54 | |
| Atlantic | NADR | 47 | 0.032 | 0.014 | 42 | 3.32 | 1.25 | 4 | 0.051 | 0.023 | 4 | 3.03 | 0.73 | 49 | 0.025 | 0.014 | 52 | 2.14 | 1.20 | 7 | 0.036 | 0.007 | 7 | 2.83 | 0.45 |
| GFST | 50 | 0.034 | 0.012 | 47 | 4.39 | 1.60 | 14 | 0.013 | 0.006 | 13 | 2.40 | 1.99 | 24 | 0.040 | 0.016 | 28 | 3.08 | 1.67 | 7 | 0.054 | 0.020 | 7 | 4.32 | 2.06 | |
| NASW | 137 | 0.031 | 0.018 | 96 | 3.84 | 2.53 | 113 | 0.029 | 0.021 | 92 | 2.13 | 1.85 | 65 | 0.036 | 0.024 | 57 | 4.09 | 2.23 | 33 | 0.041 | 0.019 | 30 | 4.66 | 1.35 | |
| MEDI | 46 | 0.013 | 0.007 | 85 | 3.29 | 1.36 | 26 | 0.005 | 0.003 | 36 | 2.65 | 1.86 | 77 | 0.032 | 0.021 | 113 | 2.57 | 1.66 | 55 | 0.040 | 0.019 | 105 | 2.64 | 1.35 | |
| NASE | 25 | 0.029 | 0.018 | 27 | 3.95 | 1.78 | 17 | 0.030 | 0.009 | 17 | 2.72 | 1.67 | 44 | 0.025 | 0.014 | 60 | 2.86 | 2.01 | 86 | 0.031 | 0.018 | 7 | 5.23 | 1.60 | |
| Pacific | PSAE | 18 | 0.035 | 0.014 | 18 | 2.48 | 0.74 | 42 | 0.032 | 0.013 | 46 | 2.54 | 0.81 | 8 | 0.025 | 0.012 | 9 | 2.35 | 1.06 | 68 | 0.032 | 0.013 | 73 | 2.50 | 0.82 |
| PSAW | 33 | 0.039 | 0.011 | 31 | 3.32 | 0.93 | 8 | 0.036 | 0.018 | 8 | 3.51 | 1.55 | 41 | 0.038 | 0.013 | 39 | 3.36 | 1.06 | 41 | 0.038 | 0.013 | 39 | 3.36 | 1.06 | |
| KURO | 83 | 0.020 | 0.009 | 81 | 3.62 | 1.64 | 61 | 0.017 | 0.007 | 60 | 2.88 | 1.41 | 82 | 0.020 | 0.007 | 86 | 3.62 | 1.70 | 10 | 0.021 | 0.009 | 9 | 4.63 | 0.52 | |
| NPPF | 134 | 0.027 | 0.013 | 144 | 3.28 | 1.46 | 111 | 0.024 | 0.013 | 114 | 2.79 | 1.23 | 96 | 0.020 | 0.008 | 100 | 3.48 | 1.65 | 10 | 0.021 | 0.009 | 9 | 4.63 | 0.52 | |
| NPSE | 134 | 0.027 | 0.013 | 144 | 3.28 | 1.46 | 111 | 0.024 | 0.013 | 114 | 2.79 | 1.23 | 96 | 0.020 | 0.008 | 100 | 3.48 | 1.65 | 10 | 0.021 | 0.009 | 9 | 4.63 | 0.52 | |
| NPSW | 83 | 0.020 | 0.009 | 81 | 3.62 | 1.64 | 61 | 0.017 | 0.007 | 60 | 2.88 | 1.41 | 6 | 0.015 | 0.005 | 5 | 3.22 | 0.59 | 10 | 0.021 | 0.009 | 9 | 4.63 | 0.52 | |
| OCAL | 10 | 0.007 | 0.002 | 10 | 1.87 | 0.59 | 12 | 0.017 | 0.006 | 46 | 2.54 | 0.81 | 1 | 0.006 | 9 | 2.35 | 1.06 | 10 | 0.021 | 0.009 | 73 | 2.50 | 0.82 | ||
| TASM | 8 | 0.053 | 0.017 | 12 | 5.10 | 1.51 | 19 | 0.053 | 0.013 | 25 | 4.68 | 2.04 | 3 | 0.029 | 0.001 | 19 | 5.99 | 1.54 | 12 | 0.053 | 0.012 | 13 | 4.52 | 1.22 | |
| SPSG | 240 | 0.021 | 0.015 | 246 | 1.79 | 1.33 | 27 | 0.022 | 0.011 | 29 | 1.35 | 0.79 | 3 | 0.029 | 0.001 | 281 | 1.77 | 1.30 | 4 | 0.025 | 0.004 | 6 | 3.16 | 1.37 | |
| Mean P-I | ||||||
| Coastal | Polar | Trades | Westerlies | Total | ||
| 47 × 106 | 57 × 106 | 141 × 106 | 131 × 106 | 376 × 106 | ||
| Antarctic | 79 × 106 | 1.06 ± 0.09 (0.88−1.21) | 4.83 ± 0.14 (4.66–5.14) | 5.88 ± 0.19 (5.58–6.20) | ||
| Atlantic | 94 × 106 | 3.13 ± 0.16 (2.89–3.33) | 1.54 ± 0.09 (1.39–1.76) | 6.52 ± 0.14 (6.24–6.73) | 3.12 ± 0.05 (3.04–3.24) | 14.3 ± 0.38 (13.7–14.9) |
| Indian | 48 × 106 | 3.82 ± 0.18 (3.55–4.10) | 4.42 ± 0.12 (4.17–4.62) | 8.24 ± 0.30 (7.72–8.70) | ||
| Pacific | 155 × 106 | 4.76 ± 0.22 (4.34–5.03) | 0.91 ± 0.06 (0.80–1.02) | 9.21 ± 0.31 (8.57–9.62) | 7.39 ± 0.16 (7.09–7.60) | 22.3 ± 0.63 (21.2–23.1) |
| Total | 376 × 106 | 11.7 ± 0.53 (10.9–12.4) | 3.51 ± 0.20 (3.12–3.85) | 20.2 ± 0.50 (19.1–20.7) | 15.3 ± 0.30 (15.0–15.9) | 50.7 ± 1.38 (48.7–52.5) |
| Mean P-I –1 Standard Deviation | ||||||
| Coastal | Polar | Trades | Westerlies | Total | ||
| 47 × 106 | 57 × 106 | 141 × 106 | 131 × 106 | 376 × 106 | ||
| Antarctic | 79 × 106 | 0.56 ± 0.04 (0.48–0.64) | 2.82 ± 0.09 (2.72–3.02) | 3.39 ± 0.11 (3.22–3.59) | ||
| Atlantic | 94 × 106 | 1.64 ± 0.08 (1.51–1.76) | 0.81 ± 0.05 (0.73–0.90) | 2.52 ± 0.06 (2.39–2.61) | 1.51 ± 0.03 (1.47–1.56) | 6.48 ± 0.19 (6.16–6.75) |
| Indian | 48 × 106 | 2.27 ± 0.10 (2.11–2.42) | 2.70 ± 0.08 (2.54–2.82) | 4.96 ± 0.17 (4.65–5.24) | ||
| Pacific | 155 × 106 | 2.35 ± 0.11 (2.12–2.49) | 0.52 ± 0.03 (0.45–0.58) | 5.13 ± 0.18 (4.75–5.34) | 4.00 ± 0.09 (3.85–4.12) | 12.0 ± 0.34 (11.4–12.5) |
| Total | 376 × 106 | 6.26 ± 0.28 (5.79–6.64) | 1.89 ± 0.10 (1.70–2.06) | 10.3 ± 0.27 (9.75–10.6) | 8.34 ± 0.17 (8.13–8.66) | 26.8 ± 0.74 (25.7–27.8) |
| Mean P-I +1 Standard Deviation | ||||||
| Coastal | Polar | Trades | Westerlies | Total | ||
| 47 × 106 | 57 × 106 | 141 × 106 | 131 × 106 | 376 × 106 | ||
| Antarctic | 79 × 106 | 1.49 ± 0.14 (1.24–1.71) | 6.71 ± 0.19 (6.48–7.14) | 8.20 ± 0.26 (7.78–8.63) | ||
| Atlantic | 94 × 106 | 4.57 ± 0.23 (4.21–4.86) | 2.27 ± 0.14 (2.05–2.61) | 10.4 ± 0.22 (9.95–10.7) | 4.70 ± 0.08 (4.58–4.87) | 21.9 ± 0.58 (20.9–22.8) |
| Indian | 48 × 106 | 5.34 ± 0.26 (4.96–5.72) | 6.07 ± 0.17 (5.71–6.34) | 11.4 ± 0.42 (10.7–12.0) | ||
| Pacific | 155 × 106 | 7.08 ± 0.32 (6.47–7.49) | 1.30 ± 0.08 (1.13–1.46) | 13.2 ± 0.44 (12.3–13.8) | 10.6 ± 0.23 (10.2–10.9) | 32.1 ± 0.92 (30.6–33.4) |
| Total | 376 × 106 | 17.0 ± 0.77 (15.8–18.0) | 5.07 ± 0.30 (4.48–5.56) | 29.6 ± 0.73 (28.1–30.4) | 22.0 ± 0.43 (21.5–22.8) | 73.7 ± 2.00 (70.8–76.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulk, G.; Platt, T.; Dingle, J.; Jackson, T.; Jönsson, B.F.; Bouman, H.A.; Babin, M.; Brewin, R.J.W.; Doblin, M.; Estrada, M.; et al. Primary Production, an Index of Climate Change in the Ocean: Satellite-Based Estimates over Two Decades. Remote Sens. 2020, 12, 826. https://doi.org/10.3390/rs12050826
Kulk G, Platt T, Dingle J, Jackson T, Jönsson BF, Bouman HA, Babin M, Brewin RJW, Doblin M, Estrada M, et al. Primary Production, an Index of Climate Change in the Ocean: Satellite-Based Estimates over Two Decades. Remote Sensing. 2020; 12(5):826. https://doi.org/10.3390/rs12050826
Chicago/Turabian StyleKulk, Gemma, Trevor Platt, James Dingle, Thomas Jackson, Bror F. Jönsson, Heather A. Bouman, Marcel Babin, Robert J. W. Brewin, Martina Doblin, Marta Estrada, and et al. 2020. "Primary Production, an Index of Climate Change in the Ocean: Satellite-Based Estimates over Two Decades" Remote Sensing 12, no. 5: 826. https://doi.org/10.3390/rs12050826
APA StyleKulk, G., Platt, T., Dingle, J., Jackson, T., Jönsson, B. F., Bouman, H. A., Babin, M., Brewin, R. J. W., Doblin, M., Estrada, M., Figueiras, F. G., Furuya, K., González-Benítez, N., Gudfinnsson, H. G., Gudmundsson, K., Huang, B., Isada, T., Kovač, Ž., Lutz, V. A., ... Sathyendranath, S. (2020). Primary Production, an Index of Climate Change in the Ocean: Satellite-Based Estimates over Two Decades. Remote Sensing, 12(5), 826. https://doi.org/10.3390/rs12050826