Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Anthocyanin-Rich Fraction from Purple Rice
2.2. Chemicals and Cell Lines
2.3. Animals
2.4. Experimental Protocol of TRAP Rat Model
2.5. Experimental Protocol of the PCai1 Xenograft Model
2.6. Immunohistochemistry
2.7. Western Blot Analysis
2.8. Cell Proliferation Assay
2.9. RNA Extraction, cDNA Preparation, and Quantitative Real-Time PCR
2.10. Cell-Cycle Analysis
2.11. Statistical Analysis
3. Results
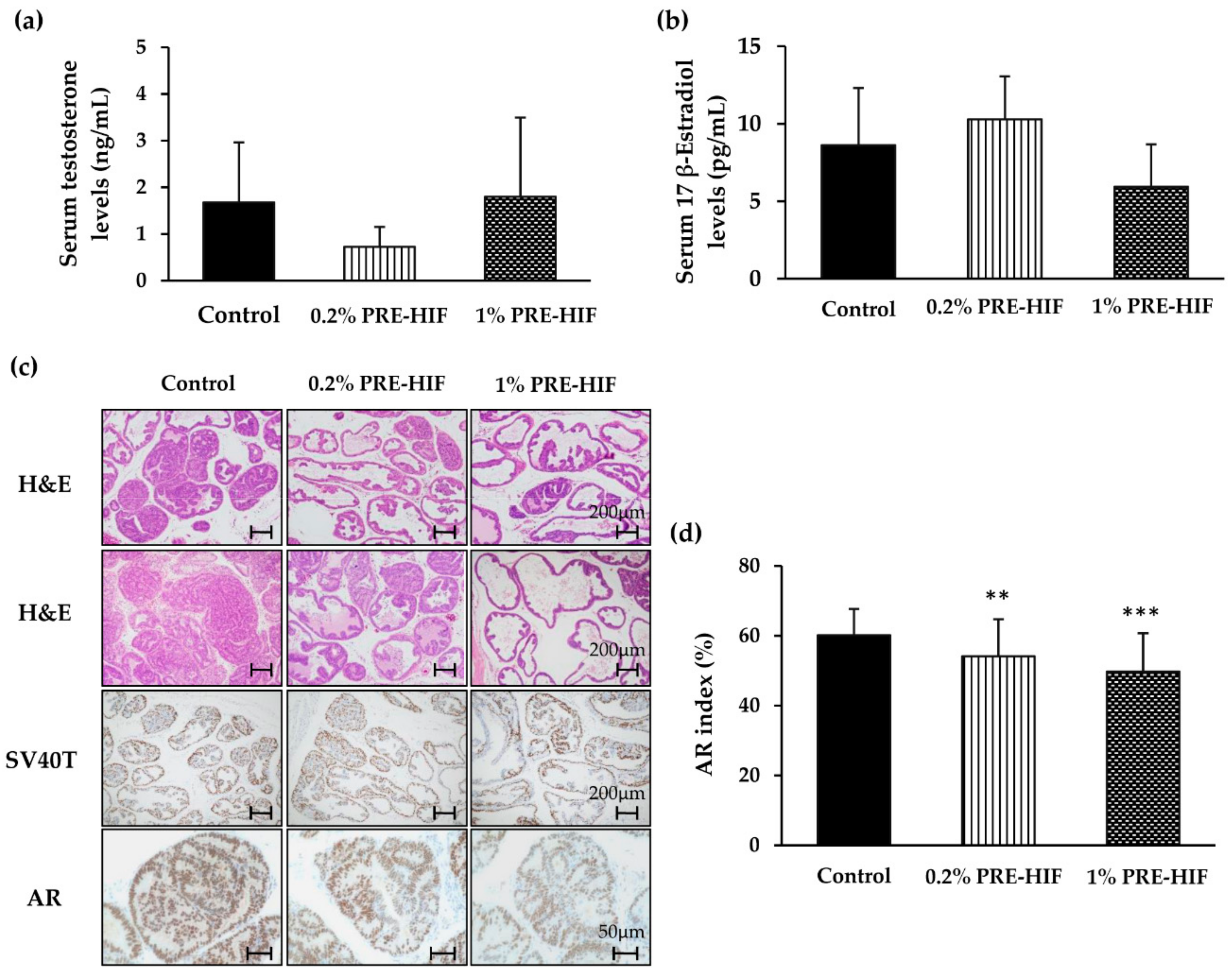
3.1. PRE-HIF Ameliorates Prostate Tumorigenesis in a TRAP Rat Model
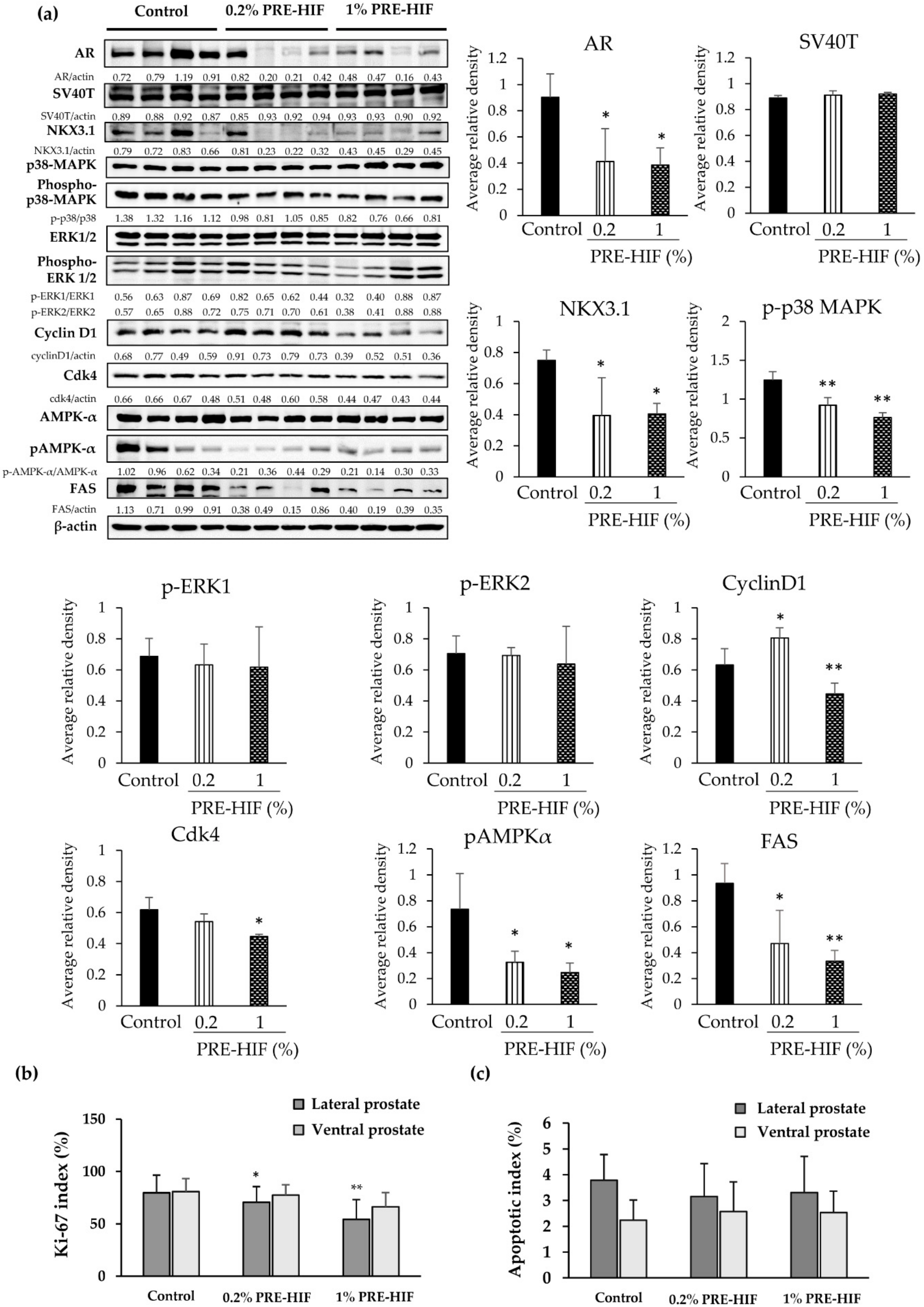
3.2. PRE-HIF Down-Regulates the Expression of Androgen Receptor and Cellular Growth and Metabolism Proteins in Lateral Prostates of TRAP Rats
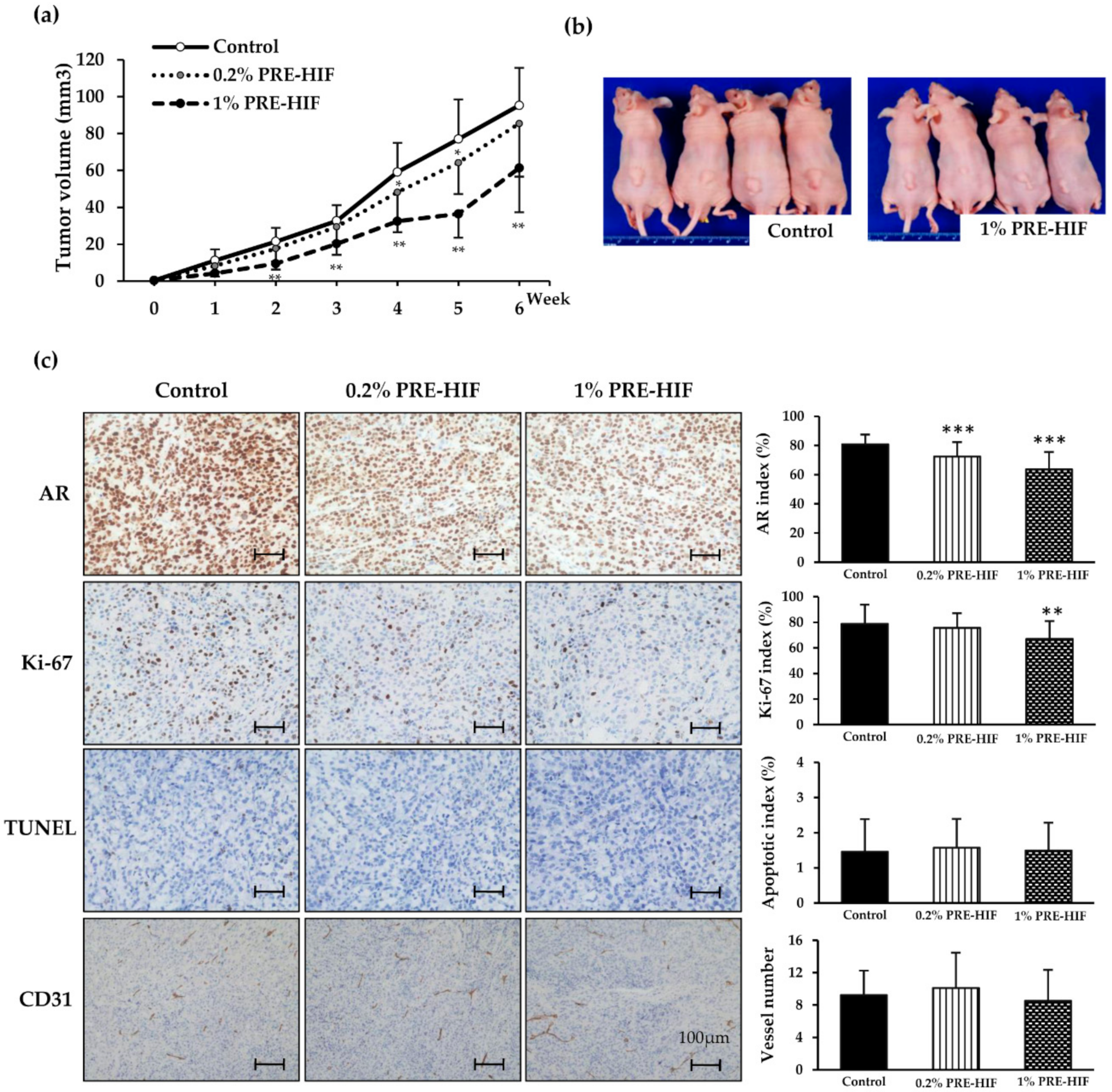
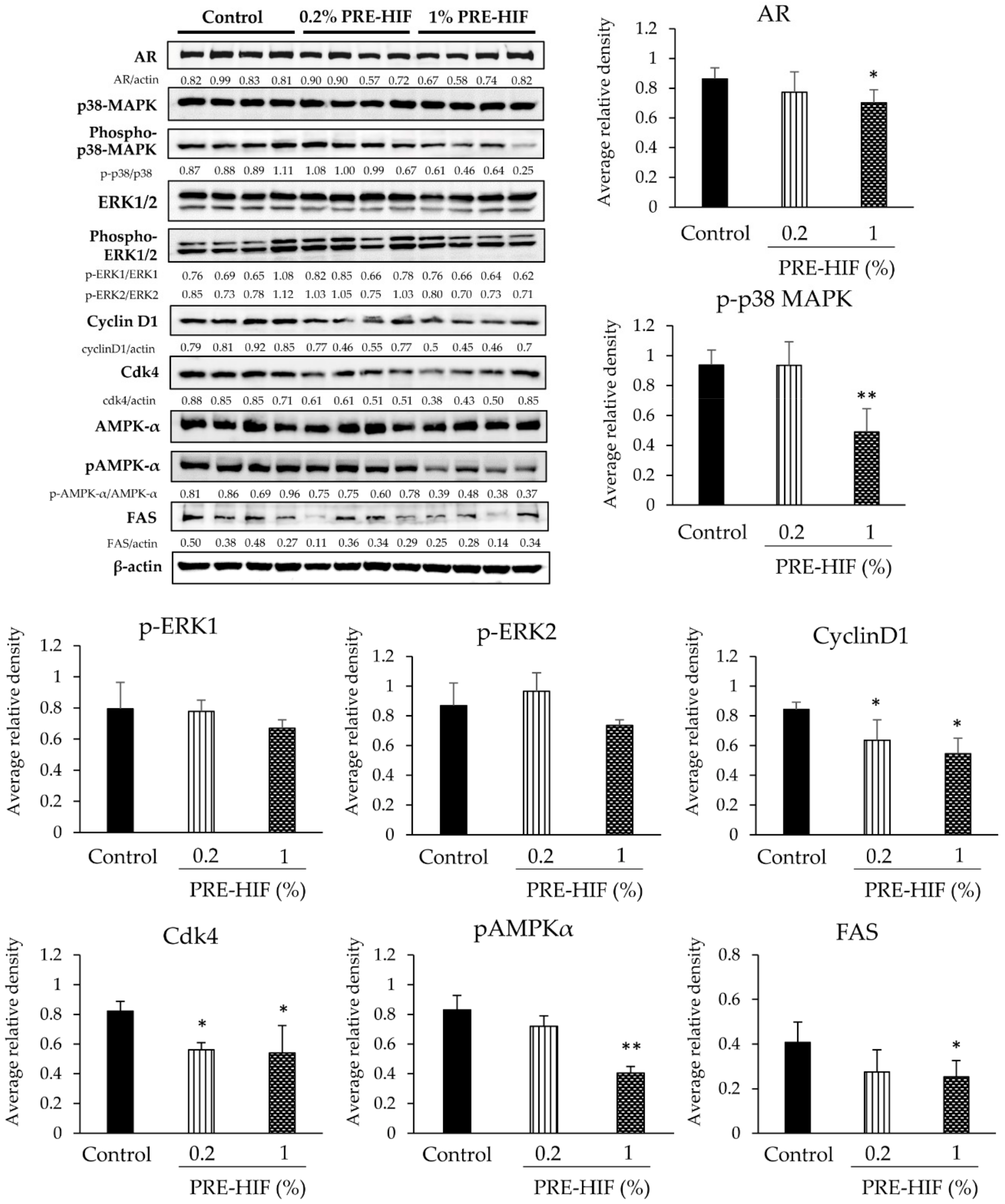
3.3. PRE-HIF Retards Tumor Growth and Inhibits Cell Growth Pathways in PCai1 Xenograft Model
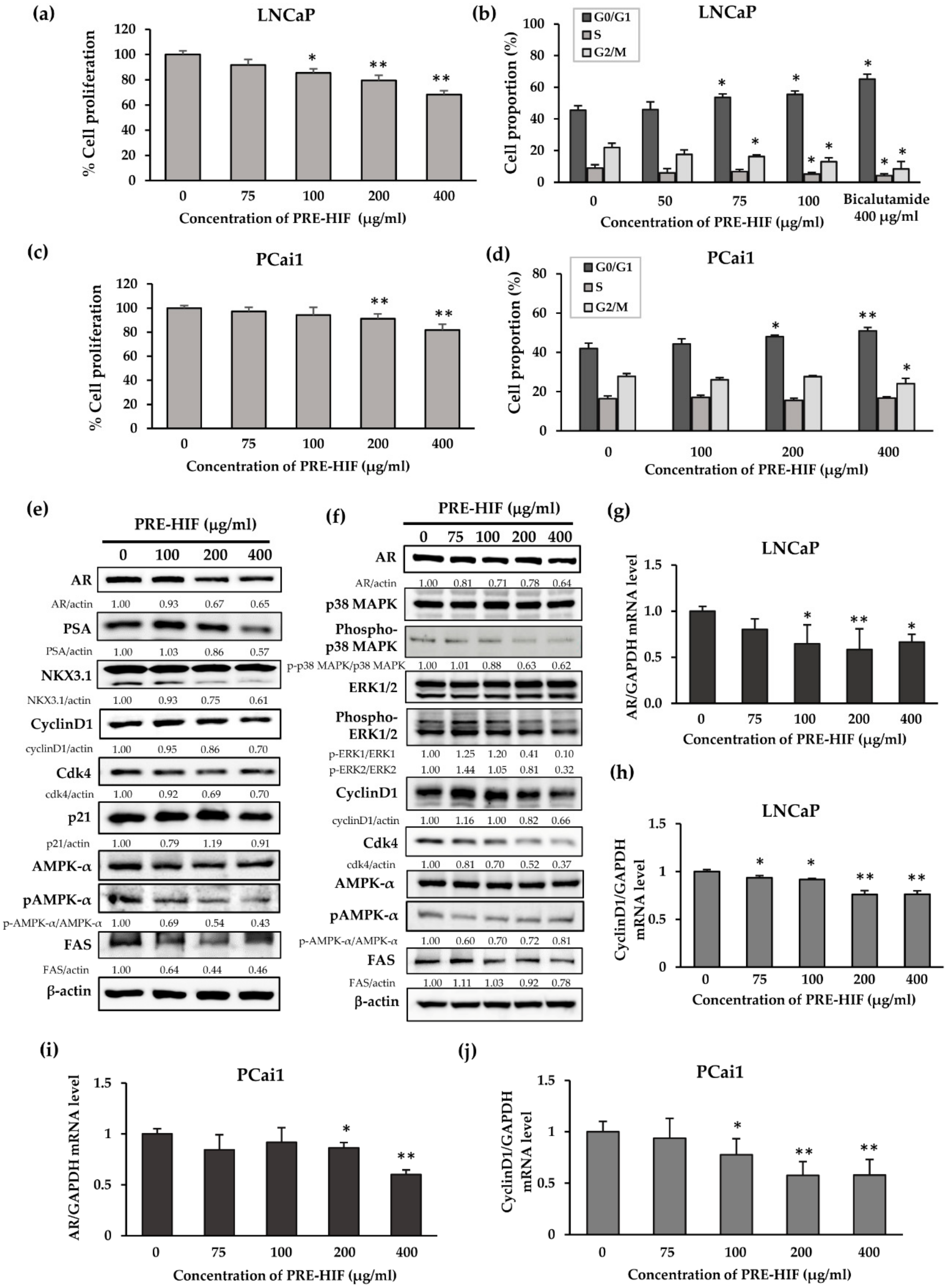
3.4. PRE-HIF Suppresses Cell Proliferation and Induces Cell-Cycle Arrest in Prostate Cancer Cell Lines
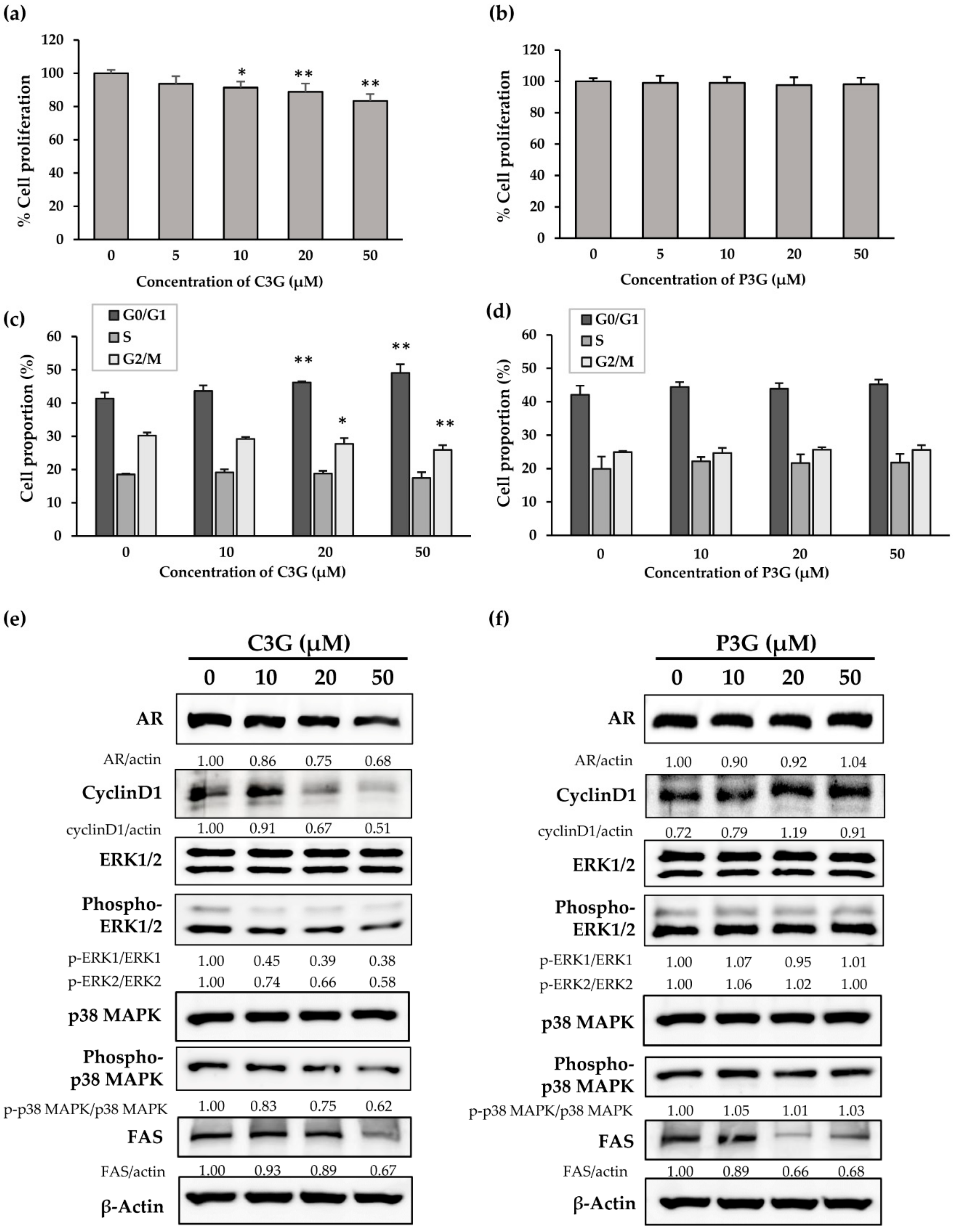
3.5. Cyanidin-3-Glucoside, but Not Peonidin-3-Glucoside, Modulates AR Expression and Attenuates ERK1/2 and p38 MAPK Activation in PCai1 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| AMPKα | Adenosine monophosphate-activated protein kinase α |
| AR | Androgen receptor |
| BPH | Benign prostatic hyperplasia |
| CRPC | Castration-resistant prostate cancer |
| CS-FBS | Charcoal-stripped FBS |
| C3G | Cyanidin-3-glucoside |
| DMSO | Dimethylsulfoxide |
| FAS | Fatty acid synthase |
| FBS | Fetal bovine serum |
| HG-PIN | High-grade prostatic intraepithelial neoplasia |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemical analysis |
| LG-PIN | Low-grade prostatic intraepithelial neoplasia |
| PRE | Crude ethanolic extract of purple rice |
| PRE-HIF | Hexane insoluble fraction of purple rice ethanolic extract |
| PRE-HSF | Hexane soluble fraction of purple rice ethanolic extract |
| PSA | Prostate-specific antigen |
| P3G | Peonidin-3-glucoside |
| Pg3G | Pelargonidin-3-glucoside |
| TRAP | Transgenic Rat for Adenocarcinoma of Prostate |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labelling |
References
- Cinar, D.; Tas, D. Cancer in the elderly. North Clin. Istanb. 2015, 2, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen deprivation therapy for prostate cancer. JAMA 2005, 294, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC 2014, 25, 42–54. [Google Scholar] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Scientific Evidence of Rice By-Products for Cancer Prevention: Chemopreventive Properties of Waste Products from Rice Milling on Carcinogenesis in Vitro and in Vivo. Biomed. Res. Int. 2017, 2017, 9017902. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Kiriya, C.; Yeewa, R.; Khanaree, C.; Chewonarin, T. Purple rice extract inhibits testosterone-induced rat prostatic hyperplasia and growth of human prostate cancer cell line by reduction of androgen receptor activation. J. Food Biochem. 2019, 43, e12987. [Google Scholar] [CrossRef]
- Yeewa, R.; Sakuludomkan, W.; Kiriya, C.; Khanaree, C.; Chewonarin, T. Attenuation of benign prostatic hyperplasia by hydrophilic active compounds from pigmented rice in testosterone implanted rat model. Food Funct. 2020. [Google Scholar] [CrossRef]
- Asamoto, M.; Hokaiwado, N.; Cho, Y.M.; Takahashi, S.; Ikeda, Y.; Imaida, K.; Shirai, T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001, 61, 4693–4700. [Google Scholar]
- Cho, Y.M.; Takahashi, S.; Asamoto, M.; Suzuki, S.; Inaguma, S.; Hokaiwado, N.; Shirai, T. Age-dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: Lack of influence of carcinogen or testosterone treatment. Cancer Sci. 2003, 94, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Nagano, A.; Mori, Y.; Kato, H.; Nagayasu, Y.; Naiki-Ito, A.; Suzuki, S.; Mori, H.; Takahashi, S. Preventive Effects of Fermented Brown Rice and Rice Bran against Prostate Carcinogenesis in TRAP Rats. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Hokaiwado, N.; Tang, M.; Ogawa, K.; Suzuki, S.; Ghanem, H.M.; Esmat, A.Y.; Asamoto, M.; Refaie, F.M.; Shirai, T. Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by leuprorelin, a luteinizing hormone-releasing hormone agonist. Cancer Sci. 2006, 97, 459–467. [Google Scholar] [CrossRef]
- Suzuki, S.; Shiraga, K.; Sato, S.; Punfa, W.; Naiki-Ito, A.; Yamashita, Y.; Shirai, T.; Takahashi, S. Apocynin, an NADPH oxidase inhibitor, suppresses rat prostate carcinogenesis. Cancer Sci. 2013, 104, 1711–1717. [Google Scholar] [CrossRef]
- Tang, M.; Ogawa, K.; Asamoto, M.; Hokaiwado, N.; Seeni, A.; Suzuki, S.; Takahashi, S.; Tanaka, T.; Ichikawa, K.; Shirai, T. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci. 2007, 98, 471–477. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Naiki, T.; Kato, H.; Iida, K.; Etani, T.; Nagayasu, Y.; Suzuki, S.; Yamashita, Y.; Inaguma, S.; Onishi, M.; et al. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis 2019. [Google Scholar] [CrossRef]
- Naiki, T.; Asamoto, M.; Toyoda-Hokaiwado, N.; Naiki-Ito, A.; Tozawa, K.; Kohri, K.; Takahashi, S.; Shirai, T. Organ specific Gst-pi expression of the metastatic androgen independent prostate cancer cells in nude mice. Prostate 2012, 72, 533–541. [Google Scholar] [CrossRef]
- Naiki, T.; Naiki-Ito, A.; Asamoto, M.; Kawai, N.; Tozawa, K.; Etani, T.; Sato, S.; Suzuki, S.; Shirai, T.; Kohri, K.; et al. GPX2 overexpression is involved in cell proliferation and prognosis of castration-resistant prostate cancer. Carcinogenesis 2014, 35, 1962–1967. [Google Scholar] [CrossRef]
- Wongjaikam, S.; Summart, R.; Chewonarin, T. Apoptosis induction in colon cancer cell lines and alteration of aberrant crypt foci in rat colon by purple rice (Oryza sativa L. var. glutinosa) extracts. Nutr. Cancer 2014, 66, 690–699. [Google Scholar] [CrossRef]
- Long, N.; Suzuki, S.; Sato, S.; Naiki-Ito, A.; Sakatani, K.; Shirai, T.; Takahashi, S. Purple corn color inhibition of prostate carcinogenesis by targeting cell growth pathways. Cancer Sci. 2013, 104, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, H.; Chen, Y.Q. Expression signature of the mouse prostate. J. Biol. Chem. 2005, 280, 36442–36451. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Chang, C.W.; Chng, K.R.; Wansa, K.D.; Sung, W.K.; Cheung, E. Integration of regulatory networks by NKX3–1 promotes androgen-dependent prostate cancer survival. Mol. Cell Biol. 2012, 32, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nakamura, S.; Tsuruma, K.; Shimazawa, M.; Shimoda, H.; Hara, H. Purple rice (Oryza sativa L.) extract and its constituents inhibit VEGF-induced angiogenesis. Phytother. Res. 2012, 26, 214–222. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Kuo, W.H.; Chiang, C.L.; Hsieh, Y.S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef]
- Yang, W.-H.; Xu, J.; Mu, J.-B.; Xie, J. Revision of the concept of anti-angiogenesis and its applications in tumor treatment. Chronic Dis. Transl. Med. 2017, 3, 33–40. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, K.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis 2010, 31, 481–488. [Google Scholar] [CrossRef]
- Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Ovatlarnporn, C.; Takahashi, S.; Limtrakul, P. Cyclohexanone curcumin analogs inhibit the progression of castration-resistant prostate cancer in vitro and in vivo. Cancer Sci. 2019, 110, 596–607. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, Y.-F.; Chen, X.-Y.; Han, B.; Li, F.; Chen, J.-Y.; Peng, X.-L.; Luo, L.-P.; Chen, W.; Yu, X.-P. Black rice-derived anthocyanins inhibit HER-2-positive breast cancer epithelial-mesenchymal transition-mediated metastasis in vitro by suppressing FAK signaling. Int. J. Mol. Med. 2017, 40, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, X.; Sun, X.; Xu, X.; Li, X.; Guo, Y.; Wang, J.; Li, X.; Yao, L.; Wang, H.; et al. Effect of PTEN loss on metabolic reprogramming in prostate cancer cells. Oncol. Lett. 2019, 17, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Frigo, D.E. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat. Rev. Urol. 2017, 14, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, R.R.; Wu, Y.; Mohler, J.L.; Ip, C. Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress. Cell. Signal. 2010, 22, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Y.; Yang, Z.; Ahmad, I.; Nixon, C.; Salt, I.P.; Leung, H.Y. AMP-activated protein kinase (AMPK) as a potential therapeutic target independent of PI3K/Akt signaling in prostate cancer. Oncoscience 2014, 1, 446–456. [Google Scholar] [CrossRef]
- Jung, S.N.; Park, I.J.; Kim, M.J.; Kang, I.; Choe, W.; Kim, S.S.; Ha, J. Down-regulation of AMP-activated protein kinase sensitizes DU145 carcinoma to Fas-induced apoptosis via c-FLIP degradation. Exp. Cell Res. 2009, 315, 2433–2441. [Google Scholar] [CrossRef]
- Park, H.U.; Suy, S.; Danner, M.; Dailey, V.; Zhang, Y.; Li, H.; Hyduke, D.R.; Collins, B.T.; Gagnon, G.; Kallakury, B.; et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol. Cancer Ther. 2009, 8, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Roskams, T.; Joniau, S.; Van Poppel, H.; Oyen, R.; Baert, L.; Heyns, W.; Verhoeven, G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 2002, 98, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; et al. Fatty acid synthase: A metabolic enzyme and candidate oncogene in prostate cancer. J. Natl. Cancer Inst. 2009, 101, 519–532. [Google Scholar] [CrossRef] [PubMed]

| Trait | Group | ||
|---|---|---|---|
| Control | 0.2% PRE-HIF | 1% PRE-HIF | |
| No. of rats | 12 | 9 | 12 |
| Initial body weights (1st Day) (g) | 180.5 ± 22.5 | 180.2 ± 31.8 | 180.3 ± 26.9 |
| Final body weights (70th Day) (g) | 609.5 ± 62.7 | 600.1 ± 65.9 | 617.2 ± 72.8 |
| Average food intake (g/rat/day) | 30.0 ± 1.8 | 29.3 ± 2.1 | 31.2 ± 1.8 |
| Average PRE-HIF intake (mg/kg/day) | 0 | 97.7 ± 7.1 | 505.0 ± 29.6 |
| Organ weights (g) | |||
| Liver | 20.7 ± 4.9 | 19.1 ± 3.1 | 20.9 ± 3.1 |
| Kidney | 3.0 ± 0.4 | 3.1 ± 0.3 | 3.1 ± 0.3 |
| Testis | 3.8 ± 0.3 | 3.7 ± 0.3 | 3.7 ± 0.3 |
| Ventral prostate | 0.27 ± 0.06 | 0.31 ± 0.07 | 0.27 ± 0.07 |
| Treatment | No. of Rats | Incidence of Adenocarcinoma (%) | % of Lesion in Prostates a | ||
|---|---|---|---|---|---|
| LG-PIN | HG-PIN | Adenocarcinoma | |||
| Ventral prostates | |||||
| Control | 12 | 12 (100%) | 7.2 ± 3.1 | 81.4 ± 7.2 | 11.4 ± 6.5 |
| 0.2% PRE-HIF | 9 | 9 (100%) | 10.5 ± 4.1 | 81.2 ± 4.1 | 8.3 ± 5.0 |
| 1% PRE-HIF | 12 | 11 (92%) | 8.3 ± 4.6 | 85.1 ± 4.6 | 6.6 ± 4.2 |
| Lateral prostates | |||||
| Control | 12 | 12 (100%) | 8.4 ± 2.9 | 85.4 ± 5.2 | 6.5 ± 3.7 |
| 0.2% PRE-HIF | 9 | 6 (67%) | 11.3 ± 5.7 | 84.7 ± 5.3 | 4.0 ± 3.5 |
| 1% PRE-HIF | 12 | 6 (50%) * | 16.3 ± 8.1 ** | 81.0 ± 7.4 | 2.7 ± 3.7 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeewa, R.; Naiki-Ito, A.; Naiki, T.; Kato, H.; Suzuki, S.; Chewonarin, T.; Takahashi, S. Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism. Nutrients 2020, 12, 558. https://doi.org/10.3390/nu12020558
Yeewa R, Naiki-Ito A, Naiki T, Kato H, Suzuki S, Chewonarin T, Takahashi S. Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism. Nutrients. 2020; 12(2):558. https://doi.org/10.3390/nu12020558
Chicago/Turabian StyleYeewa, Ranchana, Aya Naiki-Ito, Taku Naiki, Hiroyuki Kato, Shugo Suzuki, Teera Chewonarin, and Satoru Takahashi. 2020. "Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism" Nutrients 12, no. 2: 558. https://doi.org/10.3390/nu12020558
APA StyleYeewa, R., Naiki-Ito, A., Naiki, T., Kato, H., Suzuki, S., Chewonarin, T., & Takahashi, S. (2020). Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism. Nutrients, 12(2), 558. https://doi.org/10.3390/nu12020558






