The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis
Abstract
1. Introduction
2. Material and Methods
2.1. Ultrasound and Elastography
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
3. Results
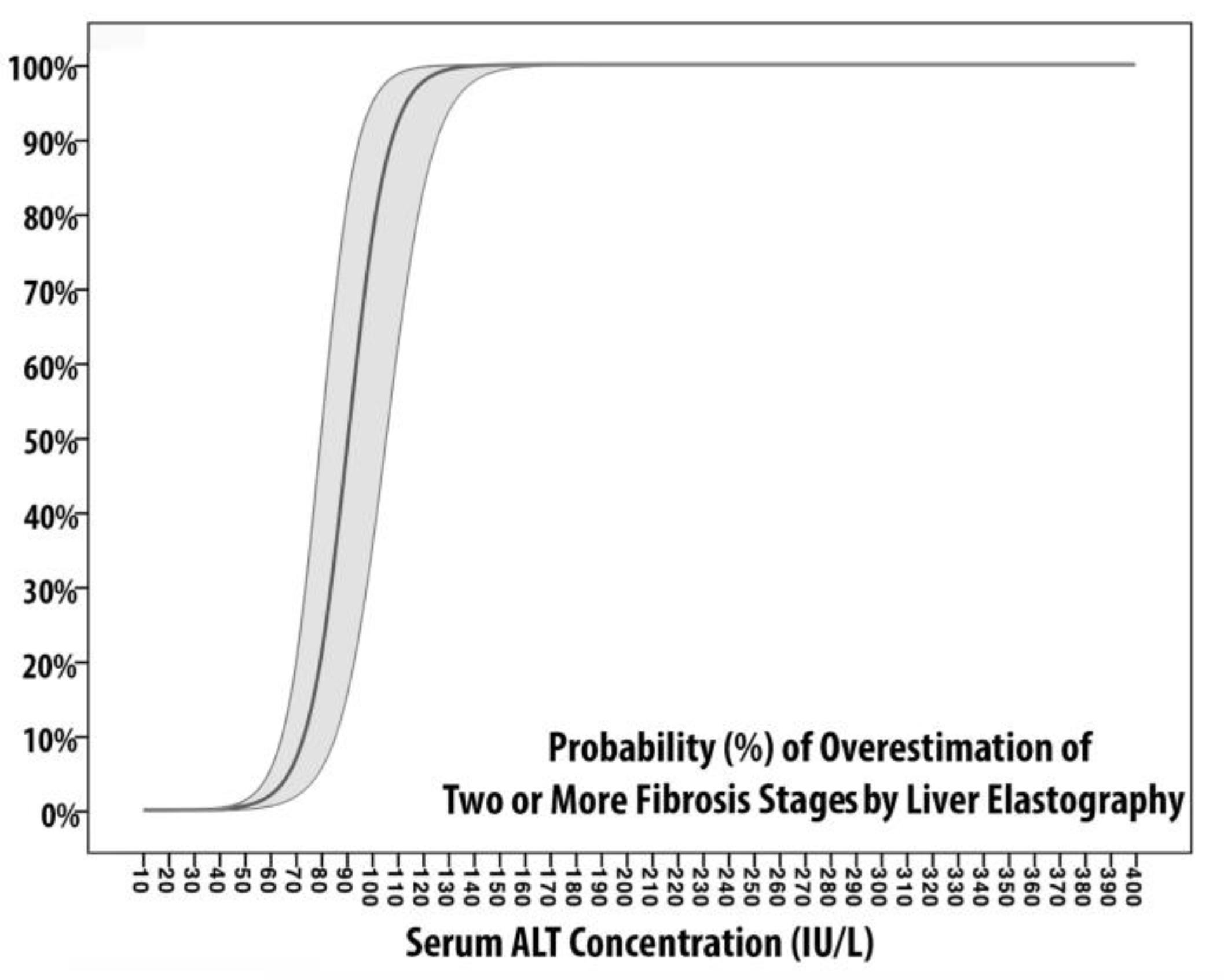
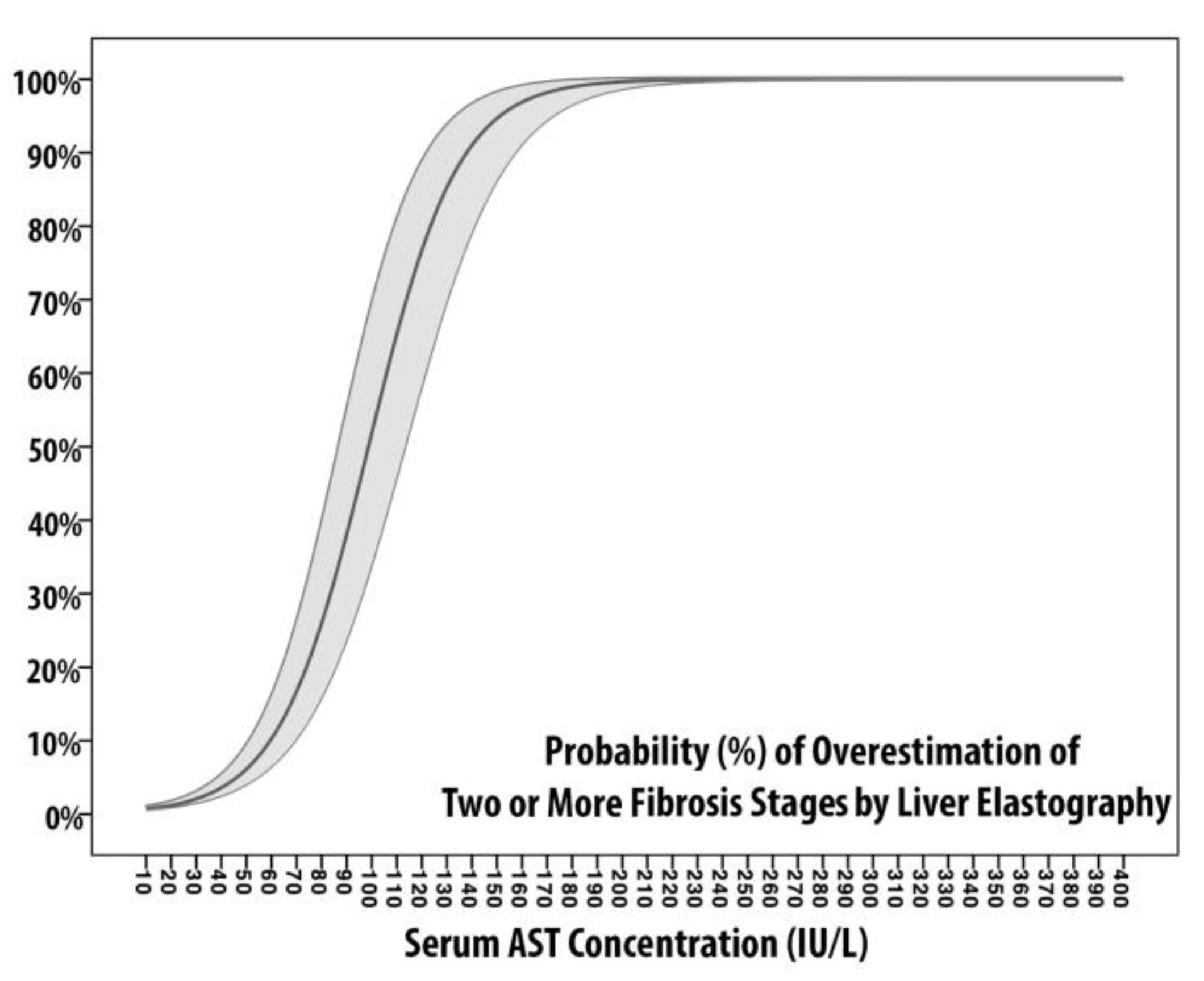
Models of Fibrosis Overestimation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCV | hepatitis C virus |
| DAAs | direct antiviral agents |
| NITs | noninvasive techniques |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ULN | upper limit of normality |
| LSM | liver stiffness measurement |
| SSM | spleen stiffness measurement |
| SVR | sustained viral response |
| pSWE | point-shear wave elastography |
References
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017. [Google Scholar] [CrossRef]
- Lingala, S.; Ghany, M.G. Natural History of Hepatitis C. Gastroenterol. Clin. North Am. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ayer, T.; Bethea, E.; Kanwal, F.; Wang, X.; Roberts, M.; Zhuo, Y.; Fagiuoli, S.; Petersen, J.; Chhatwal, J. Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: A modelling study. BMJ Open 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018. [Google Scholar] [CrossRef]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Ultrasound Q. 2016. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Zhou, Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Chan, S.C.; But, D.; Seto, W.K.; Cheng, C.; Wong, D.K.H.; Lo, C.M.; Fan, S.T.; Yuen, M.F. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am. J. Gastroenterol. 2010. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Cheng, C.; Wu, R.; Wong, D.K.H.; Yuen, M.F. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am. J. Gastroenterol. 2011. [Google Scholar] [CrossRef]
- Vispo, E.; Barreiro, P.; del Valle, J.; Maida, I.; de Ledinghen, V.; Quereda, C.; Moreno, A.; Macías, J.; Castera, L.; Pineda, J.A.; et al. Overestimation of liver fibrosis staging using transient elastography in patients with chronic hepatitis C and significant liver inflammation. Antivir. Ther. 2009, 14, 187–193. [Google Scholar] [PubMed]
- Masutti, F.; Giuffrè, M.; Santi, M.; Crosato, M.I.; Roberto, L.; Balestra, R.; Crocè, L.S. How does Trieste treat HCV in PWID? An effective coordination between addiction treatment service (SerD), the infective disease department and the liver clinic. Dig. Liver Dis. 2019, 51, e261. [Google Scholar] [CrossRef]
- Martinot-Peignoux, M.; Stern, C.; Maylin, S.; Ripault, M.P.; Boyer, N.; Leclere, L.; Castelnau, C.; Giuily, N.; Ray, A.E.; Cardoso, A.C.; et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis c virus receiving pegylated interferon and ribavirin. Hepatology 2010. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007. [Google Scholar] [CrossRef]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. Can we revise Baveno VI criteria using spleen elastography? Dig. Liver Dis. 2019, 51, e45. [Google Scholar] [CrossRef]
- Macor, D.; Giuffrè, M.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. Spleen and liver elastography as a non-invasive tool for detection of esophageal varices in patients with liver cirrhosis. Dig. Liver Dis. 2019, 51, e172. [Google Scholar] [CrossRef]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. The Role of Liver and Spleen Elastography in the Screening of Esophageal Varices in Patients with Liver Cirrhosis: Do We Really Need Endoscopy? Hepatology 2018, 68, 1466A–1467A. [Google Scholar] [CrossRef]
- Giuffrè, M.; Macor, D.; Masutti, F.; Tinè, F.; Bedogni, G.; Tiribelli, C.; Crocè, L.S. Spleen Stiffness Probability Index (SSPI): A Simple and Accurate Method to Detect Esophageal Varices in Patients with Compensated Liver Cirrhosis. Ann. Hepatol. 2019. [Google Scholar] [CrossRef]
- Ferraioli, G.; Maiocchi, L.; Lissandrin, R.; Tinelli, C.; De Silvestri, A.; Filice, C.; Above, E.; Barbarini, G.; Bruno, R.; Corona, S.; et al. Accuracy of the ElastPQ ® technique for the assessment of liver fibrosis in patients with chronic hepatitis C: A “real life” single center study. J. Gastrointest. Liver Dis. 2016. [Google Scholar] [CrossRef]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. Evaluation of spleen stiffness in healthy volunteers using point shear wave elastography. Ann. Hepatol. 2019. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Lissandrin, R.; Zicchetti, M.; Bello, B.D.; Filice, G.; Filice, C. Point shear wave elastography method for assessing liver stiffness. World J. Gastroenterol. 2014, 20, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Patti, R.; Pascut, D.; Sukowati, C.; Masutti, F.; Abazia, C.; Tinè, F.; Macor, D.; Buonocore, M.R.; Colombo, A. MicroRNAs as Regulators of Neo-Angiogenesis in Hepatocellular Carcinoma. Ann. Gastroenterol. Dig. Disord. 2018, 1, 09–016. [Google Scholar]
- Fagerland, M.W.; Hosmer, D.W. A goodness-of-fit test for the proportional odds regression model. Stat. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Gantner, F.; Bohlinger, I.; Tiegs, G.; Germann, P.G.; Wendel, A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 1995, 146, 1220. [Google Scholar] [PubMed]
- Lawson, J.A.; Fisher, M.A.; Simmons, C.A.; Farhood, A.; Jaeschke, H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology 1998. [Google Scholar] [CrossRef]
- Bajt, M.L. Protection against Fas Receptor-Mediated Apoptosis in Hepatocytes and Nonparenchymal Cells by a Caspase-8 Inhibitor in Vivo: Evidence for a Postmitochondrial Processing of Caspase-8. Toxicol. Sci. 2000. [Google Scholar] [CrossRef]
- Fitzmaurice, K.; Klenerman, P. Cellular Immunity and Acute Hepatitis C Infection. Curr. Pharm. Des. 2008. [Google Scholar] [CrossRef]
- Shen, F.F.; Wang, Y.; Wang, Y.F.; Zheng, R.D.; Xian, J.C.; Shi, J.P.; Qu, Y.; Dong, Y.W.; Xu, M.Y.; Lu, L.G. Prediction of hepatic necroinflammatory activity in patients with chronic hepatitis B by a simple noninvasive model. J. Transl. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pradat, P.; Alberti, A.; Poynard, T.; Esteban, J.I.; Weiland, O.; Marcellin, P.; Badalamenti, S.; Trépo, C. Predictive value of ALT levels for histologic findings in chronic hepatitis C: A European collaborative study. Hepatology 2002. [Google Scholar] [CrossRef]
- Xie, L.T.; Xu, D.X.; Tian, G.; Zhong, L.Y.; Zhao, Q.Y.; Ke, Q.H.; Jiang, T.A. Value of Two-Dimensional Shear Wave Elastography for Assessing Acute Liver Congestion in a Bama Mini-Pig Model. Dig. Dis. Sci. 2018. [Google Scholar] [CrossRef]
- Piecha, F.; Paech, D.; Sollors, J.; Seitz, H.K.; Rössle, M.; Rausch, V.; Mueller, S. Rapid change of liver stiffness after variceal ligation and TIPS implantation. Am. J. Physiol. Gastrointest. Liver Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Castera, L. Is it really worth adapting liver stiffness cut-offs according to AST levels? Liver Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Englert, S.; Seitz, H.K.; Badea, R.I.; Erhardt, A.; Bozaari, B.; Beaugrand, M.; Lupşor-Platon, M. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015, 35, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Santos, B.; Simón-Talero, M.; Ventura-Cots, M.; Riveiro-Barciela, M.; Esteban, R.; Augustin, S.; Genescà, J. Rapid liver and spleen stiffness improvement in compensated advanced chronic liver disease patients treated with oral antivirals. Ther. Adv. Gastroenterol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cammà, C.; Di Bona, D.; Schepis, F.; Heathcote, J.; Zeuzem, S.; Pockros, P.J.; Marceliln, P.; Balart, L.; Alberti, A.; Craxì, A. Effect of Peginterferon Alfa-2a on Liver Histology in Chronic Hepatitis C: A Meta-analysis of Individual Patient Data. Hepatology 2004. [Google Scholar] [CrossRef]
- Shiratori, Y.; Imazeki, F.; Moriyama, M.; Yano, M.; Arakawa, Y.; Yokosuka, O.; Kuroki, T.; Nishiguchi, S.; Sata, M.; Yamada, G.; et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann. Intern. Med. 2000. [Google Scholar] [CrossRef]
- Marcellin, P.; Boyer, N.; Gervais, A.; Martinot, M.; Pouteau, M.; Castelnau, C.; Kilani, A.; Areias, J.; Auperin, A.; Benhamou, J.P.; et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-α therapy. Ann. Intern. Med. 1997. [Google Scholar] [CrossRef]
- Rewisha, E.A.; Elsabaawy, M.M.; Alsebaey, A.; Elmazaly, M.A.; Tharwa, E.S. Evaluation of the role of liver and splenic transient elastography in chronic hepatitis C related fibrosis. J. Liver Dis. Transpl. 2016, 5, 3. [Google Scholar] [CrossRef]
- Leung, V.Y.; Shen, J.; Wong, V.W.; Abrigo, J.; Wong, G.L.; Chim, A.M.; Chu, S.H.; Chan, A.W.; Choi, P.C.; Ahuja, A.T.; et al. Quantitative Elastography of Liver Fibrosis and Spleen Stiffness in Chronic Hepatitis B Carriers: Comparison of Shear-Wave Elastography and Transient Elastography with Liver Biopsy Correlation. Radiology 2013. [Google Scholar] [CrossRef]
- Lurie, Y.; Webb, M.; Cytter-Kuint, R.; Shteingart, S.; Lederkremer, G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Campigotto, M.; Colombo, A.; Visintin, A.; Buonocore, M.R.; Aversano, A.; Budel, M.; Tinè, F.; Masutti, F.; Abazia, C.; et al. Spleen Stiffness/Platelets-Based Models Can Predict Presence of Esophageal Varices in Patients With Compensated Liver Cirrhosis. Dig. Liver Dis. 2019, 52, e61–e62. [Google Scholar] [CrossRef]

| Values | Baseline | End of Therapy | One-Year after SVR12 |
|---|---|---|---|
| Male, n (%) | 48 (43.6%) | ||
| Age, years | 64 (52;74) | ||
| HCV-Genotype, n (%) | |||
| Genotype 1 | 63 (57.2%) | ||
| Genotype 2 | 14 (12.7%) | ||
| Genotype 3 | 25 (22.7%) | ||
| Genotype 4 | 8 (7.3%) | ||
| BMI, (kg/m2) | 21.1 (17.7; 23.4) | 22 (18;24) | 23.2 (20.2;25.6) |
| Creatinine, (mg/dL) | 0.78 (0.70;0.84) | 0.81 (0.73;0.91) | 0.76 (0.69;0.82) |
| Glucose, (mg/dL) | 94 (85;102) † | 98 (86;102) ‡ | 105 (94;113) †,‡ |
| Total Cholesterol, (mg/dL) | 180 (170;200) † | 181 (176;210) ‡ | 193 (180;223) †,‡ |
| AST, (IU/L) | 90 (68;149) † | 35 (31;42) ‡ | 21.5 (20;26) †,‡ |
| ALT, (IU/L) | 94.5 (79;135) † | 32 (28;36) ‡ | 17.5 (15;21) †,‡ |
| GGT, (IU/L) | 44 (37;46) | 43 (35;48) | 40 (32;50) |
| ALP (IU/L) | 105 (80;130) | 100 (78;135) | 107 (89;116) |
| Total Bilirubin, (mg/dL) | 0.85 (0.75;0.99) | 0.80 (0.68;1.12) | 0.87 (0.70;1.01) |
| Albumin, (g/dL) | 4.1 (3.5;4.4) | 3.8 (3.4;4.3) | 3.9 (3.6;4.5) |
| INR | 1.10 (1.03;1.14) | 1.07 (1.04;1.09) | 1.12 (1.07;1.15) |
| PLT, (×103/L) | 151 (137.5;218) † | 165 (153;171) ‡ | 185 (152.5;242) †,‡ |
| Liver Stiffness, (kPa) | 14.10 (11.3;20) † | 7.1 (5.3;11) † |
| Patients Stratified by Degree of Fibrosis Regression | AST (IU/L) (Baseline) | ALT (IU/L) (Baseline) | Platelets (×109/L) (Baseline) | AST (IU/L) (1-Year after Therapy) | ALT (IU/L) (1-Year after Therapy) | Platelets (×109/L) (1-Year after Therapy) | % Changes in LSM from Baseline |
|---|---|---|---|---|---|---|---|
| Group 1 (G1) (n = 34) From F4 to F4 | 62 (28.5;85) | 71 (41.5;87) | 97 (80;130) | 26 (20;34) | 18 (14;29.5) | 115 (90;153) | −14.3% |
| Group 2 (G2) (n = 9) From F4 to F3 | 70 (51;90) | 82 (52;118) | 152 (140;217) | 21 (19;26.5) | 15 (13;22.5) | 188 (151;239) | −20% |
| Group 3 (G3) (n = 30) From F3/4 to F2 | 110 (90;150) | 100 (89;119) | 170 (150;240) | 20 (18;24) | 16 (15;21) | 190 (170;259) | −37% |
| Group 4 (G4) (n = 37) From F3/4 to F0-1 | 176 (149;230) | 251 (182;300) | 222 (146;291) | 22 (20;24) | 21 (17;26) | 251 (182;300) | −57.5% |
| Statistical Significance | |||||||
| Group 1 vs. Group 2 | p = 0.049 | p = 0.043 | p = 0.019 | NS | NS | p = 0.022 | NE |
| Group 1 vs. Group 3 | p = 0.03 | p = 0.001 | p = 0.001 | NS | NS | p < 0.001 | NE |
| Group 1 vs. Group 4 | p < 0.001 | p < 0.001 | p < 0.001 | NS | NS | p < 0.001 | NE |
| Group 2 vs. Group 3 | p = 0.032 | NS | p = 0.047 | NS | NS | NS | NE |
| Group 3 vs. Group 4 | p = 0.028 | p = 0.021 | p = 0.032 | NS | NS | p = 0.039 | NE |
| Patients Stratified by Degree of Fibrosis Regression | Spleen Stiffness (kPa) | Portal Vein Diameter (cm) | Portal Vein Median Flow Velocity (cm/s) | Spleen Bipolar Diameter (cm) | Spleen Area (cm2) |
|---|---|---|---|---|---|
| Group 1 (G1) (n = 34) From F4 to F4 | 45 (36;56) | 1.14 (1;1.3) | 17.8 (17.5;18) | 12.4 (11;13.6) | 54 (46.5;68) |
| Group 2 (G2) (n = 9) From F4 to F3 | 29 (27;32) | 1.01 (0.9;1.18) | 19 (15;21) | 11.5 (10.5;13.5) | 48 (38;66) |
| Group 3 (G3) (n = 30) From F3/4 to F2 | 24 (18;26) | 1.01 (0.9;1.06) | 20.4 (18.2;24) | 10.7 (10;12) | 44 (35;56) |
| Group 4 (G4) (n = 37) From F3/4 to F0-1 | 19 (16;24) | 1.01 (0.9;1.09) | 26 (17;28) | 10 (9;11) | 38 (30;44.5) |
| Statistical Significance | |||||
| Group 1 vs. Group 2 | p = 0.01 | NS | NS | NS | p = 0.039 |
| Group 1 vs. Group 3 | p = 0.001 | NS | p = 0.024 | p = 0.05 | p = 0.023 |
| Group 1 vs. Group 4 | p < 0.001 | p = 0.043 | p = 0.001 | p = 0.001 | p < 0.001 |
| Group 2 vs. Group 3 | p = 0.038 | NS | p = 0.045 | NS | p = 0.037 |
| Group 3 vs. Group 4 | p = 0.029 | NS | p = 0.031 | p = 0.044 | p = 0.036 |
| Model 1 (M1) (Overestimation of One Fibrosis Stage) | Model 2 (M2) (Overestimation of Two or More Fibrosis Stages) | |||
|---|---|---|---|---|
| Model Parameters | M1-AST | M1-ALT | M2-AST | M2-ALT |
| Linear Predictor | −0.479 + 0.005×[AST] | −1.329 + 0.018×[ALT] | −5.525 + 0.056×[AST] | −11.497 + 0.127×[ALT] |
| VIF | 2.532 | 1.712 | ||
| AIC | 210 | 209 | 89 | 88 |
| BIC | 213 | 200 | 92 | 90 |
| AUROC | 0.623 (0.4;0.685) | 0.623 (0.485;0.761) | 0.936 (0.882;0.989) | 0.958 (0.922;0.996) |
| Nagelkerke-PseudoR2 | 0.009 | 0.088 | 0.682 | 0.785 |
| Hosmer-Lemeshow p-value | 0.048 | 0.025 | 0.203 | 0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuffrè, M.; Fouraki, S.; Comar, M.; Masutti, F.; Crocè, L.S. The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms 2020, 8, 348. https://doi.org/10.3390/microorganisms8030348
Giuffrè M, Fouraki S, Comar M, Masutti F, Crocè LS. The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms. 2020; 8(3):348. https://doi.org/10.3390/microorganisms8030348
Chicago/Turabian StyleGiuffrè, Mauro, Sofia Fouraki, Manola Comar, Flora Masutti, and Lory Saveria Crocè. 2020. "The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis" Microorganisms 8, no. 3: 348. https://doi.org/10.3390/microorganisms8030348
APA StyleGiuffrè, M., Fouraki, S., Comar, M., Masutti, F., & Crocè, L. S. (2020). The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms, 8(3), 348. https://doi.org/10.3390/microorganisms8030348






