Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-stressed Broilers via Antioxidant Genes and HSP70 Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Experimental Design and Sample Collection
2.2. Meat Quality Analysis
2.3. Measurement of Redox State in the Breast Muscle
2.4. Measurement of HSP70 Levels in Breast Muscle
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Meat Quality
3.2. MDA in the Breast Muscle
3.3. SOD, CAT, GSH, GSH-Px, and T-AOC Levels in the Breast Muscle
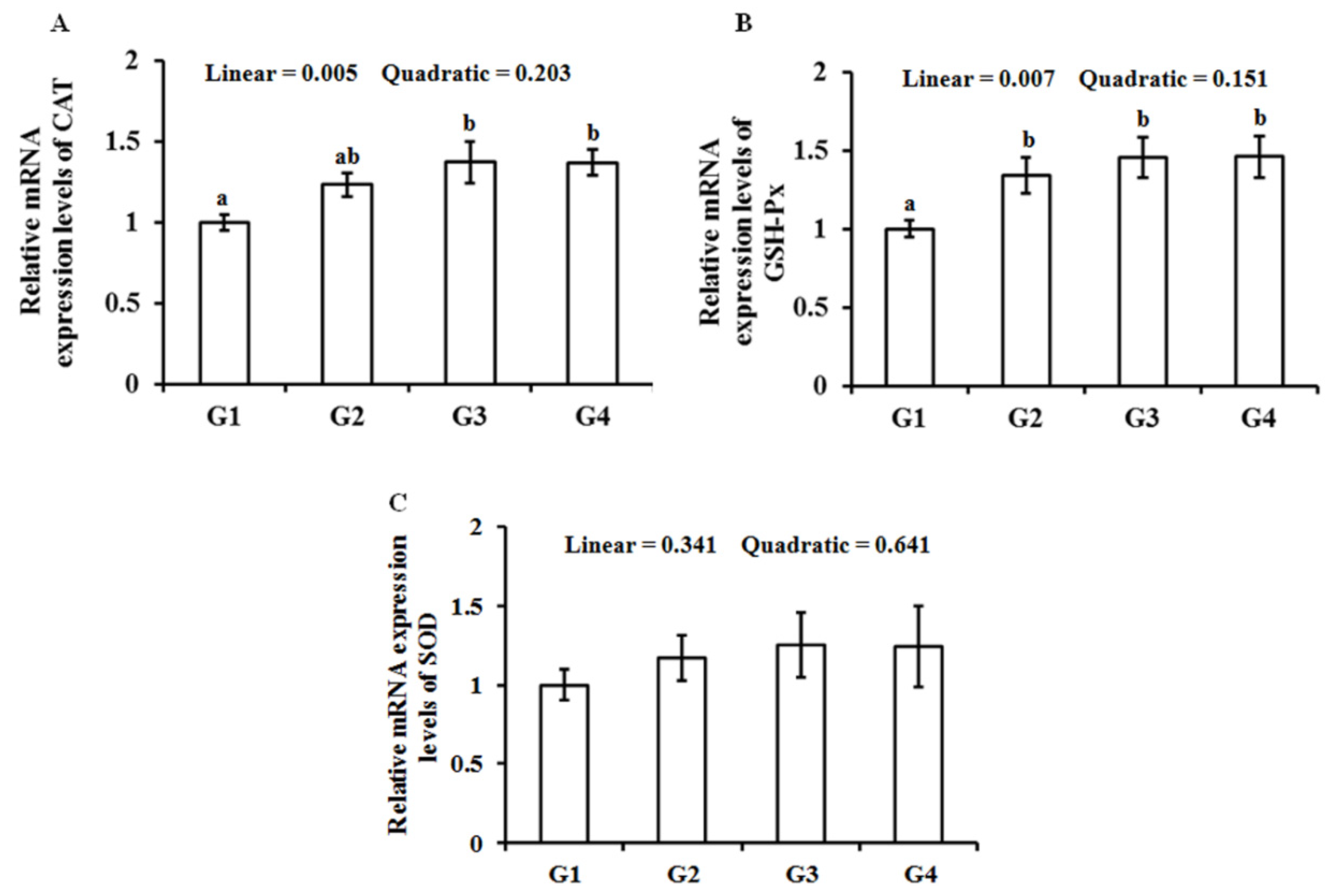
3.4. Gene Expression of Antioxidant Enzymes in the Breast Muscle
3.5. Gene and Protein Expression Levels of HSP70 in the Breast Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goo, D.; Kim, J.H.; Park, G.H.; Delos Reyes, J.B.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. An. N 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibian, M.; Ghazi, S.; Moeini, M.M. Effects of dietary selenium and vitamin E on growth performance, meat yield, and selenium content and lipid oxidation of breast meat of broilers reared under heat stress. Biol. Trace. Elem. Res. 2016, 169, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.H.; Gao, F. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agr. 2019, 99, 1066–1072. [Google Scholar] [CrossRef]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian. Pathol. 2018, 47, 616–624. [Google Scholar] [CrossRef]
- Erol, H.S.; Imik, H.; Gumus, R.; Halici, M. The effects of different amount of protein and vitamin E supplementation in rations on lipid and antioxidant metabolism of broilers exposed to heat stress. Braz. J. Poultry Sci. 2017, 19, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.A.; Jacobs, J.A.; Murugesan, G.R.; Cheng, H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018, 97, 1101–1108. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, S. A mini review on heat shock proteins (HSPs): Special emphasis on heat shock protein70 (HSP70). BN Seal J. Sci. 2017, 9, 129–138. [Google Scholar]
- Kattaia, A.A.; El-Baset, S.A.A.; Mohamed, E.M. Heat Shock Proteins in Oxidative and Nitrosative Stress; Heat Shock Proteins and Stress; Springer: Cham, Switzerland, 2018; pp. 127–138. [Google Scholar]
- Wan, X.; Ahmad, H.; Zhang, L.; Wang, Z.; Wang, T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agr. 2018, 98, 3715–3721. [Google Scholar] [CrossRef]
- Greene, E.S.; Rajaei-Sharifabadi, H.; Dridi, S. Feather HSP70: A novel non-invasive molecular marker for monitoring stress induced by heat exposure in broilers. Poult. Sci. 2019, 98, 3400–3404. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Lu, C.; Bai, K.; Zhang, L.; Wang, T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agr. Food Chem. 2015, 63, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Givisiez, P.E.N.; Ferro, J.A.; Ferro, M.I.T.; Kronka, S.N.; Decuypere, M. Hepatic concentration of heat shock protein 70 kD (Hsp70) in broilers subjected to different thermal treatments. Brit. Poultry Sci. 1999, 40, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Mohammed, A.A.; Jacobs, J.A.; Cramer, T.A.; Cheng, H.W. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult. Sci. 2020, 99, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Procopio, J.; Lagranha, C.; Gorjão, R.; Pithon-Curi, T.C. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, Q.; Wang, Y.; Li, J.; Lu, G.; Liu, Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ. Health Prev. 2019, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Shanware, N.P.; Mullen, A.R.; De Berardinis, R.J.; Abraham, R.T. Glutamine: Pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. 2011, 89, 229–236. [Google Scholar] [CrossRef]
- Dai, S.F.; Bai, X.; Zhang, D.; Hu, H.; Wu, X.; Wen, A.; He, S.J.; Zhao, L. Dietary glutamine improves meat quality, skeletal muscle antioxidant capacity and glutamine metabolism in broilers under acute heat stress. J. Appl. Anim. Res. 2018, 46, 1412–1417. [Google Scholar]
- Bai, X.; Dai, S.; Li, J.; Xiao, S.; Wen, A.; Hu, H. Glutamine improves the growth performance, serum biochemical profile and antioxidant status in broilers under medium-term chronic heat stress. J. Appl. Poult. Res. 2019, 28, 1248–1254. [Google Scholar] [CrossRef]
- Akagi, R.; Ohno, M.; Matsubara, K.; Fujimoto, M.; Nakai, A.; Inouye, S. Glutamine protects intestinal barrier function of colon epithelial cells from ethanol by modulating Hsp70 expression. Pharmacology 2013, 91, 104–111. [Google Scholar] [CrossRef]
- Gong, J.; Jing, L. Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol. 2011, 77, 488–495. [Google Scholar]
- Wang, H.; Tang, C.; Jiang, Z.; Zhou, X.; Chen, J.; Na, M.; Shen, H.; Lin, Z. Glutamine promotes Hsp70 and inhibits α-Synuclein accumulation in pheochromocytoma PC12 cells. Exp. Ther. Med. 2017, 14, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Olubodun, J.O.; Zulkifli, I.; Farjam, A.S.; Hair-Bejo, M.; Kasim, A. Glutamine and glutamic acid supplementation enhances performance of broiler chickens under the hot and humid tropical condition. Ital. J. Anim. Sci. 2015, 14, 3263. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress–induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Chen, K.; Zhao, X.; Geng, Z. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J. Sci. Food Agr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gregory, N.G. How climatic changes could affect meat quality. Food Res. Int. 2010, 43, 1866–1873. [Google Scholar] [CrossRef]
- Tang, S.; Yu, J.; Zhang, M.; Bao, E. Effects of different heat stress periods on various blood and meat quality parameters in young Arbor Acer broiler chickens. Can. J. Anim. Sci. 2013, 93, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.F.; Gao, F.; Xu, X.L.; Zhang, W.H.; Song, S.X.; Zhou, G.H. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water-holding characteristic in broilers under cyclic heat stress. Brit. Poultry. Sci. 2012, 53, 471–481. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles, hypocholesterolemic, atherogenic, and thrombogenic indices of broiler meat in the retail market. Lipids Health Dis. 2017, 16, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Geng, Z.Y.; Chen, K.K.; Zhao, X.H.; Wang, C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult. Sci. 2019, 98, 4648–4655. [Google Scholar] [CrossRef]
- Balogh, K.; Kövesi, B.; Zándoki, E.; Kulcsár, S.; Ancsin, Z.; Erdélyi, M.; Dobolyi, C.; Bata-Vidács, I.; Notai, K.; Szekeres, A.; et al. Effect of sterigmatocystin or aflatoxin contaminated feed on lipid peroxidation and glutathione redox system and expression of glutathione redox system regulatory genes in broiler chicken. Antioxidants 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Du, M.; Xu, Q.; Chen, Y.; Wen, C.; Zhou, Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.D.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Zhang, J.F.; Hu, Z.P.; Lu, C.H.; Yang, M.X.; Zhang, L.L.; Wang, T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015, 93, 1656–1665. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Kikusato, M.; Maekawa, T.; Shirakawa, H.; Toyomizu, M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Phys. A 2010, 155, 401–406. [Google Scholar] [CrossRef]
- Xing, T.; Wang, M.F.; Han, M.Y.; Zhu, X.S.; Xu, X.L.; Zhou, G.H. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Animal 2017, 11, 1599–1607. [Google Scholar] [CrossRef]
- Tang, S.; Yin, B.; Xu, J.; Bao, E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxid. Med. Cell Longev. 2018, 2018, 7014126. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Chen, K.; Lv, Y.; Huang, D.; Liu, J.; Liang, G.; Zhang, L.; Wang, F.F.; Su, C.; Zou, Y.; et al. Increased oxidative stress and plasma Hsp70 levels among gasoline filling station attendants. Toxicol. Ind. Health 2017, 33, 171–181. [Google Scholar] [CrossRef]
- Gu, X.; Hao, Y.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012, 91, 790–799. [Google Scholar] [CrossRef]


| Ingredients | % as Fed Basis | Chemical Composition | % as Fed Basis |
|---|---|---|---|
| Corn | 58.5 | ME (MJ/kg) | 12.73 |
| Soybean meal | 32.0 | Crude protein | 20.30 |
| Fish meal | 2.0 | Lysine | 1.08 |
| Starch | 1.0 | Methionine + cysteine | 0.76 |
| Soybean oil | 3.5 | Ca | 0.89 |
| CaHPO4·2H2O | 1.5 | Available P | 0.42 |
| Limestone | 0.9 | ||
| Salt | 0.3 | ||
| DL-Met | 0.1 | ||
| Vitamin and trace mineral premix 2 | 0.2 | ||
| Total | 100 |
| Gene | Primer (5′→3′) | Genbank Number |
|---|---|---|
| β-actin | F: TGCTGTGTTCCCATCTATCG R: TTGGTGACAATACCGTGTTCA | NM 205518 |
| SOD | F: GGAGGAGTGGCAGAAGT R: TAAACGAGGTCCAGCAT | NM 205064 |
| CAT | F: TATCCTTCCTGGTCTTTCTACAT R: CGCCATCTGTTCTACCTCC | NM 001031215.2 |
| GSH-Px | F: AAGTGCGAGGTGAACGG R: CGGCGACCAGATGATGTAC | NM 001277853.1 |
| HSP70 | F: GGAGGACTTTGACAACCG R: CAAGCTGTACGCAGACG | NM_001006685.1 |
| Item | Treatment | SEM 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Linear | Quadratic | ||
| pH | 5.905 a | 5.942 a | 6.063 b | 6.072 b | 0.019 | <0.001 | 0.579 |
| L* | 46.007 a | 44.394 a b | 43.463 b | 43.246 b | 0.346 | 0.002 | 0.228 |
| a* | 4.104 | 4.394 | 4.494 | 4.548 | 0.087 | 0.074 | 0.496 |
| b* | 4.904 a | 5.677 b | 5.893 b | 5.913 b | 0.121 | 0.001 | 0.055 |
| DL (%) | 4.9 a | 4.7 b | 4.7 b | 4.5 b | 0.04 | <0.001 | 0.561 |
| WLR (%) | 32.3 a | 31.0 a | 30.8 a | 28.8 b | 0. 4 | 0.001 | 0.621 |
| CL (%) | 35.9 a | 35.3 a b | 34.5 a b | 34.0 b | 0. 3 | 0.017 | 0.958 |
| Item | Treatment | SEM 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Linear | Quadratic | ||
| SOD (U/mg protein) | 79.753 | 84.852 | 92.357 | 91.232 | 3.396 | 0.19 | 0.658 |
| CAT (U/mg protein) | 2.115 a | 2.340 a b | 2.874 a b | 3.105 b | 0.146 | 0.007 | 0.991 |
| GSH (mg/g protein) | 3.174 a | 4.083 a b | 5.392 b c | 6.324 c | 0.357 | <0.001 | 0.983 |
| GSH-Px (U/mg protein) | 11.783 a | 16.187 a b | 15.963 a b | 18.456 b | 0.841 | 0.006 | 0.517 |
| T-AOC (U/mg protein) | 1.722 a | 3.084 b | 3.233 b | 2.930 b | 0.214 | 0.035 | 0.037 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Chen, L.; Dai, S.; Li, J.; Bai, X. Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-stressed Broilers via Antioxidant Genes and HSP70 Pathway. Animals 2020, 10, 404. https://doi.org/10.3390/ani10030404
Hu H, Chen L, Dai S, Li J, Bai X. Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-stressed Broilers via Antioxidant Genes and HSP70 Pathway. Animals. 2020; 10(3):404. https://doi.org/10.3390/ani10030404
Chicago/Turabian StyleHu, Hong, Liang Chen, Sifa Dai, Jiaqi Li, and Xi Bai. 2020. "Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-stressed Broilers via Antioxidant Genes and HSP70 Pathway" Animals 10, no. 3: 404. https://doi.org/10.3390/ani10030404




